Hepatitis B is a chronic viral infection of the liver leading to complications including cirrhosis and hepatocellular carcinoma. The leading cause of acquisition is vertical transmission from an infected mother to the newborn. Despite newborn immunoprophylaxis, vertical transmission may still occur in 1-14%. The aim of this article is to provide a concise review of the mechanisms and risk factors involved in vertical transmission, as well as prophylactic strategies using immunoprophylaxis and antiviral medications. Mechanisms of vertical transmission include intrauterine and perinatal transfer of virus. High HBV viral load and presence of HBeAg increases risk of transmission. Combination vaccine and hepatitis B immunoglobulin given at birth reduces risk of transmission, as does HBIG given to mothers in the third trimester. Three antivirals have been studied in pregnancy: lamivudine, telbivudine, and tenovofir. All have shown significant reduction in viral loads and vertical transmission and have favorable safety profiles. In conclusion, HBV vertical transmission is preventable through use of immunoprophylaxis and antiviral medications. Recommendation for antiviral use in third trimester in mothers whose HBV VL is greater than 1 × 106 copies/mL.
Chronic Hepatitis B virus (HBV) is estimated to affect 350-360 million worldwide and one third of the world’s population has serologic evidence of infection, past or present.1 Modes of transmission include horizontal, via contaminated blood products, injection drug use and sexual contact as well as vertical from mother to child. Vertical transmission remains a main source of persistence of HBV, particularly in endemic countries such as China, Southeast Asia and sub-Saharan Africa.2 Perinatal transmission has been estimated to account for 35-50% of chronic HBV carriers in China.3 In Canada, it is estimated that between 0.7 to 0.9 percent of the population are chronically infected and 5 percent have been infected in their lives.4
Chronic infection can lead to liver cirrhosis and associated complications from decompensated liver disease. HBV carriers are 100 times at risk of developing hepatocellular carcinoma.5 The risk of developing chronic infection is associated with age of acquisition. At birth, 80-90% of babies born to HBeAg positive mothers will become chronically infected. During the perinatal period to 6 years of age, this risk decreases to 30% and adolescence is associated with a one to 12% risk.6
Vaccination against HBV has been available since the 1980s and has been demonstrated to be both efficacious and well-tolerated.7,8 Despite effective primary prophylaxis, HBV remains a significant health problem both internationally and within Canada, especially in areas that have large communities with origins from endemic countries. Neonatal transmission, unfortunately, does still occur in Canada despite post-exposure prophylaxis and it is with this in mind that we review this topic.
The aim of this article is to provide a review of the mechanisms and risk factors involved in vertical transmission, as well as prophylactic strategies using immunoprophylaxis and antiviral medications.
Routes of Vertical TransmissionAlthough it is commonly believed that vertical transmission occurs only in the perinatal period, in fact, there are several proposed mechanisms of vertical transmission, with studies characterizing each stage from in utero to breastfeeding.
Intrauterine transmissionEarly placental pathology studies suggest infected intrauterine tissue and exposure to maternal blood to be main factors in transplacental transfer. When 32 HBeAg positive mothers were investigated, five had symptoms of a threatened abortion, three of whom delivered 6 weeks later and all the babies delivered had detectable HBV. In the other two, they delivered within one week and HBIG was effective in preventing transmission.9 When placenta has been examined during the various terms of pregnancy, increasing HBV infection rates are seen with progression of pregnancy, with HBV DNA detectable as early as 19 weeks.10 The proposed mechanism is cell-to-cell transfer, with high infection rates of villous capillary endothelial cells (OR 18.46, 95% CI = 2.83-152.78). A larger case control study also confirmed that threatened pre-term labor, HBeAg positivity and infected villous endothelial cells contributed to intrauterine infection.11
An underlying concern with the transplacental mechanism of spread is the ineffectiveness of immunoprophylaxis. A recent Chinese study of HBV postexposure neonatal immunoprophylaxis determined that intrauterine transmission occurred in 0.9% of infants born mothers who were chronic HBV carriers vs. 3.7% infants in whom transmission occurred perinatally.1 With appropriate post-exposure prophylaxis no perinatal transmissions resulted in persistent HBV infection 36 months post-partum compared to 100% of the intrauterine transmissions. HBeAg status, high maternal viremia and intrauterine infection are associated with failure of immune prophylaxis.1,12
