A 76-year-old woman was referred to our hospital due to massive gingival bleeding following teeth extraction. Laboratory findings suggested disseminated intravascular coagulopathy (DIC). Enhanced computed tomography and magnetic resonance imaging disclosed multiple hypervascular liver masses of 2-6 cm in diameter, the largest of which displaying an irregular enhancement pattern. We considered that her DIC was caused by the multiple liver masses and commenced repeated erythrocyte/fresh frozen plasma infusion and gabexate mesilate administration. However, the DIC proved uncontrollable and trans-arterial embolization could not be attempted. The patient eventually died 4 months after admission due to spontaneous hepatic tumor rupture and hepatic failure. Post-mortem hepatic tumor biopsy led to a final diagnosis of hepatic angiosarcoma with Kasabach-Merritt phenomenon (KMP). Among the 7 cases of hepatic angiosarcoma representing KMP found in the literature, mortality occurred within 4 months of the appearance of bleeding tendency primarily due to abdominal bleeding and hepatic failure. The possibility of hepatic angiosarcoma should be considered in patients with DIC and hypervascular liver tumors. Since treatment is uncertain and prognosis is poor, novel diagnostic and therapeutic advances are needed for angiosarcoma.
Disseminated intravascular coagulopathy (DIC) is characterized by massive microthrombi appearing through various mechanisms.1 Enhanced thrombolysis and consumption of coagulation factors in DIC lead to bleeding tendency, thrombocytopenia, hypofibrinogenemia, and increased circulating fibrinogen degradation product (FDP).1 Although DIC is frequently accompanied by severe infection, leukemia, and cancer cell invasion of the bone marrow, some cases are caused by tumors of vascular origin, such as cavernous hemangioendothelioma.1 Hepatic angiosarcoma is an uncommon malignant vascular tumor of the liver that is very rarely accompanied with DIC.2 We herein report a case of hepatic angiosarcoma showing Kasabach-Merritt phenomenon (KMP), review its clinical features, and compare it with existing cases in the literature.
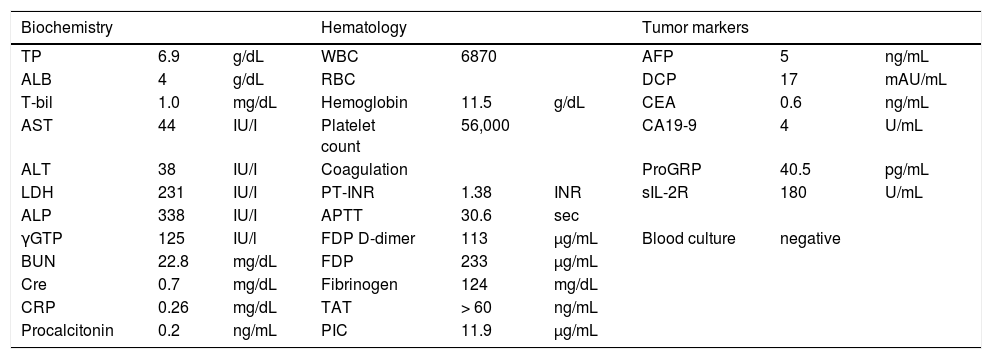
Case ReportA 76-year-old woman was referred to our outpatient clinic due to bouts of persistent or massive gingival bleeding following teeth extraction two weeks prior. On admission, she had no history of drinking, blood transfusion, medicine or supplement regimen, or occupation handling vinyl chloride or heavy metals. She did not suffer from weight loss, general fatigue, anorexia, or abdominal fullness or pain. Physical examination showed no jaundice, purpura, lymphadenopathy, or splenomegaly, but a soft liver was palpable in the epigastric region. Laboratory findings revealed decreased platelet count (56,000/μL) and circulating fibrinogen level (124 μg/mL) with elevated FDP level (233 μg/mL), which were suggestive of DIC (Table 1). Microscopic examination of peripheral blood cells detected no atypical leukocytes or fragmented erythrocytes. Serum levels of lactate dehydrogenase and the tumor markers alpha fetoprotein, des-gamma-carboxy prothrombin, carcinoembryonic antigens, carbohydrate antigen, pro-gastrin-releasing peptide, and soluble interleukin 2 receptor were all within normal ranges (Table 1). Negative blood culture results and normal levels of serum C-reactive protein and procalcitonin indicated a low possibility of underlying severe infection or inflammation (Table 1).
