Little information exists in the international scientific or medical literature about the hepatic manifestations and complications of Epstein-Barr virus (EBV). The aim of this study was to describe a series of patients with hepatic manifestations of EBV infection. Our sample population was a series of patients whose hepatic dysfunction was correlated with a documented EBV infection. Serum concentrations of IgG and IgM antibodies against the EBV viral capsid antigen (anti-EBV VCA IgG), EBV early antigen, and EBV nuclear antigen (EBV-EBNA), and heterophilic antibodies were determined. The expression of latent membrane protein (LMP 1) was also evaluated in each patient. RESULTS. The study included nine patients (six men, three women) with a mean age of 43.5 years. Five patients presented with recent clinical pictures suggestive of acute EBV infection. Five patients began with a cholestatic pattern. Two patients required liver biopsies. Those liver biopsies showed positive immunohistochemical staining for LPM 1. No fatalities were attributed to EBV infection. In conclusion, the bilirubin levels of patients with acute EBV infection differed from those reported in the medical literature. EBV infection should be considered in the differential diagnosis of patients with liver abnormalities or diverse hepatic manifestations, increased levels of aminotransferases, or a transitory cholestatic pattern with a favorable outcome.
Epstein-Barr virus (EBV) was discovered in cells from the tissue of a Burkitt’s lymphoma by Epstein, Achong, and Barr. More than 90% of healthy people carry EBV.1-3 Half these patients present with fever, lymphadenopathy, and exudative pharyngitis. Other manifestations include splenomegaly (50%), palatal petechiae, hepatomegaly (more than 20%), and jaundice (5%).4,5 Hepatitis is a common characteristic of infection by EBV, although severe hepatocellular liver injury is rare and its pathogenesis uncertain.6 In the acute phase, high serum aminotransferase levels are present in 80% of patients, whereas jaundice is observed in only 6.6% of patients.7 The main difference between EBV hepatitis and viral hepatitis A, B, or C is that the former does not infect hepatocytes, gallbladder epithelium, or vascular endothelium.8-10 Ninety percent of patients develop mild hepatitis in the second or third week of symptomatic infection, as well as high serum alkaline phosphatase levels, whereas serum bilirubin levels greater than 8 mg/dL are observed in only 3% of patients.3-5,11 Little information exists in the international scientific or medical literature about the hepatic manifestations and complications of EBV. Therefore, the aim of this study was to describe a series of patients with hepatic manifestations associated with EBV infection.
MethodsThis study was carried out at the Medica Sur Clinic and Foundation in Mexico City, Mexico, from June 1, 2003, to June 1, 2004. Our sample population was derived from a series of patients with hepatic dysfunction that correlated with a documented EBV infection. Demographic data, family history, clinical presentation, laboratory test results, abdominal ultrasound, liver biopsy (if indicated), treatment, and outcome were recorded for each patient. The results are expressed as means, ranks, standard deviations, and percentages. Laboratory tests for EBV included the detection of IgG and IgM antibodies against the EBV viral capsid antigen (anti-EBV VCA IgG; SmithKline Beecham, Van Nuys CA), viral early antigen (Epstein-Barr virus early antigen; Mayo Medical Laboratories, Rochester, MN), EBV nuclear antigen (EBV-EBNA; Mayo Medical Laboratories), and heterophilic antibodies (Monospot; Meridian Diagnostics, Cincinnati, OH). Immunohistochemical studies were performed in patients for whom liver biopsies were indicated, including assessment of the expression of latent membrane protein (LMP 1).
ResultsWe included nine patients in the study, six of whom were male and three female. Their mean age was 43.5 ± 17.15 (range, 19–73 years). The overall fatality rate was 0%.
Underlying illnessTwo patients had underlying illnesses. One male patient had ulcerative colitis and a female patient had hyperthyroidism. The diseases were controlled in both patients.
Clinical featuresFatigue and adynamia were the most common clinical features of patients upon admission, and were noted in eight patients (88.8%). Other symptoms, which were either identified on admission or developed during hospitalization, are shown in Table I. Gastrointestinal symptoms, including abdominal pain, nausea, and diarrhea were noted in seven patients (77.7%) at baseline. Fever and respiratory symptoms were present in five (55.5%) and four (44.4%) patients, respectively, on admission. On hematological tests, cytopenia involving one or two cell lines simultaneously was observed in four patients (44.4%), and leukopenia (white cell count < 4,000 cells/μL) and thrombocytopenia (< 150,000 platelets/μL) were the two most common hematological abnormalities on admission. Hepatic abnormalities, identified by elevated serum liver enzyme and/or bilirubin levels, were found in seven patients (77.7%). Coagulopathy with prolonged prothrombin time was noted in one patient (11.1%) (Table II).
