Liver cirrhosis is characterized by increased intrahepatic resistance, splanchnic vasodilation/angiogenesis, and formation of portosystemic collateral vessels. Collaterals can cause lethal complications such as gastroesophageal variceal hemorrhage. Homocysteine is linked to vascular dysfunction and angiogenesis and higher levels have been reported in cirrhotic patients. It is also known that folic acid supplementation reverses the effects of homocysteine. However, the treatment effect in cirrhosis has yet to be investigated.
Material and methodsLiver cirrhosis was induced in Sprague-Dawley rats with common bile duct ligation (CBDL). The CBDL rats randomly received (1) vehicle; (2) dl-homocysteine thiolactone (1g/kg/day); (3) dl-homocysteine thiolactone plus folic acid (100mg/kg/day); or (4) folic acid. On the 29th day, hemodynamic parameters, liver and renal biochemistry, protein expressions of proangiogenic factors, mesenteric vascular density and portosystemic shunting were evaluated.
ResultsIn the cirrhotic rats, homocysteine increased mesenteric vascular density and the severity of shunting. It also up-regulated the protein expressions of mesenteric vascular endothelial growth factor (VEGF) and phosphorylated-endothelial nitric oxide synthase (p-eNOS). These effects were reversed by folic acid treatment (P<0.05).
ConclusionFolic acid ameliorated the adverse effects of homocysteine in the cirrhotic rats, which may be related to down-regulation of the VEGF-NO signaling pathway.
Liver cirrhosis with portal hypertension is characterized by increased intrahepatic resistance due to a distortion of intrahepatic vasculature caused by fibrosis, regeneration nodules and enhanced vasoconstrictive vascular tone [1–3]. In contrast, the extrahepatic vascular beds, especially splanchnic and systemic vasculature, are vasodilated with splanchnic hyperemia and increased blood flow, leading to elevated portal inflow and pressure. Portosystemic collaterals then develop in order to divert the stagnant portal blood from the hypertensive portal system and such collaterals are prone to rupture with massive hemorrhage. Among them, gastroesophageal varices are the most notorious and the control of variceal bleeding significantly influences the prognosis of cirrhotic patients.
Recent studies have reported that angiogenesis is involved in the development and maintenance of splanchnic hyperemia and portosystemic collaterals in cirrhosis and portal hypertension, and that vascular endothelial growth factor (VEGF) plays a prominent role in the process. In portal hypertensive rats, anti-VEGF receptor-2 (VEGFR-2) monoclonal antibody and the inhibitor of VEGFR-2 autophosphorylation have been shown to inhibit the formation of portosystemic collaterals and alleviated the severity of portal hypertension [4].
Homocysteine is formed during the metabolism of methionine, an essential amino acid derived from dietary protein [5]. Due to the adverse vascular effects [6], hyperhomocysteinemia is a known risk factor for coronary heart disease and peripheral vascular diseases [7]. Surprisingly, the plasma homocysteine level has been shown to be higher in cirrhotic patients [8], regardless of the etiology of cirrhosis [9]. An inverse correlation has also been reported between concentrations of homocysteine and folate [9]. Furthermore, a recent study reported an association between hyperhomocysteinemia and the severity of cirrhosis and that this exerted a negative impact after liver transplantation [10].
Several studies have investigated the vascular influences of homocysteine, including angiogenesis. However, these studies were not performed on cirrhotic subjects and the results are controversial: homocysteine has been reported to increase the synthesis of VEGF [11–13]. On the contrary, some studies indicated that homocysteine impaired angiogenesis [14–16]. Indeed, the relevant influences in cirrhosis deserve further investigation.
Folic acid supplementation has been used to treat hyperhomocysteinemia and endothelial dysfunction [17,18]. In patients with peripheral arterial disease and diabetes, the administration of folic acid and vitamins B1, B6, B12 have been shown to significantly reduce homocysteine levels and down-regulate VEGF expression in leukocytes [19]. In a human study, the plasma level of dimethylarginine, an endogenous inhibitor of NO, was shown to be positively correlated with plasma homocysteine and negatively correlated with folic acid levels [20]. Of note, plasma folate concentration have been shown to be lower in cirrhotic patients compared with controls [21], in whom inadequate dietary intake and urinary folate loss are considered to be responsible for folate deficiency in patients with chronic liver disease [22]. Since previous studies have indicated higher homocysteine levels in cirrhotic patients, it would seem to be reasonable to give such patients a higher dose of folic acid to combat the adverse effects of homocysteine.
Taken together, the current literature indicates the diverse actions of homocysteine on angiogenesis, the action of folate on homocysteine, and high homocysteine and low folate levels in cirrhotic patients. In addition, due to the lack of relevant studies on cirrhosis with portal hypertension, this study was designed to investigate the impacts of homocysteine and folic acid treatment on mesenteric angiogenesis and severity of portosystemic collaterals in rats with common bile duct ligation (CBDL)-induced chronic liver injury.