Method of delivery/perinatal transmissionAs perinatal infection is well-recognized to occur, it would appear plausible that the method of childbirth may affect the likelihood of neonatal infection. The studies looking at the effect of vaginal delivery vs. caesarian section, however, have not consistently shown one method to be associated with less risk than the other. A study by Lee et al showed that vaginal delivery was associated with higher rates of infection (19.9 vs. < 6%, p < 0.03) despite immunoprophylaxis.13 Vaccine efficacy requires at least one year of follow-up and this study was limited as it ended at 6 months post-delivery. When follow-up is extended to one year post-delivery, rates of chronic infection are not significantly different between the vaginal delivery, forceps and caesarian section (7.3%, 7.7%, 6.8%, p = 0.89).14
Other factors such a premature rupture of membranes, premature birth, low birth weight, meconium staining and poor Apgar score (< 7) have not been associated with immunoprophylaxis failure.15
HBV transmission risk and breastfeeding
Breast milk is well known to carry HBV, as detected by presence of HBsAg16 or HBV PCR17 and has been long speculated to be a potential source of transmission to infants. However, the risk of breastfeeding has long been called into question. In an age before immunoprophylaxis, no significant increased risk was seen in breastfeeding as a method of transmission compared to non-breast fed infants.18
Contact with broken skin on breast sores may be a risk factor for transmission19 however, in the era of immunoprophylaxis at birth, all recent studies including a meta-analysis have not demonstrated any risk with breast feeding.20,21
Risk Factors for Vertical TransmissionDifferent risk factors have been identified that increases the risk of HBV mother to child transmission.
HBeAgHBeAg is a HBV viral protein and its presence in serum signifies increased host infectivity and correlates with higher viral loads. Studies looking at its association with vertical transmission demonstrate that the presence of HBeAg correlates with increased vertical transmission rates among HBV carrier mothers.
Case control studies from India and Canada demonstrate that HBeAg positivity leads to vertical transmission of 65 – 78% whereas in mothers that were HBeAg negative, the rate of transmission was significantly lower at 9-23%.22,23 Another study showed that positive HBeAg has an OR of 17 for vertical transmission.11 Therefore, HBeAg is a significant factor increasing rates of HBV vertical transmission.
HBV DNA viral loadHBV viral load (VL) reflects the degree of maternal viremia and detectable VL is a significant risk factor in vertical transmission. It is determined via modern PCR assays that quantify the amount of HBV DNA present in a sample. The pathologic correlate for increased risk of intrauterine transmission was demonstrated in studies with immunohistologic staining of placental tissue for evidence of HBV. Infectivity across the placenta occurs progressively through cell layers from maternal to fetal sides and the depth of tissue infection is linearly related to HBV VL.11 Higher HBV VL also confers increased risk of transmission compared to lower DNA titres.24,25 Multiple studies demonstrate that the risk of intrauterine infection is increased with the increasing VL; however, there is marked variability. Cut off values range from 105-108 copies/mL as the level of viremia at which vertical transmission is associated.25–27 In Wiseman, et al.’s study, the rate of transmission was 9% for those above 1.0 × 108 copies/mL, whereas no transmission was found below this threshold.25 Compared to HBeAg, HBV VL is thought to be a stronger independent predictor of vertical transmission from logistic regression studies.24
Other factorsThe HBV genotype does not appear to significantly correlate with vertical transmission in a case control study.23 Threatened preterm labor is a significant risk factor for HBV transmission (OR 5.44, CI 1.15-25.67), whereas threatened abortion and multiparity is not.11 Conversely, the presence of cord blood HBV DNA is associated with higher rates of preterm labor;28 however, the presence of cord viremia was not a risk factor for vertical transmission.28
Invasive diagnostic testing may contribute a theoretical increased risk of vertical transmission during pregnancy, most likely owing to disruption of normal tissue barriers leading to fetal contamination with maternal blood. Prenatal testing with amniocentesis appears to be low risk for transmission of HBV; however, the available evidence is lacking.29 Those with HBeAg positivity at time of amniocentesis have a non-significant trend towards increased transmission.29 No data for chorionic villous sampling or cordocentesis is currently available.
ImmunoprophylaxisImmunoprophylaxis is the utilization of hepatitis B immunoglobulins (HBIG) and/or HBV vaccine for the purpose of preventing perinatal mother to child HBV transmission. “Joint immunoprophylaxis” refers to the strategy of using both HBIG and vaccine together.