Clinical and demographic characteristics of patients.
| Variables | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 |
|---|---|---|---|---|---|---|---|---|---|
| Sex | M | M | F | F | F | M | M | M | M |
| Age (yr) | 41 | 33 | 23 | 57 | 44 | 46 | 19 | 56 | 73 |
| Evolution (days) | 3 | 7 | 15 | 7 | 5 | 3 | 30 | 43 | 30 |
| Symptoms: | |||||||||
| Adynamia | + | + | + | + | + | – | + | + | + |
| Anorexia | – | – | – | – | – | – | – | – | + |
| Abdominal pain | + | – | + | + | + | + | – | + | – |
| Nausea | – | – | – | + | + | + | + | + | – |
| Diarrhea | – | – | – | + | – | – | – | + | – |
| Upper respiratory tract infection | + | + | + | – | – | + | – | – | – |
| Cephalalgia | + | – | – | – | + | – | – | – | – |
| Fever | + | + | + | – | + | + | – | – | – |
| Jaundice | + | – | – | – | + | + | + | + | + |
| Hepatodynia | + | + | + | + | + | + | – | + | – |
| Hepatomegaly | + | – | – | – | + | – | + | – | – |
| Splenomegaly | – | – | – | – | – | – | + | – | – |
| Rash | – | – | – | – | – | + | – | – | – |
| Paresthesia | – | – | – | – | – | – | + | – | – |
| Pruritus | – | – | – | – | – | – | – | + | – |
M, male; F, female; Yr, years.
Biochemical characteristics of patients at baseline.
| Variables | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 |
|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 14.3 | 14.5 | 14.7 | 14 | 7.9 | 12.8 | 16.6 | 14.4 | 11.8 |
| Platelets (mm-3) | 157,000 | 156,000 | 318,000 | 289,000 | 457,000 | 133,000 | 184,000 | 209,000 | 238,000 |
| Leukocytes (mm-3) | 8,000 | 3,500 | 11,100 | 5,100 | 5,900 | 11,600 | 9,600 | 8,300 | 6,000 |
| Lymphocytes (mm-3) | 880 | 1,435 | 444 | 1,900 | 1,121 | 1,276 | 1,344 | 1,411 | 1,440 |
| Atypical lymphocytes (%) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Monocytes (mm-3) | 960 | 280 | 1,110 | 500 | 250 | 560 | 384 | 498 | 120 |
| Prothrombin time (s) | 13.7 | 11.2 | 13.3 | 10.8 | 18.7 | 13.7 | 10.6 | 13.5 | 14 |
| Albumin (g/dL) | 2.4 | 3.46 | 3.21 | 3.7 | 3.02 | 2.85 | 3.74 | 2.49 | 2 |
| Bilirubin (mg/dL) | 14.03 | 0.6 | 1.04 | 0.45 | 6.69 | 1.8 | 4.22 | 26.33 | 26 |
| Bilirubin direct (mg/dL) | 8.74 | 0.14 | 0.21 | < 0.1 | 4.52 | 0.83 | 0.12 | 15.46 | 16 |
| ALT (UI/L) | 109 | 65 | 91 | 33 | 605 | 362 | 18 | 49 | 99 |
| AST (UI/L) | 232 | 34 | 105 | 33 | 621 | 292 | 16 | 74 | 143 |
| Alkaline phosphatase (UI/L) | 633 | 144 | 74 | 78 | 159 | 111 | 214 | 166 | 151 |
| GGT (UI/L) | 1,009 | 160 | 58 | 68 | 95 | 180 | 20 | 64 | 60 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gammaglutamyl transpeptidase.
Serology tests for EBV were performed in all patients. Five patients (55.5%) presented a recent clinical picture suggestive of acute EBV infection (patients 1, 6, 7, 8, and 9) (Table III). Five patients (55.5%) began with cholestasis (patients 1, 5, 7, 8, and 9), and one of them underwent the molecular adsorbent recycling system (MARS) because of severe cholestasis. Patient 5 was thought to represent a reactivation or atypical presentation of EBV infection, because of the presence of only VCA IgG and EBNA antibodies. However, an acute process was confirmed by hepatic inflammation identified on biopsy. The remaining three patients (33.3%), patients 1, 2, and 3, had recently reported an upper respiratory tract infection, followed by a clinical picture suggestive of EBV infection. These patients showed few biochemical changes, with the exception of patient 2 who had high levels of serum alkaline phosphatase and gammaglutamyl transpeptidase (GGT), with an EBV serological profile positive for VCA IgG and EBNA. EBV infection was diagnosed in this patient by discounting other etiologies.