2Material and methods2.1Animal modelMale Sprague-Dawley rats weighing 240–270g at the time of surgery were used. Secondary biliary cirrhosis was induced 4 weeks after common bile duct ligation (CBDL) as previously described [23]. Animals were allowed an acclimatization period for 1 week at the Animal Center of Department of Medical Research, Taipei Veterans General Hospital. Rats were housed at constant temperature (25±2°C), humidity (60±10%) and a light/dark (12/12h) cycle. Access to food and water throughout the experimental period was allowed ad libitum. This study was approved by Taipei Veterans General Hospital Animal Committee (grant numbers: IACUC2013-151, IACUC2013-191, and IACUC2013-194). All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health, the United States (NIH publication 86-23, revised 1985).
2.2Measurement of systemic and portal hemodynamicsMean arterial pressure (MAP), heart rate (HR) and portal pressure (PP) were measured by catheterization of the right femoral artery and ileocolic vein respectively, which were connected to Spectramed DTX transducer (Spectramed Inc., Oxnard, CA, U.S.A.). MAP, HR and PP were continuously recorded on a multi-channel recorder (model RS 3400, Gould Inc., Cupertino, CA, U.S.A.).
Superior mesenteric artery (SMA) flow and portal vein (PV) flow were measured by a pulsed-Doppler flow transducer (T206 small animal blood flow meter, Transonic System Inc., Ithaca, NY, U.S.A.). Cardiac output (CO) was measured by thermodilution, as previously described [24]. Cardiac index (CI, ml/min/100g body weight (BW)) was calculated as CO per 100g BW. Stoke volume (SV) was calculated as CO divided by HR (ml/beats). Systemic vascular resistance (SVR, mmHg/ml/min/100g BW) was calculated as MAP divided by CI, and SMA resistance (mmHg/ml/min/100g BW) was calculated as (MAP-PP)/SMA flow per 100g BW.
2.3Portosystemic shunting analysisPortosystemic shunting was determined as previously described, substituting color for radioactive microspheres [25]. Portal-systemic shunting was calculated as lung/(liver+lung) microspheres. Color microspheres have been shown to provide similar results to radioactive microspheres [26].
2.4Immunofluorescent study for mesenteric vascular densityMesenteric angiogenesis was quantified using CD31-labeled microvascular networks in rat mesenteric connective tissue windows according to previous studies [25], [27].
2.5Western analysisProtein expressions were analyzed by Western blotting as previously described [28]. Blots were incubated with the primary antibodies [inducible NO synthase (iNOS), endothelial NO synthase (eNOS), phospho-eNOS (p-eNOS), cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX-2), platelet-derived growth factor receptor β (PDGFRβ): Cell Signaling Technology, Beverly, MA, U.S.A.; VEGF, phospho-VEGFR-2 (p-VEGFR2), platelet-derived growth factor (PDGF): Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.]. The blots were then incubated with the appropriate secondary antibodies (peroxidase-labeled anti-rabbit IgG or anti-mouse IgG, Cell Signaling Technology). The specific proteins were detected by enhanced chemiluminescence (Immobilon Western Chemiluminescent HRP Substrate, Merk Millipore Co., Billerica, MA, U.S.A.). With a computer assisted video densitometer and digitalized system (BioSpectrum® 600 Imaging System, Ultra-Violet Products Ltd., Upland, CA, U.S.A.), the blots were scanned, photographed and then the signal intensity (integral volume) of the appropriate band was analyzed.
2.6Measurement of biochemical parameters of liver and renal injuryAlanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, blood urea nitrogen (BUN), and creatinine levels were measured with a Cobas Mira analyzer (GMI Inc., Ramsey, MN, U.S.A.).
2.7Drugsdl-Homocysteine thiolactone and folic acid were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, U.S.A.).
2.8Data analysisAll results are expressed as mean±S.D. Statistical analyses were performed using an unpaired Student's t-test or ANOVA with LSD test as appropriate. Results are considered statistically significant at a two-tailed P-value less than 0.05. SPSS version 21 statistical software package for Windows (SPSS Inc., Chicago, IL, USA) was used for all analyses.
2.9Study protocolCommon bile duct ligation was performed in male Sprague-Dawley rats. CBDL rats developed significant liver fibrosis 2 weeks after CBDL and liver cirrhosis 4 weeks after CBDL [23]. CBDL rats were subdivided to receive the following treatments since the 14th to 28th day after surgery: (1) vehicle (V group); (2) dl-homocysteine thiolactone (1g/kg/day, oral gavage, H group); (3) dl-homocysteine thiolactone with folic acid (100mg/kg/day according to a previous study [29], oral gavage, H+F group); (4) folic acid (F group). On the 29th day after CBDL, two series of experiments using four independent treatment groups for each series were performed: first series (n=10 for each group at the time of CBDL): measurement of the severity of portosystemic shunting using color microsphere method; second series (n=10 for each group at the time of CBDL): (a) BW and hemodynamic measurements including: MAP, HR, PP, CO, CI, SV, SVR, SMA flow, PV flow; (b) measurements of plasma levels of ALT, AST, total bilirubin, BUN, and creatinine; (c) mesenteric protein expressions of VEGF, VEGFR2, p-VEGFR2, PDGF, PDGFR-β, eNOS, p-eNOS, iNOS, COX-1, COX-2 with Western blot analysis; (d) mesenteric vascular density assessment with immunofluorescence.