HBIG in the newbornHBIG is a plasma derived product from pooled HBV-immune donors who have high antibody titres against HBsAg. It is typically administered to the newborn of a HBV positive mother at the time of birth as an intramuscular injection. The pre-formed donor antibodies provide immediate but temporary passive immunization for the newborn.30 A metaanalysis showed that HBIG significantly reduced the rate of HBV transmission (OR 0.5, CI 0.41-0.6) compared to placebo when given to the newborn at time of birth.31 The safety profile is excellent and most trials report only mild non-specific events.31
HBIG in the HBV infected motherIn addition to routine joint immunoprophylaxis for the newborn, a meta-analysis of 37 RCT’s demonstrates efficacy for HBIG use in the mother prior to delivery to reduce vertical transmission, presumably from intrauterine HBV exposure.32 Multiple doses of HBIG in the third trimester (typically 100-200 lU IM at 28, 32, 36 weeks gestation) appear to reduce mother to child transmission via reduction of maternal viral load and increase in passive fetal immunity from placental antibody transfer in-utero. Compared to placebo, there is lower risk of transmission (indicated by HBV viral load, OR 0.15, CI 0.07-0.3; by HBsAg, OR 0.22, CI 0.17-0.29) with 3rd trimester HBIG use. The study also noted higher rates of newborn anti-HBs positivity (OR 11.79, CI 4.69-29.61) suggestive of higher protection levels in those whose mothers received HBIG. In follow up at 9-12 months, the HBIG group had lower rates of persistent infection (OR 0.33, CI 0.21-0.51) in comparison to placebo.32 The authors conclude that HBIG use in the third trimester to be an efficacious and safe modality to reduce the risk of HBV vertical transmission.
HBV vaccineThe hepatitis B vaccine has been available since 1981 using plasma-derived methods and today, different formulations and production are commercially available.33 Most modern HBV vaccines are manufactured via recombinant DNA techniques yielding purified HBsAg viral protein, which is administered as an intramuscular injection in multiple doses according to schedule. The vaccine is typically given routinely to newborns of HBV carrier mothers in three separate doses: at birth, 1 month, and 6 months. Exposure of viral protein induces adaptive immunity leading to sustained lifetime production of antibodies against HBV, specifically anti-HBs. Because the HBV vaccine only contains purified viral protein, active infection is not a risk. No differences were found between recombinant and plasma derived HBV vaccines in terms of efficacy.31
In terms of efficacy, a meta-analysis of multiple HBV vaccine trials reported a significant reduction of HBV transmission with the vaccine compared to placebo (RR 0.28, CI 0.2-0.4).31 In addition, vaccine alone without addition of HBIG at birth may confer protection rates of > 83%.34 In addition to providing efficacious post-exposure prophylaxis to the newborn, there are associated long-term benefits to the child. In a ten-year follow-up of a large cohort (n = 972) of Taiwanese and American children, vaccinated at birth, it was reported that of the 85% who developed a successful response to the vaccine, only 3 developed chronic HBV infection by age 10. In contrast, of the vaccine failures, the majority (84.1%) were chronically infected by 12 months of age.35
Combination immunoprophylaxisJoint immunoprophylaxis using both HBIG and HBV vaccine has demonstrated benefits in reduction mother to child transmission. Meta-analysis of 3 RCT’s found that HBIG plus vaccine used together significantly reduced HBV infection rates in the newborn when compared to placebo (RR 0.08, CI 0.03-0.17).31 In comparison, HBIG plus vaccine compared to vaccine alone has been reported to have superior efficacy (RR 0.54, CI 0.41-0.53). Therefore joint immunoprophylaxis using both HBIG and vaccine together is superior to either used alone for reducing HBV transmission rates and is generally the standard of care.
Failure of immunoprophylaxisFailure of immunoprophylaxis, or “breakthrough” infection, is defined as persistent HBV infection of the newborn, who has received vaccination and/or HBIG, indicated by a positive HBsAg or measurable HBV viral load at 9-12 months postpartum. The reported failure rates range from 1-14%12,15,27 despite immunoprophylaxis. The mechanism underlying immunoprophylaxis failure appears to be from intrauterine transmission where the fetus is already infected in-utero and thus negating the subsequent protective effects of vaccine or HBIG.1
Factors associated with failure include HBeAg positivity and high HBV viral loads.15,23,27 DNA levels above 1.0 × 108 copies/ml is associated with immunoprophylaxis failure.27 A meta-analysis of three randomized clinical trials from the 10 year Netherlands neonatal vaccination program reported a 100% efficacy of immunoprophylaxis if the HBV viral load is less than 150 pg/mL (approximately 107 copies/mL) and only 68% if greater that that threshold.36 Although the reported failures rates of combination post-exposure immunoprophylaxis vary greatly, depending on geographic location, time of the study, and the quantification of the HBV viral loads is dependent on the assays used at the time, it is clear that failure does occur in a significant minority of cases and that a high viral load is more likely to result in failure.
