Hepatic carcinosarcoma (HCS) is defined as a malignant tumor containing an intimate mixture of carcinomatous and sarcomatous elements. Here, we report the case of a 72-year-old man who developed HCS from an otherwise normal liver. The patient had no history of alcohol abuse or hepatitis B or C infection. An enhanced abdominal CT scan revealed a 9-cm heterogeneous tumor, with enhancement during the arterial phase and delayed wash-out in the latter phases. Also, a marked elevation in alpha-fetoprotein level (15,164 ng/mL; normal range, < 10 ng/mL) was noted. He underwent resection of liver segments V and VI under a pre-operative diagnosis of atypical hepatocellular carcinoma (HCC). The diagnosis of HCS was made based on thorough pathologic examination with a panel of immunohistochemical staining. Following surgery, the patient made an uneventful recovery, and at present, 16 months post-surgery, he remains well with no evidence of tumor recurrence. In conclusion, pre-operative diagnosis of HCS is difficult and radical resection in the early stage is encouraged to improve the prognosis of these patients.
Hepatic carcinosarcoma (HCS) is a very rare type of tumor, which has been defined by Ishak, et al., as a malignant tumor containing an intimate mixture of carcinomatous (either hepatocellular or cholan-giocellular) and sarcomatous elements. Pre-operative diagnosis of HCS is difficult, as the image findings of HCS are nonspecific and biopsies often lead to incorrect interpretation. The prognosis of HCS is significantly poorer than hepatocellular carcinoma (HCC) and cholangiocellular carcinoma owing to the aggressiveness of the sarcomatous elements. At present, little is known about the pathogenesis, epidemiology, or risk factors associated with HCS. Here, we report a further case of HCS, together with a review of the literature.
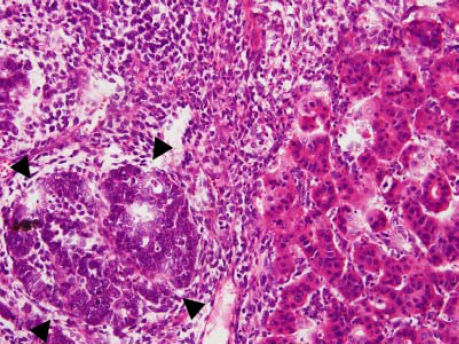
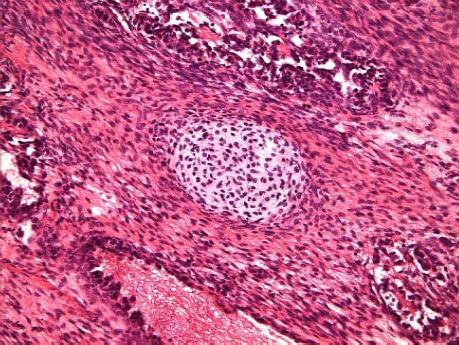
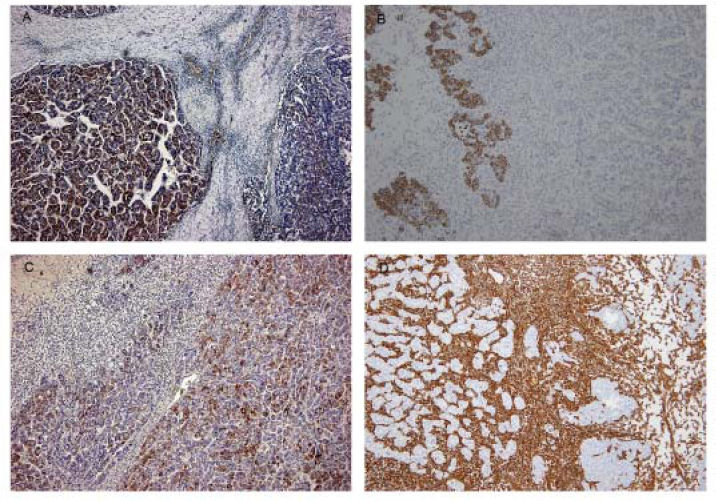
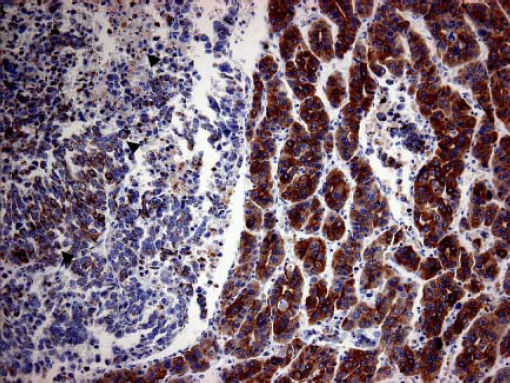
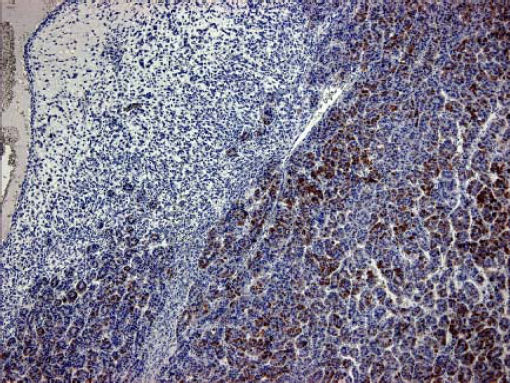
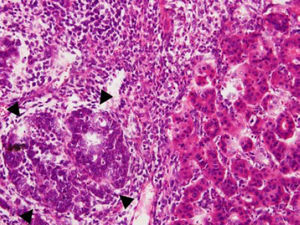
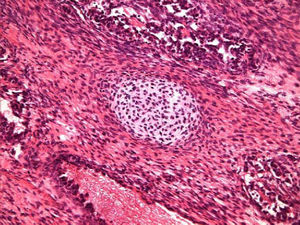
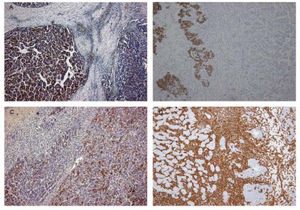
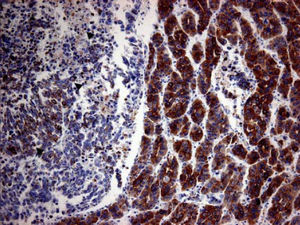
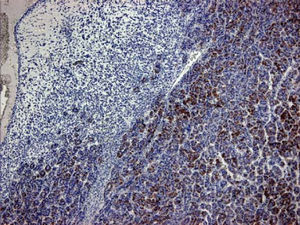