3Results3.1HemodynamicsHomocysteine significantly reduced the BW (g) as compared with the vehicle (control) group [vehicle (V, n=8) vs. homocysteine (H, n=7) vs. homocysteine plus folic acid (H+F, n=5) vs. folic acid (F, n=8)]: 395.0±36.1 vs. 338.6±23.4 vs. 367.6±29.6 vs. 369.6±34.7; V vs. H, P=0.001.
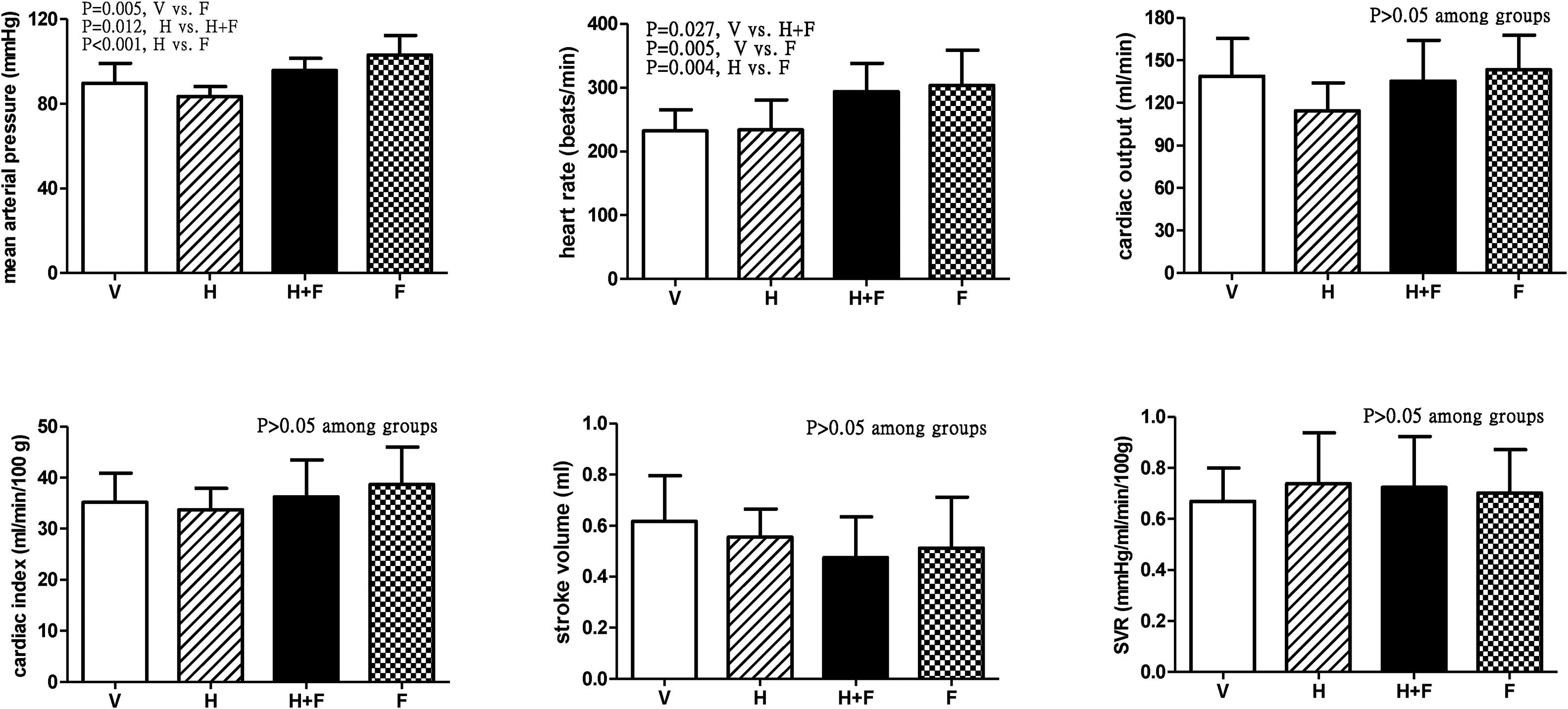
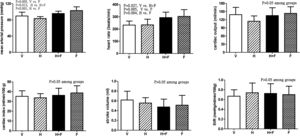
Fig. 1 shows the systemic hemodynamic data of the study groups after the received treatment. Folate significantly elevated MAP and HR. The MAP values (mmHg) after each different treatment were 89.9±9.4 (V) vs. 83.4±4.8 (H) vs. 95.8±5.7 (H+F) vs. 103.2±9.2 (F) (V vs. F, P=0.005; H vs. H+F, P=0.012; and H vs. F, P<0.001). While HR values (bpm) were 232.3±33.3 (V) vs. 233.7±47.2 (H) vs. 293.8±44.4 (H+F) vs. 303.3±55.5 (F) (V vs. H+F, P=0.027; V vs. F, P=0.005; and H vs. F, P=0.004). There were no significant differences in other hemodynamic parameters among the groups, including CO, CI, SV, and SVR (all P>0.05 among groups).
