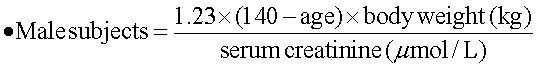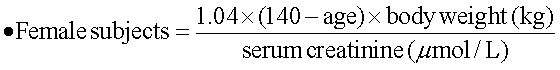Background and aim. In patients with chronic hepatitis C receiving Peg interferon/ribavirin (PEG-IFN/RBV) who do not achieve ≥ 2log-reduction in HCV-RNA at week 12 (null responders, NR) and in those with ≥ 2log-decrease but detectable at week 24 (partial responders, PR) the probability to achieve the sustained virological response (SVR) is almost null. The aim of this study was to investigate the efficacy of individualized schedule of progressively increased RBV doses in the setting of PEG-IFN/RBV treatment. Material and methods. PR or NR to PEG-IFN/RBV instead of discontinuing treatment were enrolled to receive increasing doses of RBV until a target theoretical concentration ([tRBV]) of ≥ 15 Mmol/L (by pharmacokinetic formula based on glomerular filtration rate). HCV-RNA was assessed every 4 weeks and, if detectable, RBV dose was gradually increased until negativization. Twelve weeks later, patients with detectable HCV-RNA discontinued therapy while those with undetectable HCV-RNA continued for further 48 weeks. Results. Twenty genotype-1 patients (8 NR and 12 PR) were enrolled. After 12 weeks 9 (45%) were still HCV-RNA positive and were discontinued, while remaining 11 had undetectable HCV-RNA. One stopped treatment for side effects. Ten completed treatment. Five (all PR) achieved SVR. Side effects incidence was similar to that observed during PEG-IFN/RBV. Conclusions. In conclusion, RBV high doses, according to individualized schedule, increase SVR in PR on a similar extent to that of triple therapy but without increase of side effects. Such treatment should be considered in PR with no access or intolerant to protease inhibitors (PI).
The main goal of the antiviral therapy for chronic hepatitis C (CHC) is the achievement of sustained virological response (SVR), defined as undetectable serum HCV-RNA 24 weeks after the cessation of treatment. The dual therapy with Peg-interferon/ ribavirin (PEG-IFN/RBV) in CHC allows to achieve the SVR in 40 to 50% of subjects infected by genotype 1.1–3
According to serum HCV-RNA decline, the response to PEG-IFN/RBV can be classified as:
- •
Null response (NR), if the decline is < 2 log10 after 12 weeks.
- •
Partial response (PR), if the decline is ≥ 2 log10 but still detectable after 24 weeks, and
- •
Relapse, if serum HCV-RNA is undetectable at the end of treatment (EOT) but reappears after treatment cessation.
European guidelines4 suggest to discontinue therapy in NR and PR, since the probability to achieve the SVR is considered to be almost null. The recent introduction of treatment regimens combining protease inhibitors (PI) with PEG-IFN/RBV has substantially changed the landscape of HCV genotype 1 management for both treatment-naïve and experienced patients. Nevertheless, approximately 25-35% of treatment-naïve and up to 60% of experienced do not respond even to the triple therapy.5–8 It is likely that the lack of response to the triple therapy depends on the poor sensitivity to PEG-IFN/RBV, with appearance of PI resistant viruses. This outlines the importance of an optimal response to PEG-IFN/RBV to allow the combination therapies with PI displaying their maximal efficacy, avoiding, at the same time, the selection of resistant strains. Moreover, in addition to the high rate of moderate/ severe side effects, a serious limitation to the use of triple therapies may arise from their high cost. Therefore, the optimization of dual therapy with PEG-IFN/ RBV is still of great importance.