Case ReportA 72-year-old man was referred after suffering right hypochondrial pain and poor appetite for 1 month. Physical examination revealed a mass in the right upper quadrant of the abdomen. He had no history of alcohol abuse and serological tests for hepatitis B and C were negative. Abdominal ultrasonography showed a well-defined mass (9 cm in diameter), containing a cystic component, in liver segments V and VI. An enhanced CT scan of the tumor revealed heterogeneous enhancement during the arterial phase and delayed wash-out in the latter phases. There was no radiological evidence of a synchronous primary tumor, pulmonary metastases, or lymphadenopathy. Analysis of serum tumor markers revealed an elevated alphafetoprotein (AFP) level of 15,164 ng/mL (normal range: < 10 ng/mL); however, both carbohydrate antigen 19–9 and carcinoembryonic antigen levels were within normal ranges. Under a pre-operative diagnosis of atypical HCC, resection of liver segments V and VI was performed. Macroscopically, the resected tumor measured 10 cm in the largest dimension. The main portion had a grayish yellow soft component, and areas of hemorrhage and necrosis were found in the cut surface. Histologically, the solid part of the tumor had a mixed structure comprising a carcinomatous component, which was arranged in trabecular and tubular patterns, scattering with nests of undifferentiated epithelial cells, and a sarcomatous component, which was mainly composed of spindle-shaped cells (Figure 1) with focal immature cartilage formation (Figure 2). The surrounding parenchyma showed no cirrhotic change. Immunohistochemical (IHC) staining revealed that the carcinomatous cells were positive for AFP (Figure 3A), HepPar-1 (Figure 3C), CAM5.2 (Figure 4), and TTF-1 (Figure 5) but negative for vimentin (Figure 3D). The sarcomatous cells were positive for vimentin (Figure 3D), but negative for HepPar-1 (Figure 3C) and CK AE1/AE3 (Figure 3B). There were nests of undifferentiated epithelial cells with neuroendocrine differentiation found in the carcinomatous component (Figure 1, arrowheads), which were positive for CK AE1/AE3 (Figure 3B), CAM5.2 (Figure 4), synaptophysin and chromogranin A. These findings led to a diagnosis of HCS with focal neuroendocrine differentiation. Following surgery, the patient recovered uneventfully, and at present, 16 months after surgery, he remains well with no evidence of tumor recurrence.