Systemic hemodynamic parameters of cirrhotic rats with vehicle (V), homocysteine (H), homocysteine plus folic acid (H+F) or folic acid (F) administration. The number of each group was 8, 7, 5, 8, respectively. SVR: systemic vascular resistance. Folic acid significantly increased mean arterial pressure and heart rate as compared with the vehicle and homocysteine (P<0.05).
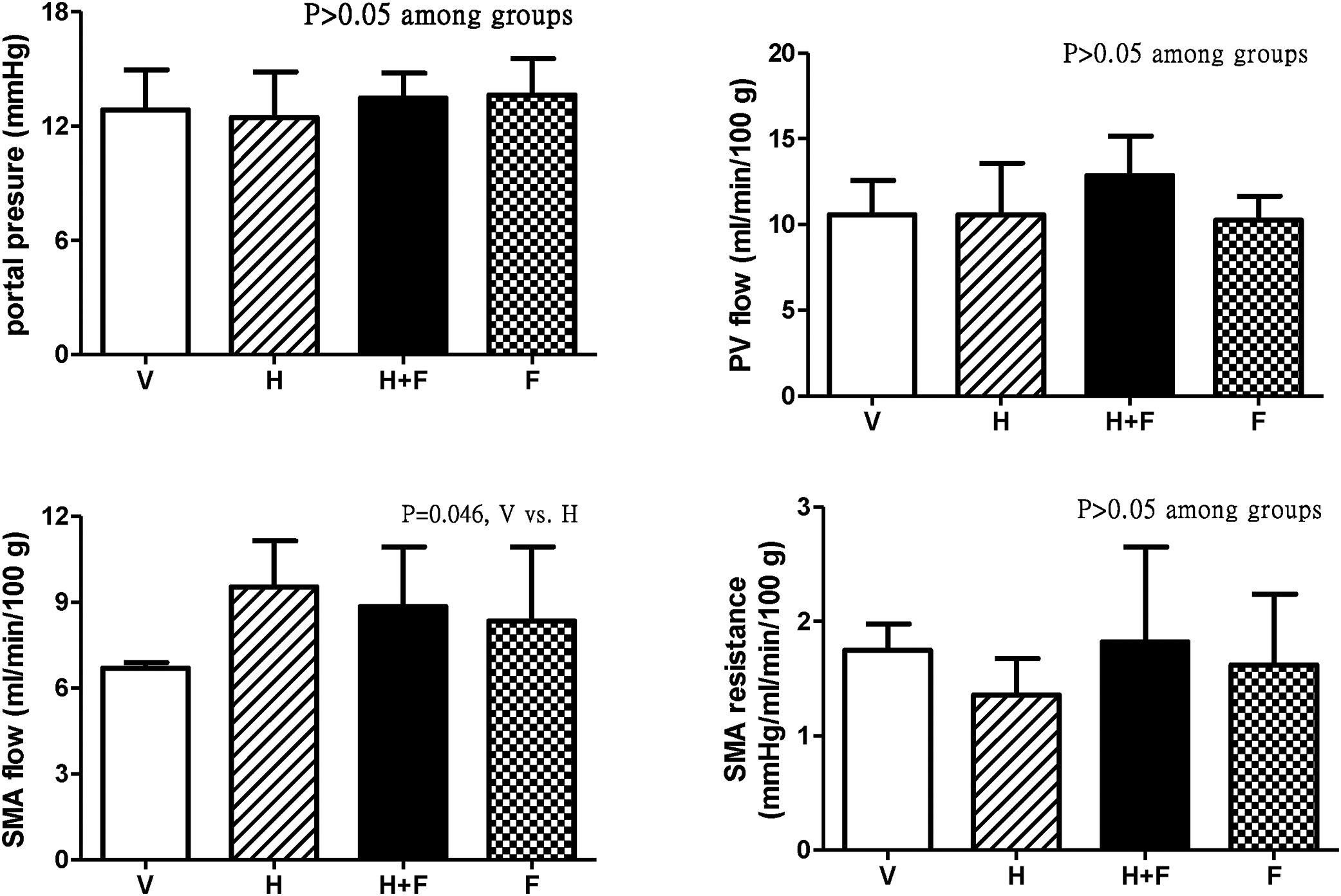
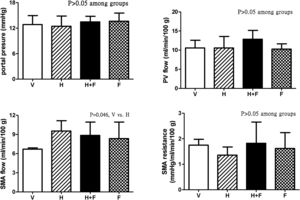
Fig. 2 shows the splanchnic hemodynamic data of the study groups after the received treatment. There was no significant difference of PP, PV flow and SMA resistance among groups (P>0.05) except that homocysteine increased SMA flow (ml/min/100g) as compared with vehicle, which was tended to be reduced by folic acid [6.7±0.2 (V) vs. 9.5±1.6 (H) vs. 8.8±2.1 (H+F) vs. 8.3±2.6 (F); V vs. H, P=0.046].