Prophylaxis with Antiviral AgentsAs proportion of HBV transmissions are not prevented despite joint immunoprophylaxis as outlined above, antiviral agents can be utilized to further decrease the risk of vertical transmission. By decreasing the maternal viral load, placental transmission is decreased and leads to lower intrauterine infection rates.32 Several antiviral agents are currently used for treating chronic HBV infections in non-pregnant patients; however, additional considerations must be taken into account because antiviral medications, while safe and well tolerated in the adult, may pose teratogenic risks to the developing fetus. It is important to appreciate that the antiviral agents effective against HBV do not have a licensed indication in pregnancy and their use in pregnancy is regarded as off-label.
If considered for use, antiviral agents are typically initiated in the late second or third trimester of pregnancy. The rationale is that intrauterine transmission mainly occurs after 28 weeks gestation, with placental studies showing little transmission in the 1st and 2nd trimesters.10,38 In addition, the third trimester is a relatively safe window for maternal drug use generally, as fetal organogenesis is largely completed by this stage and thus risk of teratogenicity is reduced. These time points are approximate and the clinician will also need to consider the initial viral load which, in a healthy young adult, can be in excess of one hundred million copies/ml, and the time to delivery.
The Antiretroviral Pregnancy Registry keeps data on various drugs that have been used in all trimesters of pregnancy and their fetal safety has been evaluated. The existing data on overall birth defect rate with lamivudine or tenofovir is 2.8% (CI 2.5-3.2%), which is not significantly different from population baseline (p = 0.90).39 In addition, the birth defect rate is similar across all three trimesters.39
The indication for use of antiviral agents has not been firmly established as thresholds for increased transmission can be seen with a large range of susceptible HBV viral loads. As outlined previously, viral loads of 105-108 copies/mL is associated with increased risk of vertical transmission.25–27 Therefore, it is reasonable to consider initializing antiviral drug therapy > 105 copies/mL in the late second or third trimester for a greater safety margin depending on viral load.
It is also important to keep in mind the fact that the pregnant patient with chronic HBV is at risk for reactivation flares during the pregnancy. Unvaccinated pregnant patients are also not immune to HBV and, if exposed, may contract acute HBV. In these circumstances, antiviral therapy may also be necessary to treat the pregnant mother, as well as the fetus40 or an acute reactivation flare of chronic HBV.41,42
In the anecdotal cases cited, lamivudine, as mono-therapy or as part of combination therapy, was used in early to late second trimester with successful stabilization of the mother’s liver failure and with no complications to the pregnancy or sequelae to the newborn. These cases illustrate the need to follow maternal liver biochemistry throughout pregnancy. The importance of monitoring liver biochemistry during pregnancy is underscored by a report of acute reactivation during pregnancy resulting in termination of pregnancy and urgent liver transplantation.43
A related clinical question is in regards to women with chronic HBV who are on chronic antiviral therapy at the time of pregnancy. A common practice is to discontinue the antiviral agents, for fear of teratogenicity especially during the first and early second trimesters. A small study from Korea44 has reported that approximately half of patients will develop elevations in serum ALT up to 5 times the upper limit of normal, although no hepatic decompensation was reported. Not unexpectedly, the majority of patients developed active HBV viremia after withdrawal of antiviral agents. The risk of a flare of ALT was highest in those who had an elevated ALT prior to starting antiviral agents pre-pregnancy.
It is important to keep in mind that patients who required antiviral agents before their pregnancy are a different group from the majority of chronic healthy carriers with clinically quiescent disease. This latter group constitutes the majority of patients and it is well-recognized that most pregnant HBV carriers will have normal liver biochemistry throughout their pregnancy although a significant minority who are HBeAg seropositive may experience a hepatitis flare post-pregnancy.45 Clearly the pregnant patient with HBV will need to have close monitoring of liver biochemistry with a view to antiviral therapy if clinically necessary. Monitoring of liver biochemistry will also be necessary post-partum and mothers for whom antiviral therapy for neonatal prophylaxis was initiated may need to continue with the antiviral agents for a few months after delivery.