In the current practice, RBV is administered according to body weight, usually between 800 and 1,400 mg daily, but data on the relationship between the oral dose and the resulting plasma concentrations are limited. A plasma RBV concentration higher than 15 ^mol/L has been shown to improve the SVR rate in genotype 1 patients with high baseline viral load.9
RBV is mainly eliminated by renal filtration.10 Some studies have shown the importance of renal function for maintaining an appropriate RBV plasma concentration, indicating that the RBV optimal dose should be calculated not only on the basis of body weight but also on the patient’s renal function.11,12 Recently, a large-scale analysis reported that in HCV genotype 1 patients treated with PEG-IFN alpha-2b and RBV, the relapse rate was dose-dependently correlated with RBV dose, irrespective of age, sex, early viral kinetics or the dose of PEG-IFN alpha-2b.13 Furthermore, in a hard-to-cure population such as liver transplanted patients with HCV recurrence, a high SVR rate was attained by utilizing a concentration-guided RBV dosing, calculated according to renal function and body weight.14 Finally, the data published by Lindahl, et al.15 explored the safety and efficacy of individualized schedule of high dose RBV in previously untreated patients with chronic hepatitis C, proving its feasibility in the latter clinical context.
Based on these premises, this study aimed to assess whether high doses of RBV (hdRBV) increase the virological response in genotype 1 difficult-to-treat patients presenting a NR or PR during a course of PEG-IFN/RBV treatment. In order to set the oral dose of RBV, we used a pharmacokinetic formula based on the glomerular filtration rate (GFR).
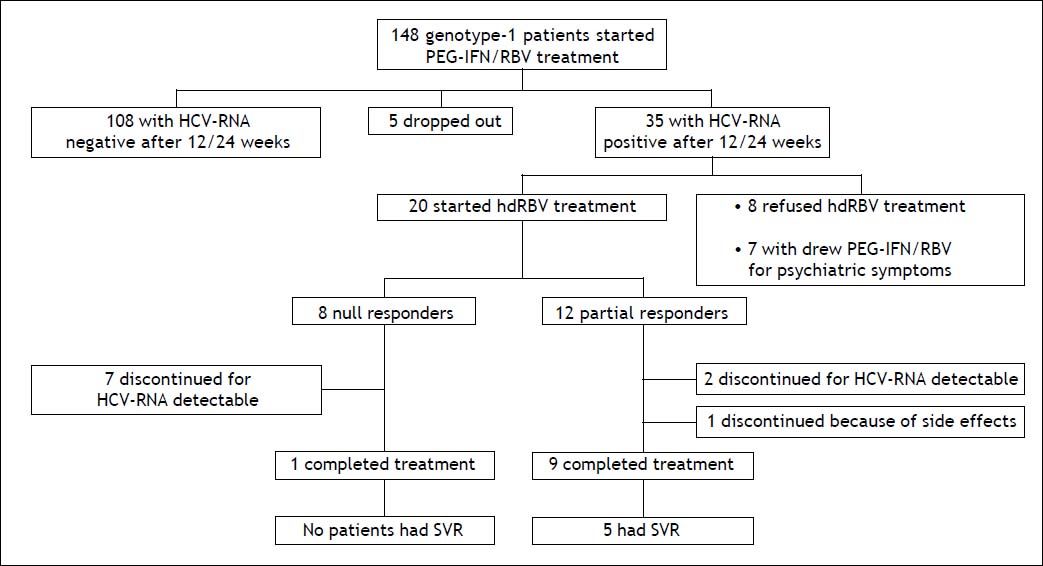
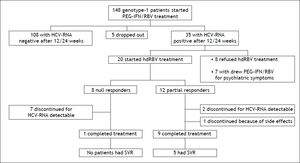
Material and MethodsSelection of patients and study designBetween October 2007 and October 2010, 148 consecutive patients ≥ 18 years, with HCV genotype 1 infection, both treatment naïve and experienced, were referred to our Outpatient Clinics for Liver Disease of our Department to receive PEG-IFN/RBV combination treatment (Figure 1). The exclusion criteria for standard PEG-IFN/RBV treatment prescription were: co-infection with hepatitis B virus or human immunodeficiency virus, any other cause of liver disease, evidence of hepatocellular carcinoma, previous diagnosis of severe depression or other severe psychiatric disorders, significant cardiac and/ or renal disease, seizure disorders, severe retinopathy, pregnancy, evidence of alcohol or drug abuse, low hemoglobin level (< 12 g/dL for women or < 13 g/dL for men), conditions at high risk of developing anemia or for which the occurrence of anemia could represent a vital risk, neutrophil granulocyte count < 1,500 cells/mm3, platelet count < 75,000/ mm3, creatinine > 1.5 times the upper limit of normal values. The stage of liver fibrosis was assessed by performing liver biopsy and classified according to Metavir score.16 The standard treatment consisted in PEG-IFN alpha-2a (180 µg/weekly subcutaneously) or PEG-IFN alpha-2b (1.5 µg/kg/weekly subcutaneously) plus oral