Laboratory data on admission.
| Biochemistry | Hematology | Tumor markers | ||||||
|---|---|---|---|---|---|---|---|---|
| TP | 6.9 | g/dL | WBC | 6870 | AFP | 5 | ng/mL | |
| ALB | 4 | g/dL | RBC | DCP | 17 | mAU/mL | ||
| T-bil | 1.0 | mg/dL | Hemoglobin | 11.5 | g/dL | CEA | 0.6 | ng/mL |
| AST | 44 | IU/I | Platelet count | 56,000 | CA19-9 | 4 | U/mL | |
| ALT | 38 | IU/I | Coagulation | ProGRP | 40.5 | pg/mL | ||
| LDH | 231 | IU/I | PT-INR | 1.38 | INR | sIL-2R | 180 | U/mL |
| ALP | 338 | IU/I | APTT | 30.6 | sec | |||
| γGTP | 125 | IU/l | FDP D-dimer | 113 | μg/mL | Blood culture | negative | |
| BUN | 22.8 | mg/dL | FDP | 233 | μg/mL | |||
| Cre | 0.7 | mg/dL | Fibrinogen | 124 | mg/dL | |||
| CRP | 0.26 | mg/dL | TAT | > 60 | ng/mL | |||
| Procalcitonin | 0.2 | ng/mL | PIC | 11.9 | μg/mL |
AFP: alpha fetoprotein. ALB: albumin. ALP: alkaline phosphatase. ALT: alanine transaminase. APTT: activated partial thiromboplastin time. AST: aspartate aminotransferase. BUN: blood urea nitrogen. CA19-9: carbohydrate antigen 19-9. CEA: carcinoembryonic antigen. Cre. creatinine. CRP: C-reactive protein. DCP: des-gamma-carboxy prothrombin. FDP: fibrinogen degradation product γGTP: gamma-glutamyltranspeptidase. LDH: lactate dehydrogenase. PIC: plasmin-α2 plasmin inhibitor complex. ProGRP: pro-gastrin-releasing peptide. PT-INR: international normalized ratio of prothrombin time. RBC: red blood cells. sIL-2R: soluble interleukin 2 receptor. TAT: thrombin-antithrombin complex. T-bil: total bilirubin. TP: total protein. WBC: white blood cells.
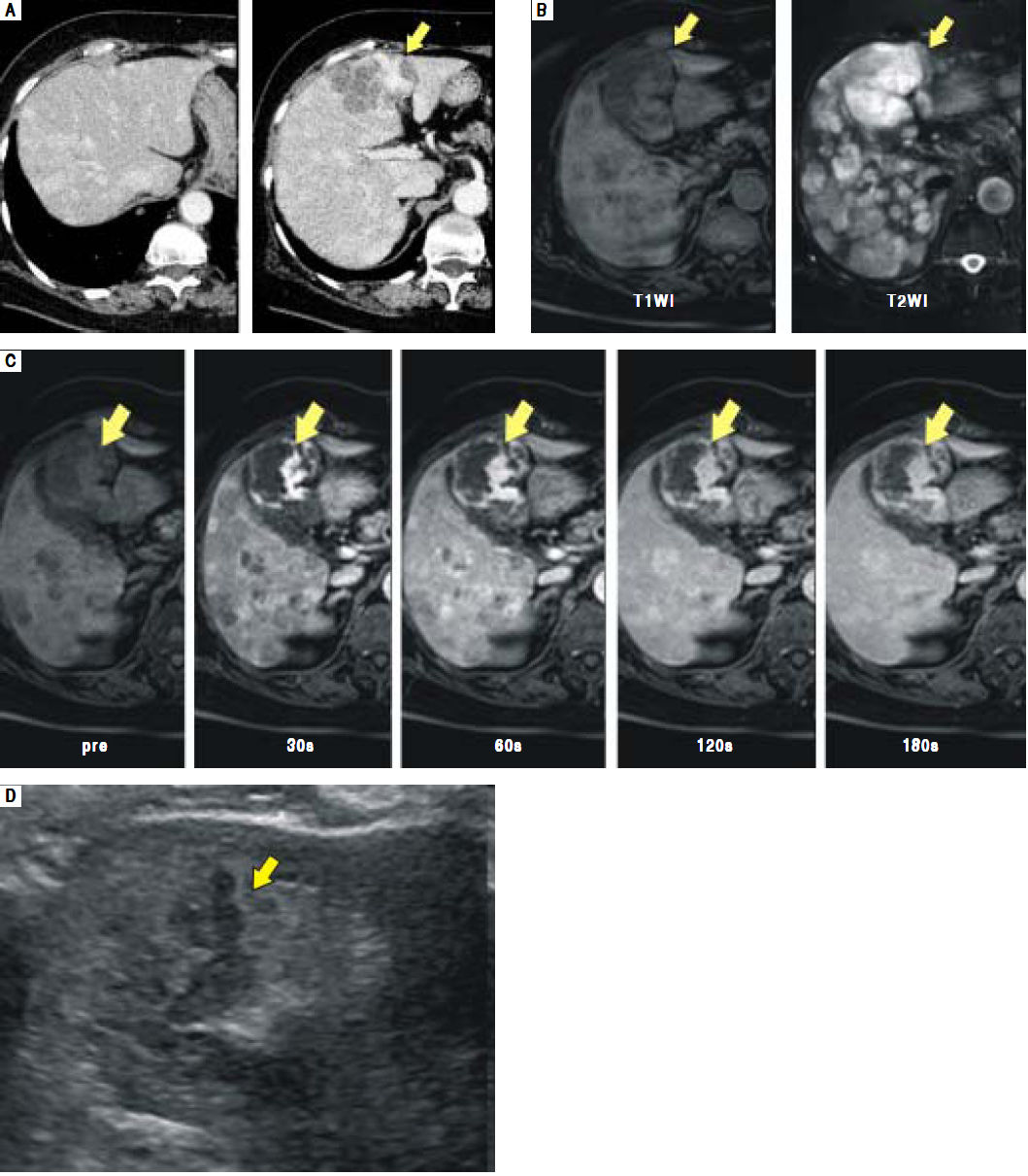
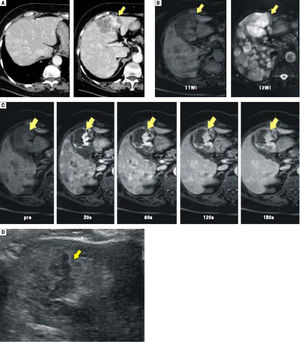