Immunohisto-chemical study of the tumor. Carcinomatous element was positive for AFP on the left (A). Nests of undifferentiated epithelial cells were positive for CK AE1/ AE3 in places, whereas the hepatocelluar carcinoma and the spindle-shaped cells were negative (B). Carcinomatous element was positive for HepPar-1 on the right (C). The spindle-shaped cells were positive for vimentin (D).
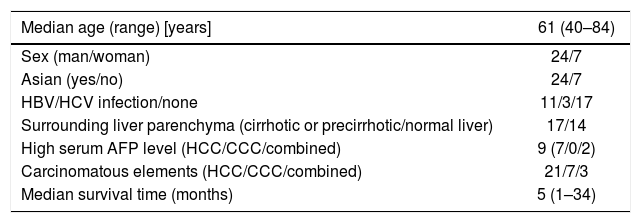
HCS is a very rare malignancy with only 31 cases,1–26 including the present case, having been reported in the literature to date. The median age of patients presenting with this tumor is 61 years (range: 40–84 years), with a predominance of Asian ethnicity (77%) and male gender (77%) (Table 1).
| Median age (range) [years] | 61 (40–84) |
|---|---|
| Sex (man/woman) | 24/7 |
| Asian (yes/no) | 24/7 |
| HBV/HCV infection/none | 11/3/17 |
| Surrounding liver parenchyma (cirrhotic or precirrhotic/normal liver) | 17/14 |
| High serum AFP level (HCC/CCC/combined) | 9 (7/0/2) |
| Carcinomatous elements (HCC/CCC/combined) | 21/7/3 |
| Median survival time (months) | 5 (1–34) |
CCC: Cholangiocarcinoma.
HCS has also been referred to as hepatoblastoma, malignant mixed tumor, spindle cell carcinoma, or sarcomatoid carcinoma, which are spindle cell variants of other more common carcinomas.1,10,17,27 According to the World Health Organization definition, HCS is “a malignant tumor containing an intimate mixture of carcinomatous (either hepatocellular or cholangiocellular) and sarcomatous elements.”28 However, Craig, et al.27 recommended that HCS would be better defined as both HCC and a non-spindle cell sarcoma, and excluded non-hepatocytic epithelial elements. Both the World Health Organization and Craig, et al. claim that HCS should be distinguished from sarcomatoid carcinoma, also known as spindle cell carcinoma, which is defined as a carcinoma with foci of spindled epithelial cells.23,27–29
Though the current literature raises doubts on HepPar1 specificity, HepPar1 had an acceptable specificity to HCC, except for some metastatic adenocarcinoma with hepatoid differentiation.30,31 Hepatoid adenocarcinoma of stomach or gall bladder was known to demonstrate HepParl reactivity and produce large amounts of AFP when metastasize to the liver.32 The absence of an extrahepatic primary tumor and multiple lesions in the liver suggests a primary liver tumor with a speculated HCC component.
A panel of IHC staining for HepPartl, AFP, CAM5.2 and TTF-1 are useful in distinguishing HCC from metastatic carcinomas or cholangiocarcinomas. TTF-1 cytoplasmic staining positively correlated with the trabecular pattern of well-differentiated HCC in our case (Figure 5).33 Also, diffused and strong cystoplasmic CAM5.2 staining was observed in hepatocellular carcinoma, whereas Golgi pattern CAM5.2 staining was noted in the undifferentiated epithelial cells with neuroendocrine differentiation (Figure 4). Sarcomatous element was immuno-negative for cytokeratin AE1/ AE3 staining and immuno-positive for vimentin, suggestive of sarcoma origin instead of sarcomatoid change of the carcinomatous element. In our case, the tumor consisted of an intimate mixture of hepatocellular carcinomatous and sarcomatous elements that met both the WHO criteria for HCS and the more restricted definition of Craig, et al.