weight-based RBV in two separated doses (for the association with PEG-IFN alpha-2a: 1,000 mg/day for patients weighing < 75 kg and of 1,200 mg/day for patients weighing ≥ 75 kg; for the association with PEG-IFN alpha-2b: 800 mg/day for patients weighing < 65 kg, 1,000 mg/day for patients with a body weight ranging from 65 to 85 kg and 1,200 mg/day for patients weighing 85 kg or more). The choice of the type of PEG-IFN was at the discretion of the attending physicians. Subjects who had been receiving 100% of expected PEG-IFN/ RBV doses were considered suitable for the enrollment in the study protocol with hdRBV. Prior to the enrolment the informed consent was obtained from each patient.
After 12 weeks of treatment for NR and 24 weeks for PR, instead of discontinue the therapy, the daily RBV dose was increased according to a pharmacokinetic formula mainly based on renal function, while PEG-IFN was continued without dose modifications. The RBV dose was calculated every 4 weeks to reach a calculated RBV theoretical concentration (tRBV) of at least 15 µmol/L, accordingly to the data obtained by Jen, et al.9 and the administered RBV dose was gradually increased until HCV-RNA negativization. Treatment was discontinued if HCV-RNA was still detectable after 12 weeks from hdRBV initiation, while continued for further 48 weeks if HCV-RNA was undetectable (< 9 UI/mL) at the same time point. The follow-up lasted 24 weeks after the EOT.
Ribavirin dose increase according to renal functionThe estimated renal function was calculated with a modified formula of the Cockroft-Gault equation:17
As suggested by Bruchfeld, et al.,11 the following formula was used to arrange the RBV daily oral dose in order to ensure a target blood concentration of about 15 µmol/L:
- •
New dose of RBV to be administered (mg) = 0.244 x target RBV concentration (i.e. 15 µmol/L) x dose interval x RBV clearance (clRBV).
The clRBV was calculated according to the following formula:
- •
clRBV (µmol/L) = 0.122 x GFR (mL/min) + 0.0414 x body weight (kg).
Serum HCV-RNA was determined every 4 weeks by quantitative PCR assay [(Versant® bDNA 3.0 (lower detection limit 615 IU/mL)], and qualitative PCR assay [(Versant® TMA (lower detection limit 9 IU/mL), ©Siemens, Milano, Italy)].
HCV genotype was determined by a commercially available line-probe assay (INNO-LiPA®, Innogenetics, Antwerp, Belgium). SVR was defined as undetectable serum HCV-RNA at the end of the 24-week follow-up period. After receiving informed consent, the IL-28B polymorphism (rs12979860) was retrospectively determined on DNA extracted from stored whole blood samples with a commercial kit according to the manufacturer’s protocol (Maxwell 16, Promega, Italy).
Safety monitoringOnce the hdRBV protocol was started, visits and laboratory determinations were performed every 4 weeks during the first 12 weeks and every 12 weeks thereafter until the EOT. Hematological adverse events were monitored by regular blood sampling on each visit. During the visits the investigators interviewed all subjects for a regular adverse events reporting in concern to the patient’s functional health and well-being perception.