Enhanced computed tomography (CT) disclosed multiple hypervascular liver masses of 2-6 cm in diameter (Figure 1A). On magnetic resonance imaging (MRI), the largest tumor was T1 hypointense and T2 hyperintense (Figure 1B). Dynamic contrast enhanced MRI revealed irregular areas of enhancement at the periphery (Figure 1C) that were hypoechoic on routine ultrasound (Figure 1D).
A.Enhanced CT scan shows multiple enhanced liver masses measuring 2-6 cm in diameter. The largest tumor in S4 measures 6 cm and exhibits an irregular enhancement pattern (arrow). B. The largest tumor in S4 shows T1 hypointensity and T2 hyperintensity on MRI (arrows). C and D. The largest tumor in S4 is seen with an irregular enhancement pattern on enhanced MRI (C, arrows) and as hypoechoic on ultrasonography (D, arrows).
Based on the above findings, we considered the possibility of a tumor of vascular origin, such as hemangioendothelioma or angiosarcoma. In spite of repeated packed red blood cell and fresh frozen plasma transfusion along with gabexate mesilate administration, the patient’s gingival bleeding did not stop and alveolar hemorrhage appeared 43 days after admission. A relationship between the multiple liver tumors and DIC was suspected, but primary liver tumor treatment, such as trans-arterial embolization (TAE), could not be attempted due to the patient’s poor general condition and uncontrollable systemic bleeding tendency; bleeding would persist for several days following routine blood sampling from the peripheral vein. Spontaneous hepatic tumor rupture and hepatic failure eventually occurred and she died 120 days after admission.