Splanchnic hemodynamic parameters of cirrhotic rats with vehicle (V), homocysteine (H), homocysteine plus folic acid (H+F) or folic acid (F) administration. The number of each group was 8, 7, 5, 8, respectively. As compared with the vehicle group, homocysteine significantly increased SMA flow (P=0.046), which was tended to be reduced by folic acid. SMA: superior mesenteric artery; PV: portal vein.
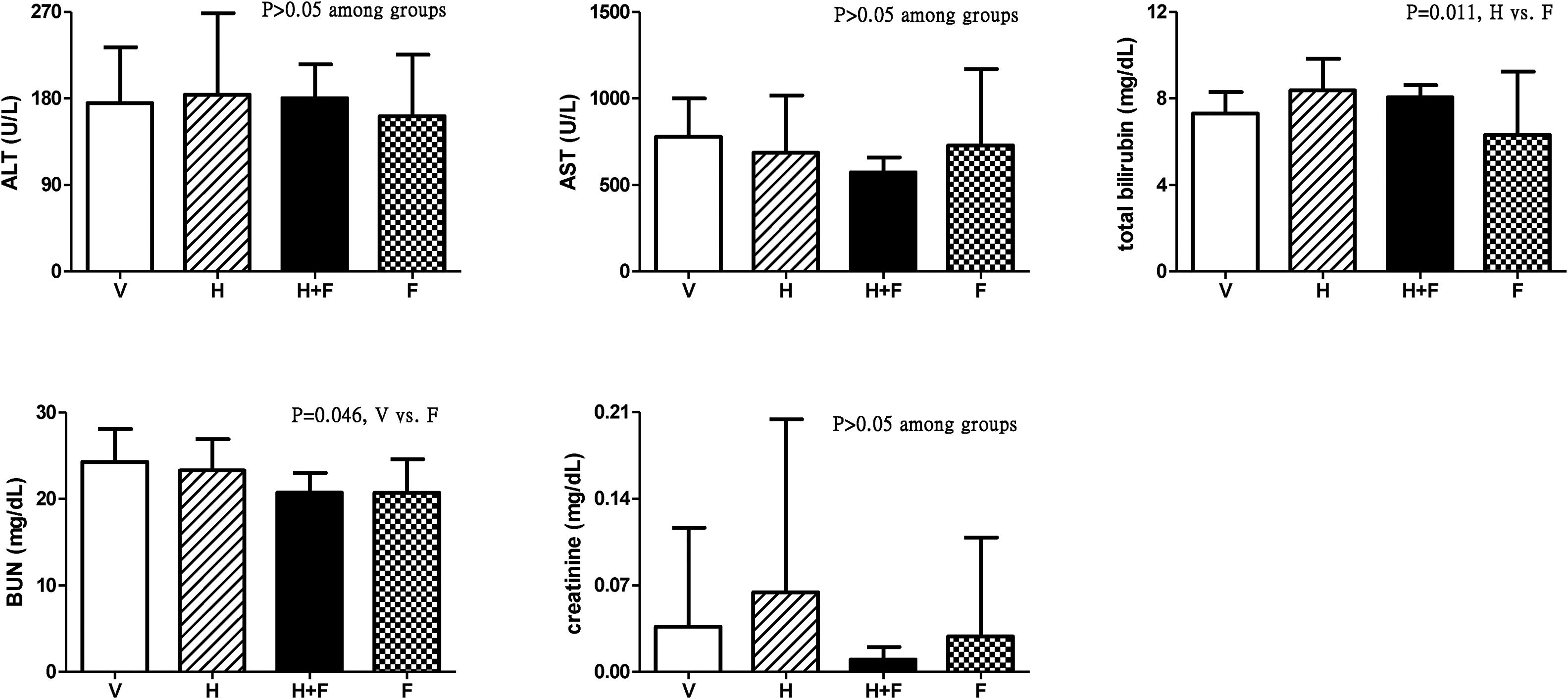
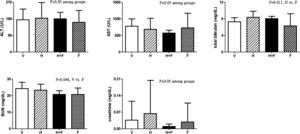
Fig. 3 shows the liver and renal biochemistry data [V (n=8) vs. H (n=7) vs. H+F (n=5) vs. F (n=8)]. Folic acid did not significantly influence ALT, AST or Cr levels but it decreased BUN and total bilirubin levels as compared with the vehicle and homocysteine groups: BUN (mg/dL): 24.3±3.8 vs. 23.3±3.6 vs. 20.7±2.3 vs. 20.7±3.9, V vs. F, P=0.046; total bilirubin (mg/dL): 7.31±1.00 vs. 8.39±1.45 vs. 8.06±0.56 vs. 6.33±2.92, H vs. F, P=0.011.
Plasma levels of liver and renal biochemistry in cirrhotic rats with vehicle (V), homocysteine (H), homocysteine plus folic acid (H+F) or folic acid (F) administration. The number of each group was 8, 7, 5, 8, respectively. Folic acid significantly reduced the BUN and total bilirubin levels (P<0.05). ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen.