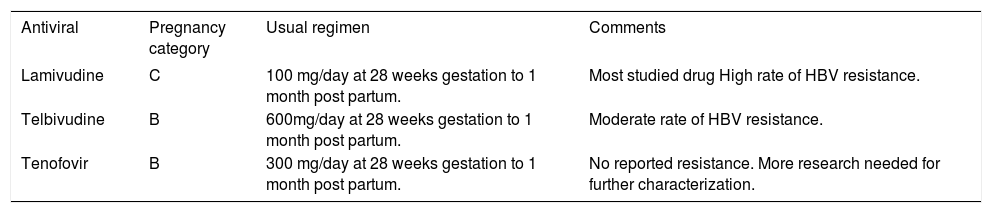
This last section will focus on individual antivirals that have evidence to support its use in pregnancy. Table 1 provides a summary.
Antivirals for HBV vertical transmission in pregnancy. Antivirals used for vertical transmission prophylaxis, Food and Drug Administration pregnancy categories, usual regimen, and additional information.
| Antiviral | Pregnancy category | Usual regimen | Comments |
|---|---|---|---|
| Lamivudine | C | 100 mg/day at 28 weeks gestation to 1 month post partum. | Most studied drug High rate of HBV resistance. |
| Telbivudine | B | 600mg/day at 28 weeks gestation to 1 month post partum. | Moderate rate of HBV resistance. |
| Tenofovir | B | 300 mg/day at 28 weeks gestation to 1 month post partum. | No reported resistance. More research needed for further characterization. |
Lamivudine is a Pregnancy Category C drug under the US Food and Drug Administration classification indicating that animal reproduction studies have reported adverse effects and that there is an absence of controlled trials in pregnant women but potential benefits may make use appropriate despite risks.46 It belongs to a drug class known as nucleoside reverse transcriptase inhibitor (NRTI) and acts to inhibit HBV DNA replication. The usual studied regimen is 100 mg/day starting at 28 weeks gestation to one month post partum.37 Lamivudine use in the third trimester demonstrates a 12 – 23.7% decreased incidence of intrauterine infection in the newborn compared to placebo according to a meta-analysis by Shi, et al.37 There was also a significant decrease of HBV DNA viral load in the lamivudine arm compared to placebo.47 Depending on the method of HBV diagnosis in the newborn, the OR is 0.38 CI 0.15-0.94 p = 0.04 for presence of HBsAg, and OR 0.22 CI 0.12-0.40 p < 0.001 for presence of HBV DNA. At 9-12 months, there is a 1.4-2% lower mother to child transmission rate, with OR of 0.31 CI 0.15-0.63 p < 0.01 for HBsAg diagnosis and OR of 0.2 CI 0.1-0.39 p < 0.01 for HBV DNA diagnosis.37 Another meta-analysis suggests that starting lamivudine at 28 weeks is more efficacious than starting at 32 weeks, with decreased rates of mother to child interruption of HBV infection.48 Importantly, no significant adverse effects were observed during the course of treatment and follow-up.37,48
The safety of lamivudine use prior to pregnancy that carried on during the early and final stages of pregnancy has also been recently studied.49 In 92 pregnancies, only two fetal abnormalities were noted: a scalp hemangioma and cerebral palsy. The investigators noted that the fetal abnormality rate was not greater than in mothers who did not receive lamivudine and concluded that lamivudine was safe in the first trimester of pregnancy and throughout pregnancy.
Despite the reported efficacy of lamivudine in reducing the risk of HBV transmission, the drug has limitations which make it a suboptimal choice for pregnant women. Lamivudine is well-reported to have a low threshold for the development of resistance with a 24% incidence of resistance after one year of use in non-pregnant patients, and a 70% incidence after four years.50 The development of lamivudine resistance can also increase the risk of resistance to other HBV antiviral agents.51 When one considers lamivudine’s relative low potency compared to other HBV antiviral agents,52 the fact that the target population in pregnancy would have a high viral load and the increased likelihood of developing antiviral resistant strains of HBV with increasing viral loads, then it is clear that lamivudine is a suboptimal choice with potential downstream risks to the mother in terms of future antiviral use post-pregnancy.