Immunological markers of patients.
| Variables | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 |
|---|---|---|---|---|---|---|---|---|---|
| Heterophilic Abs | – | – | – | – | – | – | – | – | – |
| Anti-EA | + | – | – | – | – | – | – | – | – |
| Anti-viral capsid IgG EBV | + | + | + | + | + | + | + | + | – |
| Early diffuse Ag EBV | – | – | – | – | – | + | – | – | – |
| EBNA Abs | + | + | + | + | + | + | + | + | + |
| Anti-viral capsid IgM EBV | – | – | – | – | – | – | – | – | – |
| Hepatitis virus serology | Anti-VHA IgG | – | Anti-VHA IgG | – | Anti-VHA IgG | Anti-VHA IgG | – | – | – |
Abs, antibodies; EA, early antigen; Ig, immunoglobulin; EBV, Epstein-Barr virus; Ag, antigen; EBNA, EBV nuclear antigen.
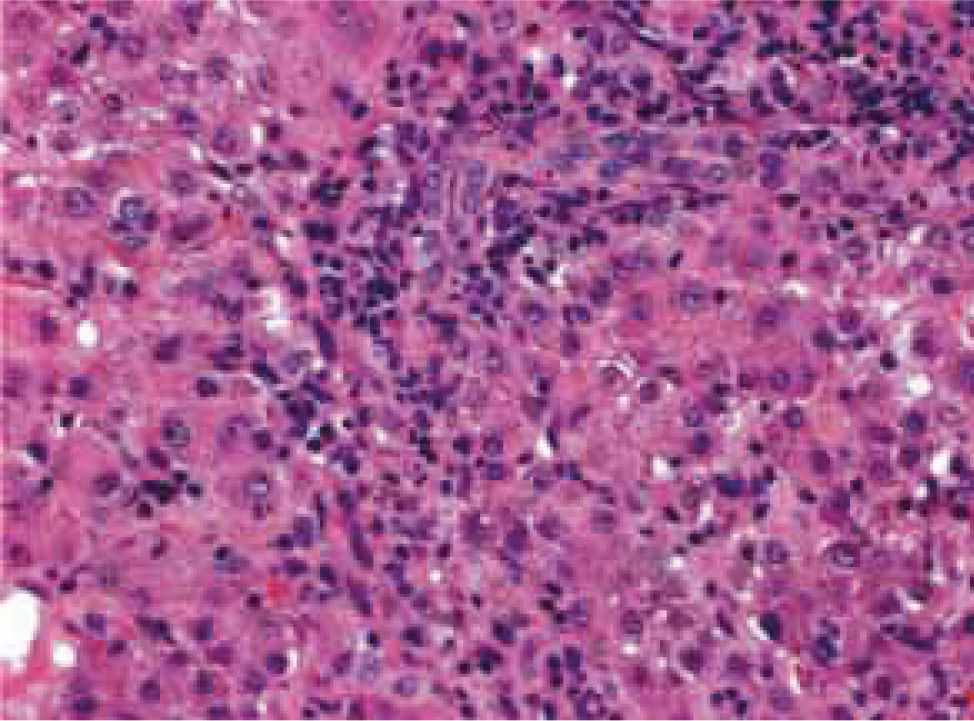
Two patients (22.2%) underwent biopsies due to uncertain diagnoses and severe clinical pictures at presentation (patients 5 and 9). Their liver biopsies showed inflammatory changes with many apoptotic hepatocytes and cholestasis in a pattern suggestive of viral cellular infection. Positive immunohistochemical staining for LMP 1 in EBV-positive lymphocytes and epithelium demonstrated infection by this virus (Figure 1) as well as the clinical manifestations of infection.
Hepatitis A viral infection was found in patient 8, who presented one month earlier without complications. During hospitalization, pleural and pericardial effusions were documented in patient 6.
Treatments and outcomesAll patients were treated with supportive care, and empirical antibiotic treatments were used initially only in some patients. However, once bacterial, parasitical, and/or fungal etiologies were discounted, treatments were continued only as symptomatic support where indicated. In some patients, ursodeoxycholic acid, adenosylmethionine, and cholestyramine were administered as therapeutic agents to treat cholestasis (patients 1, 5, 8, and 9). Patient 6 had complications of pneumonia and pleural and pericardial effusions, which were treated with cephalosporin and aminoglycoside antibiotics and surgical intervention, respectively. Patient 9 displayed a persistent progressive increase in bilirubin, which was treated with two sessions of MARS, which successfully reduced to bilirubin levels. No fatalities were attributed to EBV infection. Eighty-eight percent of patients had favorable outcomes, with symptomatic support only.