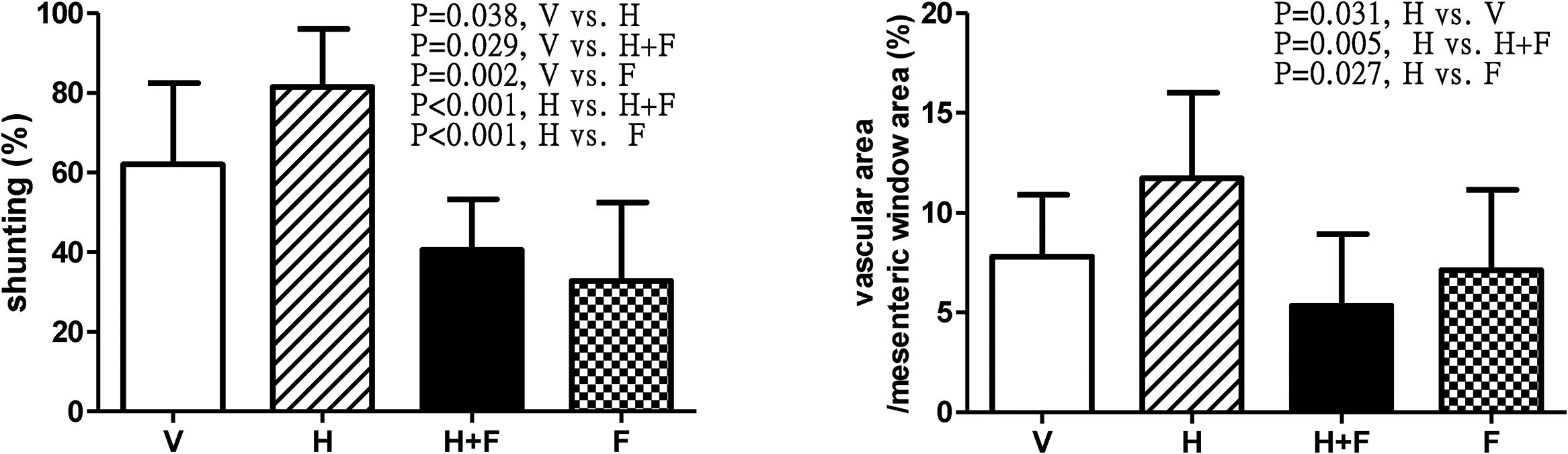
Fig. 4 depicts the shunting [V (n=10) vs. H (n=8) vs. H+F (n=6) vs. F (n=8)] and mesenteric window vascular area ratio [V (n=8) vs. H (n=7) vs. H+F (n=5) vs. F (n=8)]. Homocysteine significantly increased the shunting ratio (%) [62.06±20.37 (V) vs. 81.49±14.58 (H) vs. 40.51±12.79 (H+F) vs. 32.80±19.68 (F); V vs. H, P=0.038; V vs. H+F, P=0.029; V vs. F, P=0.002; H vs. H+F, P<0.001; and H vs. F, P<0.001], which was reduced by folic acid. The mesenteric vascular density (vascular area/mesenteric window area (%): 7.81±3.09 vs. 11.73±4.27 vs. 5.35±3.58 vs. 7.12±4.02, H vs. V, P=0.031; H vs. H+F, P=0.005; H vs. F, P=0.027) also exhibited the same statistical trend.
Shunting ratio and mesenteric vascular density in cirrhotic rats with vehicle (V), homocysteine (H), homocysteine plus folic acid (H+F) or folic acid (F) administration. The number of each group was 10, 8, 6, 8 for shunting study and 8, 7, 5, 8 for vascular density study, respectively. As compared with vehicle, homocysteine significant increased shunting ratio and vascular area per unit area of mesenteric window (P<0.05), which were reversed by folic acid (P<0.05).