Kojiro, et al.34 observed that sarcomatoid carcinomas were significantly more common in patients with HCC who had undergone transarterial embolization or hepatic arterial infusion. Further, the serum level of AFP is lower in patients with sarcomatoid carcinoma than in patients with pure HCC. In the literature, none of the reported HCS patients had been treated with anticancer therapeutics prior to diagnosis. In addition, the serum AFP level was high in HCS with HCC elements (Table 1). Therefore, HCS with HCC elements may be distinguished from sarcomatoid carcinomas, based on the history of no previous anticancer treatment and a higher serum AFP level.
According to the reported cases in the literature, the tumor diameter ranged from 2.6 to 21 cm with a mean of 11.1 cm. There was no predilection for right liver lobe involvement as Shu, et al.21 had described. The typical CT findings of HCS may show a lobulated, low-attenuation mass with necrotic portions or a cystic component and subtle rim enhancement, dense rocky calcifications, or post-contrast gradually delayed enhancement.17, 21, 35 CT findings have been described in 17 out of the 31 cases reported in the literature and have revealed the following: necrotic or cystic portions (39%), rim enhancements (10%), and calcifications (13%). In our case, enhanced CT revealed heterogeneous enhancement during the arterial phase with hypodense central areas, suggesting either a necrosis or a prolonged enhancing pattern in the solid part of the tumor. These findings are not typical of HCC but are comparable with the unique findings for HCS.
The pathogenesis of HCS remains unknown. Previous reports have suggested two theories to explain how this rare tumor develops. One theory holds that HCS arises from a multi-potent hepatic progenitor or stem cell, which differentiates into both carcinomatous and sarcomatous neoplasms to produce a “combination” tumor.7,36 The alternative theory posits that conventional HCC transforms or dedifferentiates into sarcomatous components to form a “conversion” tumor,4 which is based on the observation of transitional zones. Approximately 64% of HCS cases have reported cirrhotic liver due to previous chronic hepatitis B or C infection,20 suggesting that HCS has similar risk factors to conventional HCC, and thus supporting the latter theory. In contrast, our patient developed HCS from an otherwise normal liver, and he had no history of hepatitis B or C infection. This supports the theory that the tumor developed from a multi-potent hepatic progenitor or stem cell, which then differentiates into both carcinomatous and sarcomatous neoplasms, rather than undergoing transformation from HCC.
The prognosis for HCS is very poor, with the majority of patients dying within 1 year, and with a median survival time of only 5 months (Table 1). A factor contributing to this poor outcome is the high prevalence of vessel involvement and metastasis, owing to the aggressiveness of the sarcomatous elements.18,37 HCS patients may be given a relatively optimistic prognosis if a radical resection is performed in the early stage, similar to conventional HCC. However, Garcez-Silva, et al.16 reported the case of a patient with an early-stage presentation of HCS who died of aggressive recurrence after liver transplantation, suggesting that liver transplantation may be a relative contraindication for HCS. In our case, serum levels of AFP decreased markedly (from 15,164 ng/mL to 2.67 ng/mL) after radical resection and there was no CT evidence of recurrence 18 months after this surgery. The patient is expected to have a favorable prognosis.
In summary, the occurrence of HCS very rare with only 31 cases having been reported in the literature. The median age of the HCS patients was approximately 61 years old and these patients were predominantly Asian and male. The pathogenesis of HCS remains unknown; however, the risk factors are similar to those for conventional HCC. Although pre-operative diagnosis of HCS is difficult, some of these tumors have had unique CT findings regarding necrotic or cystic portions, rim enhancement, and calcifications. Radical resection of HCS in the early stage is encouraged to improve the prognosis of these patients.
Abbreviations- •
AFP: alpha-fetoprotein.
- •
CCC: cholangiocarcinoma.
- •
HCC: hepatocellular carcinoma.
- •
HCS: hepatic carcinosarcoma.
- •
IHC: immunohistochemical
None of the authors have any conflicts of interest to declare.