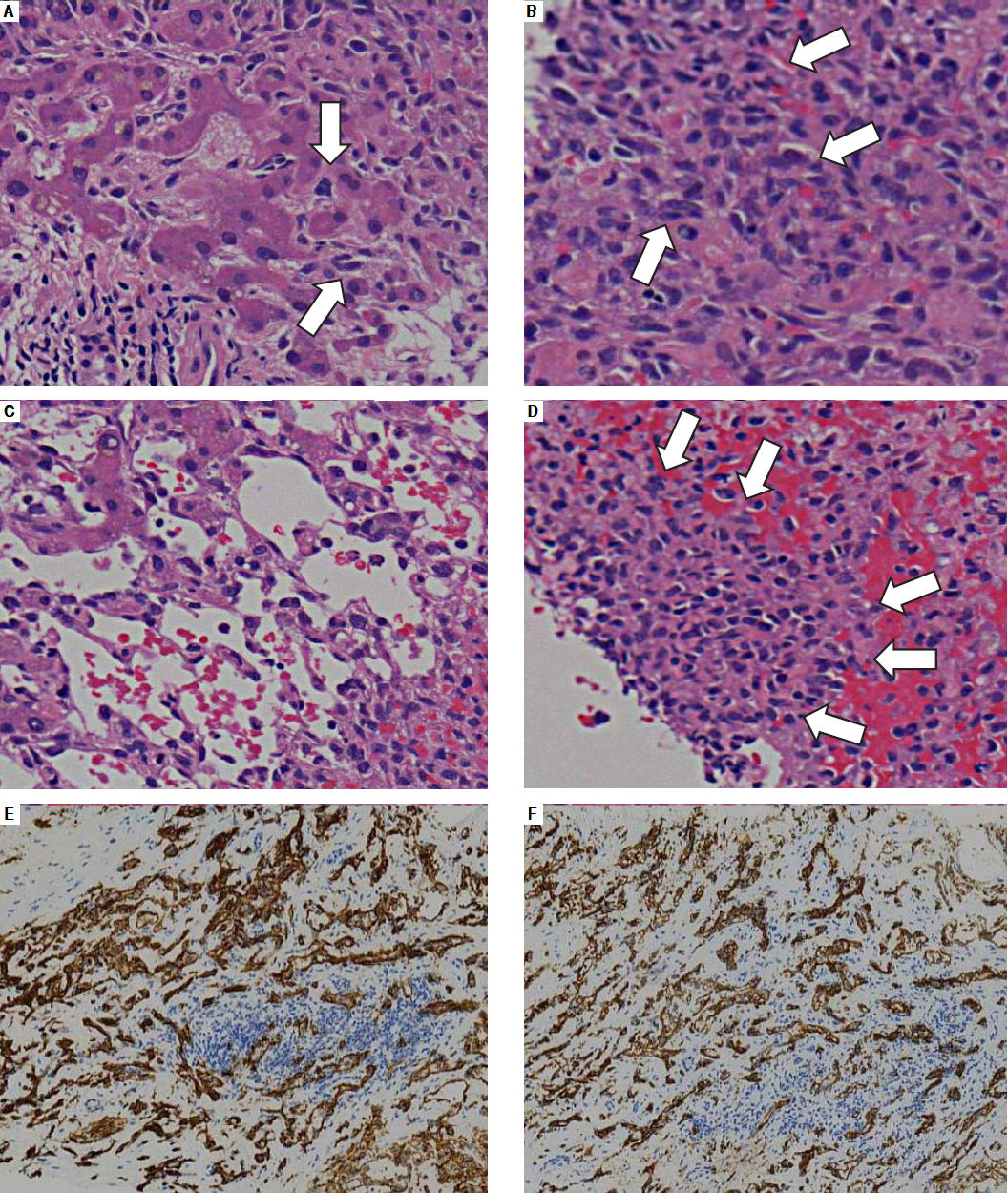
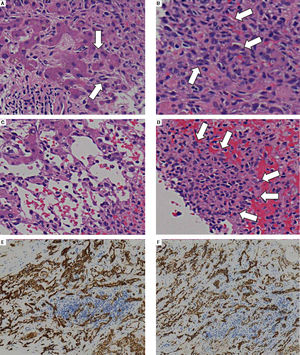
Post-mortem hepatic tumor biopsy revealed various pathological findings. Liver parenchyma with mild invasion of tumor cells exhibited dilated hepatic sinusoids lined by hypertrophied endothelial cells displaying atypical hyperchromatic nuclei (Figure 2A). In severely involved areas, these bizarre cells had proliferated in dilated sinusoids, causing liver cell plate atrophy (Figure 2B and C) and forming clusters in areas which the hepatocytes had disappeared entirely (Figure 2D). Immunohistochemical analysis revealed CD31 and CD34 positivity in tumor cells, leading to the final diagnosis of hepatic angi-osarcoma (Figure 2E and F).