TelbivudineTelbivudine is classified as a Pregnancy Category B with the US FDA indicating no or minimal risk of fetal effects in animal studies, but well controlled human studies are lacking or animal studies report adverse fetal effects but well controlled human studies have not.39 Telbivudine is a synthetic thymidine nucleoside analog and is a reverse transcriptase inhibitor with activity against HBV.
Telbivudine has been reported to have greater potency than lamivudine in HBV clinical trials.53 Although resistance to telbivudine does occur, the risk is considerably less than lamivudine: 5% at one year, 11% at two years.54
The usual dose used in pregnancy is 600mg/day starting at week 28 of gestation to one month after delivery.55 Six studies were included in a metaanalysis evaluating the efficacy of telbivudine versus placebo in the third trimester of pregnancy for reducing the transmission of HBV.55 The telbivudine arms have a significantly lower HBV DNA viral load compared to placebo.55 Compared to placebo, the telbivudine groups had significantly lower HBV transmission rates at birth and at follow-up at 9-12 months. Depending on the method of HBV diagnosis, telbivudine demonstrates a RR of 0.18 CI 0.08-0.4 for HBV DNA positivity and RR of 0.31 CI 0.2-0.49 for presence of HBsAg at birth. At follow-up at 9-12 months, there is a RR of 0.09 CI 0.04-0.22 for HBV DNA viral load and RR of 0.11 CI 0.04-0.31 for HBsAg (55). Compared to placebo, telbivudine did not have differences in major adverse effects.55
TenofovirTenofovir is a Pregnancy Category B drug under the US FDA.39 It belongs to the NRTI class and acts on HBV reverse transcriptase. Tenofovir is a potent HBV antiviral agent56 and tenofovir, unlike all other HBV antiviral drugs, has not been reported to be associated with drug resistance.57
This drug has been used in the non-pregnant population as a first line agent against HBV because of its potency and favorable resistance profile; however, studies into its use in pregnancy, in the setting of HBV, are limited. Nevertheless, tenofovir has demonstrated efficacy and safety in trials with HIV mono-infected and HIV/HBV co-infected mothers.58 The neonatal safety of tenofovir exposure, starting before and throughout pregnancy, has recently been confirmed in a large HIV study in Africa.59 Tenofovir was not associated with an increased risk of stillbirths, congenital abnormalities, infant renal dysfunction or low infant weight up to two years of follow-up. A small retrospective case study in New York involving eleven HBV viremic and HBeAg positive mothers demonstrated a significant reduction in viral loads following tenofovir use in the third trimester.60 All eleven infants born were negative for HBsAg at 7-9 month follow-up. No adverse effects were noted in the series. The usual dosing of tenovofir is 300 mg/day in the third trimester starting at 28 weeks of gestation and continued until one month post-partum.
While the study is promising for tenofovir’s efficacy in preventing HBV vertical transmission, further studies including randomized controlled trials are needed to fully elucidate the drug’s role in this setting.
Conclusions and RecommendationsIt is the authors firm belief that, in 2013, vertical transmission of HBV is completely preventable and that even a single case of transmission is unacceptable. Post-exposure immunoprophylaxis consisting of hepatitis B immune globulin and HBV vaccination must be provided to every newborn of a HBV carrier mother. All pregnant HBV carriers must have liver biochemical monitoring during pregnancy and post-pregnancy. Re-activation HBV flares during and post-partum can be treated with antiviral therapy. For those patients with a high viral load, for whom there is a significant risk of failure of post-exposure immunoprophylaxis, antiviral agents can be started in the late second or third trimester of pregnancy. Although there is no consensus in regards to a HBV viral load threshold for starting antiviral therapy, the authors suggest a threshold of one million copies/mL.
Abbreviations- •
ALT: alanine transaminase.
- •
Anti-HBs: hepatitis B surface antibody.
- •
DNA: deoxyribonucleic acid.
- •
FDA: Food and Drug Administration (USA).
- •
HBeAg: hepatitis B e antigen.
- •
HBIG: hepatitis B immunoglobulin.
- •
HBsAg: hepatitis B surface antigen.
- •
HBV: hepatitis B virus.
- •
HIV: human immunodeficiency virus.
- •
NRTI: nucleoside reverse transcriptase inhibitor.
- •
PCR: polymerase chain reaction.
- •
RCT: randomized controlled trial.
- •
VL: viral load.
No grants or financial support to declare.