DiscussionEBV infection is highly prevalent, and more than 90% of the world’s population carry EBV throughout their lives. In most patients, primary infection occurs subclinically during childhood.2 However, when the disease is acquired during adolescence or adulthood, there are nonspecific symptoms, although some reports have integrated a distinctive symptomatic triad of fever, sore throat, and lymphadenopathy.1 However, the patients described in the present report did not show these characteristics. Hepatitis caused by EBV is common, mild, and self-limiting, although fulminant hepatic failure has been reported in 17 patients worldwide, with an overall mortality of 85%.12 In the present series, five (55%) of the nine patients with documented EBV infection presented with severe cholestasis, and increased levels of total bilirubin of > 26 mg/dL. Those patients were treated with MARS and we observed a decrease in hepatic encephalopathy indicated by a clearance of toxins, an increase in mean arterial pressure, and improvement in liver and renal function, as was observed in patient 9. In these patients, bilirubin levels differed somewhat from those reported in the scientific and medical literature. Whereas jaundice is rare as a presenting feature, apparent in only 6.6% of patients in many series,13-15 most of our patients (66%) showed high aminotransferase levels that decreased gradually with complete clinical recovery. Although EBV is not a hepatotrophic virus, mildly elevated serum aminotransferase levels are not uncommon (90% of patients), which is consistent with parenchymal liver injury rather than with decreased bile flow.13,16 Severe cholestasis is rare, and in those patients in whom it is present, the mechanism of cholestasis is unknown. We believe that EBV inhibits MRP2, which is the main bilirubin transporter. Furthermore, high levels of GGT activity were observed, suggesting virus-induced self-limiting cholangiocyte damage. On the other hand, serum levels of aminotransferases are only mildly elevated.17 Other herpes viruses, such as cytomegalovirus, have been demonstrated to infect bile duct epithelial cells, as well as hepatocytes. However, this is not a consistent finding and was not documented in the present series of patients. These nine patients were immunocompetent with no underlying liver disease that might account for the rare development of severe hepatic dysfunction. However, one patient was treated with MARS because of an adverse clinical development and progressive liver damage. MARS is a therapeutic modality that appears to be safe in patients with severe cholestasis.18 In another patient, EBV manifestations were aggravated by the presence of pleural and pericardial effusions, both of which complications are rare according to data from the world scientific and medical literature. Therefore, acute EBV infection should be considered in the differential diagnosis of patients with transient cholestasis and increased alkaline phosphatase levels, whose symptoms peak in the second week after symptom onset.
Two patients described in the present report, representing 22% of cases, showed increased gallbladder wall thickening on abdominal ultrasound. This finding has been reported previously in association with EBV infection, and may reflect the severity of hepatitis.19,20
All patients were treated with supportive care. In this series, no steroids were used because this therapeutic approach is controversial. However, some reports have shown a moderate clinical benefit in patients with hepatitis associated with EBV, suggesting the importance of immunological injury in the pathogenesis of EBV-associated hepatitis.21 Another important finding was that all patients were negative for heterophilic antibodies. These are not specific reactants directed against EBV. According to other reports, approximately 15% to 20% of patients with EBV-associated IgM are negative for heterophilic antibodies. The appropriate interpretation of EBV in terms of the markers VCA IgG and IgM, early antigen, and EBNA is important because their presence or absence reflects the stage of evolution of the disease, as either acute, recent, chronic, or persistent. We were able to identify an early stage of the disease in two patients characterized by the presence of early antigen. In another two patients, biopsies positive for LMP 1 of EBV and high titers of antibodies directed against IgG VCA and EBNA suggested possible chronic active EBV infections. Because EBV must reactivate from latency to complete its life cycle, this situation is rare. The liver is the most common target organ, and has a very poor prognosis; patients die from hematological or non-hematological disorders within a few years. In these patients, a polymerase chain reaction assay can be used to detect the gene for BZLF1, which can be detected from one copy of EBV DNA. Studies of EBV DNA titers have suggested that it may correlate with disease activity more accurately than do serological data.21
In conclusion, EBV infection must be considered in the differential diagnosis of patients who present with hypertransaminasemia or cholestasis suggestive of this disease or with diverse hepatic manifestations together with increased aminotransferase levels or transitory cholestasis and high levels of alkaline phosphatase (with a characteristic peak in the second week after the onset of symptoms), with a favorable outcome. It is important to highlight the major role that MARS plays in the medical treatment of some patients who develop severe cholestasis or acute liver failure.