A.Areas with mild tumor cell invasion show dilated hepatic sinusoids lined by hypertrophied endothelial cells with atypical hyperchromatic nuclei (arrows). B and C. With progressive involvement, the sinusoids dilate and fill with malignant endothelial cells (B, arrows) with liver cell plate atrophy (C). D. Areas in which hepatocytes have entirely disappeared sometimes exhibit solid malignant cell growth (arrows). E and F. Immunohistochemical analysis. The tumor cells are positive for the endothelial markers CD31 (E) and CD34 (F).
Hepatic angiosarcoma is a malignant mesenchymal tumor with a very low incidence of 0.14–0.25 per million inhabitants that represents 1.8% of all primary liver tumors.3 Several etiological factors have been implicated in this disease, including exposure to thorotrast, vinyl chloride, copper, radium, sex steroids, and arsenic compounds.4 Although the present case contained none of these elements, numerous other reports support the existence of hepatic angiosarcoma without such factors.2
The symptoms of hepatic angiosarcoma are largely nonspecific. Abdominal pain is the most common complaint, followed next by weakness, fatigue, and weight loss.2 Moreover, there are no physical or laboratory findings characteristic of this disease. The lack of specific findings makes accurate diagnosis of hepatic angiosarcoma difficult.
Unexplainable DIC may be a clue in identifying hepatic tumors of vascular origin. In 1940, Kasabach and Merritt described a new syndrome in a boy with kaposiform hemangioendothelioma, severe thrombocytopenia, anemia, and consumption coagulopathy.3 KMP with angiosarcoma was later detected in the skin,4 breast,5 and bone.6 Recently, ultrastructural examination of kaposiform hemangioendothelioma samples with KMP revealed trapping of platelets, erythrocytes, lymphocytes, and macrophages in tumor cells and intra-tumoral channels.7 Many thrombi were generated and a large amount of platelets and coagulation factors were consumed in the tumor, leading to DIC.7 Judging from the clinical course and pathological biopsy findings in the present case, we considered that the DIC caused by multiple hepatic angiosarcoma was KMP.
Due to the nonspecific physical/laboratory findings of hepatic angiosarcoma, imaging modalities play a critical role in its diagnosis. Angiosarcoma has various hallmark features in CT/MRI that reflect its heterogeneous histologic composition. When angiosarcoma appears as multiple nodular lesions, most include enhancement foci to enable distinction from benign hemangiomas showing nodular enhancement.8 Hemorrhagic lesions may appear in cases of very large angiosarcoma.9 Our patient exhibited irregular areas of enhancement at the periphery of the largest lesion. Although we could not obtain a specimen, we surmised that it corresponded to cavernous areas lined by malignant cells and ensuing hepatocyte atrophy.