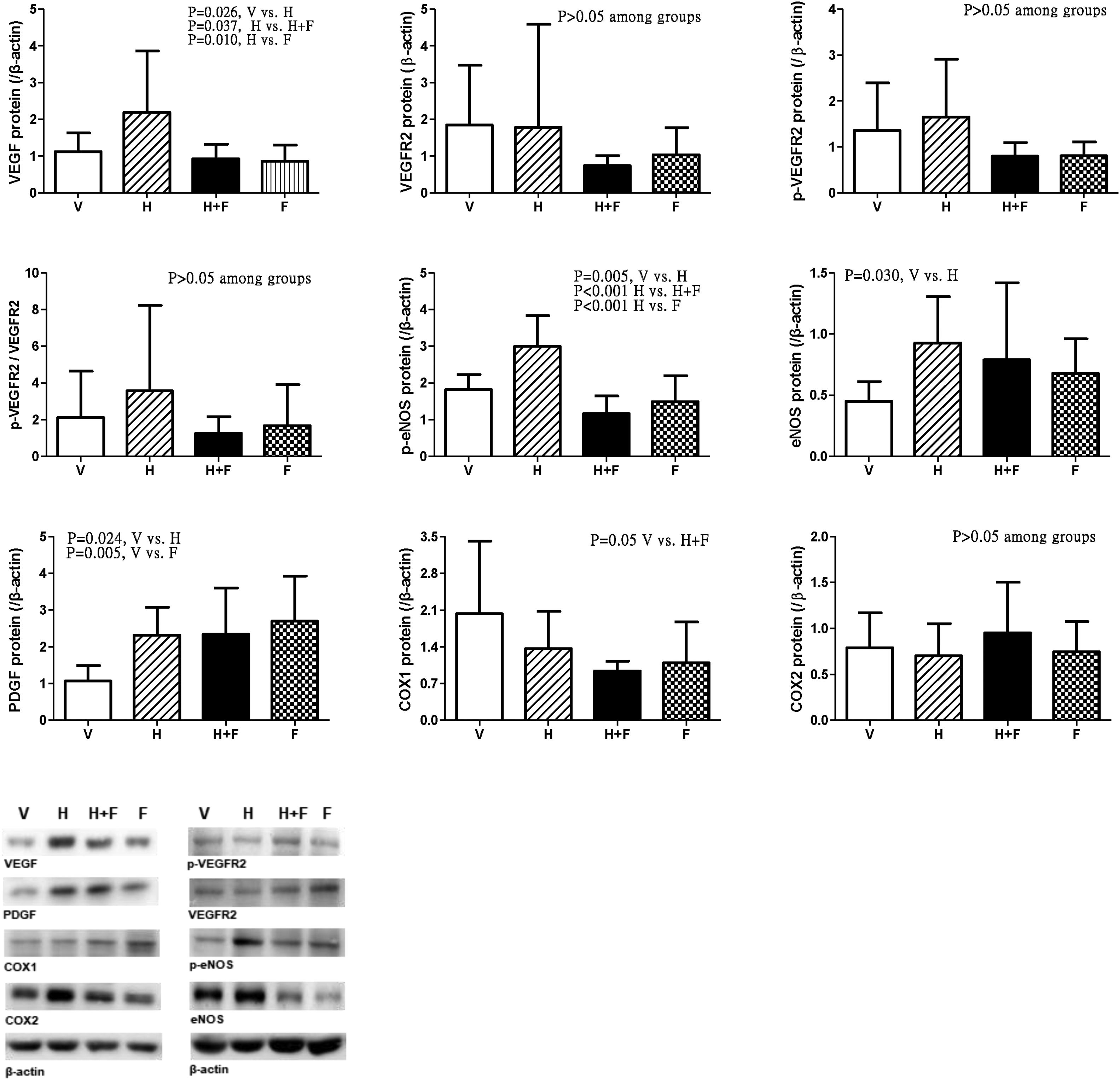
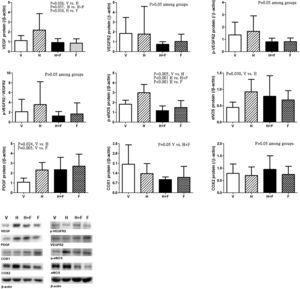
Fig. 5 discloses the protein expressions of mesenteric proangiogenic factors [V (n=8) vs. H (n=7) vs. H+F (n=5) vs. F (n=8)]. There were no significant differences of VEGFR-2, p-VEGFR2 and COX2 expressions. Homocysteine significantly up-regulated VEGF and p-eNOS expressions, which were down-regulated by folic acid [VEGF (/β-actin): 1.12±0.51 (V) vs. 2.19±1.67 (H) vs. 0.93±0.40 (H+F) vs. 0.86±0.44 (F), V vs. H, P=0.026; H vs. H+F, P=0.037; H vs. F, P=0.010; p-eNOS: 1.82±0.41 vs. 3.00±0.83 vs. 1.16±0.48 vs. 1.49±0.71, V vs. H, P=0.005; H vs. H+F, P<0.001; H vs. F, P<0.001]. Homocysteine up-regulated eNOS expression as compared with vehicle [eNOS: 0.45±0.16 vs. 0.93±0.38 vs. 0.79±0.63 vs. 0.68±0.28, V vs. H, P=0.030]. There were no significant differences in COX1 expressions among the study groups [COX1: 2.03±1.38 vs. 1.37±0.71 vs. 0.94±0.19 vs. 1.09±0.78, V vs. H+F, P=0.050]. Homocysteine tended to increase the p-VEGFR2/VEGFR2, which was tended to be reduced by folate (P>0.05).
Mesenteric proangiogenic factors protein expressions in cirrhotic rats with vehicle (V), homocysteine (H), homocysteine plus folic acid (H+F) or folic acid (F) administration. The number of each group was 8, 7, 5, 8, respectively. As compared with vehicle, homocysteine significantly increased VEGF and p-eNOS expressions (P<0.05), which were down-regulated by folic acid (P<0.05).