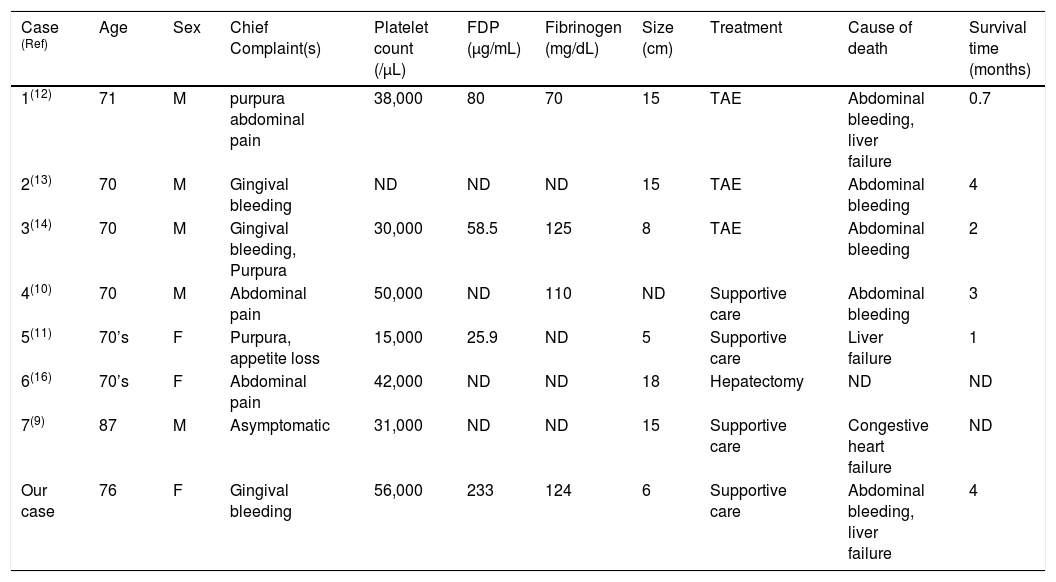
There is uncertainty on how patients with KMP should be treated. The clinical features, treatment, and outcomes of 7 case reports of hepatic angiosarcoma with KMP are presented in table 2. Patient age ranged from 70 to 87 years and initial symptoms primarily included gingival bleeding, purpura, and abdominal pain. All cases possessed thrombocytopenia and large tumors of more than 5 cm in diameter. Tumor resection and TAE were considered risky because of uncontrollable bleeding tendency. Moreover, TAE was insufficient in most cases and required additional treatments. Survival was less than 4 months due to rapid tumor progression, with intraperitoneal bleeding and liver failure representing the main causes of death. Although there are no established therapeutic strategies for angiocarcinoma at present, several reports have demonstrated the potential of anti-angiogenic agents, such as anti-VEGF drugs, tyrosine kinase inhibitors, such as sunitinib, and combinations of such molecular targeted agents as sorafenib and sunitinib to be at least partially effective.15 The establishment of novel therapeutic interventions is needed.
Case reports of hepatic angiosarcoma with Kasabach-Merritt phenomenon.
| Case (Ref) | Age | Sex | Chief Complaint(s) | Platelet count (/μL) | FDP (μg/mL) | Fibrinogen (mg/dL) | Size (cm) | Treatment | Cause of death | Survival time (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1(12) | 71 | M | purpura abdominal pain | 38,000 | 80 | 70 | 15 | TAE | Abdominal bleeding, liver failure | 0.7 |
| 2(13) | 70 | M | Gingival bleeding | ND | ND | ND | 15 | TAE | Abdominal bleeding | 4 |
| 3(14) | 70 | M | Gingival bleeding, Purpura | 30,000 | 58.5 | 125 | 8 | TAE | Abdominal bleeding | 2 |
| 4(10) | 70 | M | Abdominal pain | 50,000 | ND | 110 | ND | Supportive care | Abdominal bleeding | 3 |
| 5(11) | 70’s | F | Purpura, appetite loss | 15,000 | 25.9 | ND | 5 | Supportive care | Liver failure | 1 |
| 6(16) | 70’s | F | Abdominal pain | 42,000 | ND | ND | 18 | Hepatectomy | ND | ND |
| 7(9) | 87 | M | Asymptomatic | 31,000 | ND | ND | 15 | Supportive care | Congestive heart failure | ND |
| Our case | 76 | F | Gingival bleeding | 56,000 | 233 | 124 | 6 | Supportive care | Abdominal bleeding, liver failure | 4 |
F: female. FDP: fibrinogen degradation product M: male. ND: not described. Ref: reference. TAE: transcatheter arterial embolization.
In conclusion, we report a rare case of hepatic angiosarcoma that had manifested as bleeding tendency. The possibility of hepatic angiosarcoma should be considered for patients with DIC and liver tumor.
Abbreviations- •
CT: computed tomography.
- •
DIC: disseminated intravascular coagulopathy.
- •
FDP: fibrinogen degradation product.
- •
KMP: Kasabach-Merritt phenomenon.
- •
MRI: magnetic resonance imaging.
No funding source to declare.
Conflict of InterestNo conflict of interest exists.