Hyperdynamic circulatory status is characterized by systemic hypotension and extrahepatic vasodilatation. Compatible with our previous study [30], homocysteine did not affect MAP and HR in cirrhotic rats in this study. However, the addition of folic acid to homocysteine and the single administration of folic acid significantly increased MAP and HR in cirrhotic rats. MAP is positively correlated with HR and SV. Since the SV was not influenced by folate, the elevation of MAP might be related to an increase in HR. Although it has been reported that in male smokers, moderate amount of folic acid and vitamins supplement increased the vagal control of HR (+23%; P<0.05) [31], interpolating results from different study designs should be made with caution. Based on the currently available results, we could only note that the hemodynamic influence of folic acid in cirrhotic subjects with portal hypertension has not been reported previously and our finding suggests that folic acid may be able to reverse the profound systemic hypotension in portal hypertension with hyperdynamic circulation. Furthermore, the homocysteine-induced elevation of SMA flow tended to be reduced by folic acid. The reduction of blood flow in splanchnic vascular bed may lessen the drive to develop portosystemic collaterals.
The influences of homocysteine on angiogenesis have been debated. For instance, it has been found that in homocysteine-treated cultured cells, there was a 1.3-fold increased secretion of VEGF [11]. Another study showed that homocysteine up-regulated VEGF mRNA expression in a dose- and time-dependent manner in macrophages, followed by increased VEGF secretion [12]. Hyperhomocysteinemia also increased VEGF expression in rat retina [13]. In contrast, Nagai et al. found that the plasma concentration of homocysteine was inversely correlated with the development of collateral circulation in patients with coronary artery disease [14]. Moreover, hyperhomocysteinemia impaired ischemia-induced angiogenesis and collateral vascular formation in a rat model with hindlimb ischemia, and that this may have been mediated by reduced NO bioavailability in a hyperhomocysteinemic state [15]. A recent study also demonstrated that homocysteine induced growth arrest in human endothelial cells [16]. The differences in these results may be related to the differences in study design with various diseases models and subjects.
Interestingly, the current study found that the shunting degree, which represents the severity of collaterals, was significantly reduced by folic acid in cirrhotic rats, with or without hyperhomocysteinemia. In addition, the mesenteric vascular density and VEGF expression were also down-regulated. Therefore, folic acid reduced shunting in two steps: first, by decreasing splanchnic blood flow and mitigating the stress to form portosystemic collaterals; second, by reducing splanchnic angiogenesis related to VEGF down-regulation. In addition, the mesenteric protein expression of p-eNOS, representing eNOS activation, was also upregulated by homocysteine and reversed by folate. Interestingly, it has been demonstrated in cultured endothelial cells that folic acid activated eNOS via the modulation of eNOS phosphorylation [32]. Furthermore, eNOS is the downstream effector of VEGF in angiogenesis [33]. Although previous studies have suggested the anti-angiogenesis effect of homocysteine [15,16], the homocysteine concentrations were 0.2–5mmol/L, much higher than those in patients with hyperhomocysteinemia. Our previous investigation also revealed that in cirrhotic rats with homocysteinemia, the plasma concentration was 18.4±0.9μM [30], much lower than those of the aforementioned studies [15,16]. More portosystemic collaterals were also noted as compared with the vehicle-administered to cirrhotic rats [30], supporting the angiogenesis effect of homocysteine in this disease entity. Therefore, folic acid may overcome homocysteine-induced pathological angiogenesis in cirrhosis via down-regulating VEGF-eNOS signaling pathway. Of note, the techniques used to survey the tissue derangements caused by cirrhosis in animal studies are usually invasive and harmful, such as the microsphere method to determine shunting and CD31 immunofluorescent staining for mesenteric angiogenesis. Such methods are not readily applied to patients because of the associated organ damages. Hence, non-invasive modalities have been developed, such as the diffusion-weighted MR imaging, for the prediction of esophageal varices and liver fibrosis [34–37] and they are believed to be promising tools for clinical investigations and applications.
Folic acid also significantly reduced total bilirubin level in cirrhotic rats. Since bilirubin is a surrogate marker of liver function, the finding provides the beneficial evidence of folic acid use in cirrhosis. To sum up, folic acid reversed homocysteine-induced systemic hypotension in cirrhotic rats. The severity of shunting and mesenteric angiogenesis were also mitigated, which may be related to VEGF-eNOS pathway downregulation. Folic acid may be a feasible candidate to alleviate portosystemic collaterals in cirrhosis. Nevertheless, further clinical investigation is required.AbbreviationsALT alanine aminotransferase aspartate aminotransferase blood urea nitrogen body weight common bile duct ligation cluster of differentiation 31 cardiac index cardiac output cyclooxygenase endothelial NO synthase folic acid-treated group dl-homocysteine thiolactone-treated group dl-homocysteine thiolactone with folic acid-treated group heart rate mean arterial pressure platelet-derived growth factor receptor β phosphorylated-endothelial nitric oxide synthase portal pressure portal vein superior mesenteric artery stoke volume systemic vascular resistance vascular endothelial growth factor VEGF receptor-2 vehicle-treated group
The authors have no conflicts of interest to declare.
This work was supported by a grant from Taipei Veterans General Hospital, Taipei, Taiwan (VGHUST103-G1-2-3), which was incorporated in the Veterans General Hospitals University System of Taiwan Joint Research Program, Taiwan. We gratefully acknowledge Chia-chi Lu for her excellent technical assistances. We also thank the Clinical Research Core Laboratory and Animal Center of Department of Medical Research, Taipei Veterans General Hospital for experimental space and facilities.