Erythropoietin (EPO) and blood transfusion were used in case of anemia. Hemoglobin was monitored by regular blood testing until a stable value was reached. EPO supplementation was started in case of hemoglobin values falling below 10 g/dL and the blood transfusion was disposed for hemoglobin values < 8 g/dL. Patients were scheduled to be discontinued from therapy if hemoglobin values fell below 8 g/dL despite EPO supplementation and/or more than one blood transfusion a week were needed. PEG-IFN dose adjustments and/or discontinuations were performed according to manufacturer’s instruction.
Statistical analysisParametric and non-parametric tests were used, as appropriate. In particular, quantitative variables were expressed by mean ± standard deviation (or as median with range if more appropriate) and categorical variables as absolute and relative frequencies. Groups of quantitative and qualitative variables were compared using the Mann-Whitney and the Fisher-exact tests, respectively. A p-value < 0.05 was considered as statistically significant. Data handling and analysis were performed with SPSS software for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA).
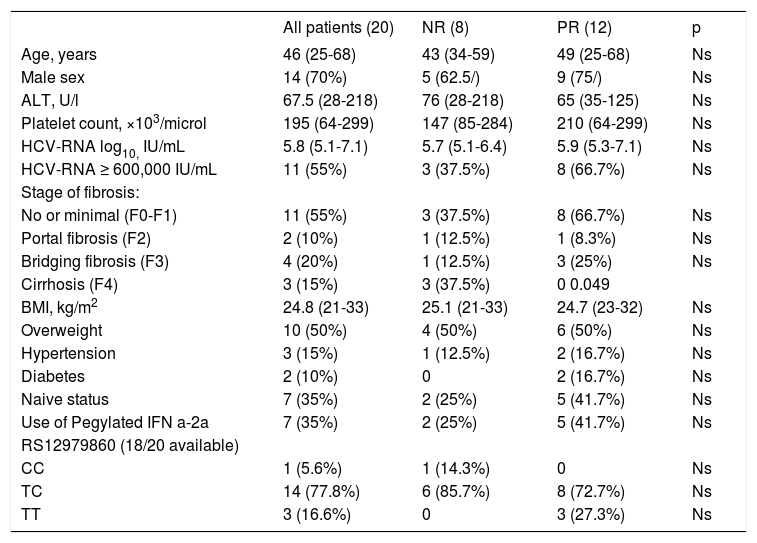
ResultsCharacteristics of study patientsAmong 148 evaluated patients (Figure 1), 5 dropped-out from the treatment since they missed the scheduled visits, so the necessary virological data were not available. One hundred-eight patients were not enrolled due to undetectable HCV-RNA after 12 or 24 weeks of treatment. Thirty five resulted NR or PR at the latter time points; among these 8 refused to participate to the hdRBV protocol and 7 withdrew the antiviral treatment due to psychiatric symptoms. Therefore, 20 patients (8 NR and 12 PR) were enrolled into the hdRBV study protocol. Table 1 shows the main characteristics of the patients at baseline, before starting the standard antiviral treatment. They were prevalently males (70%) with a median age of 46 years (range, 25-68). Apart from genotype 1, all patients had at least one or more negative predictive factors of response to antiviral therapy. Up to 30% had a histological diagnosis of bridging fibrosis or cirrhosis and, among cirrhotics, none was decompensated or had previous decompensation episodes. Overweight or obesity were present in 50% of population while diabetes in 10%. Thirteen patients (65%) had previously failed at least one course of antiviral therapy. Only 1 patient had a CC genotype at IL-28B SNP position rs12979860. There were no significant differences between NR and PR groups, except for prevalence of cirrhosis (Table 1). Mean GFR was 102 ml/min ±26.9 (range, 60.1-180.36).
Baseline characteristics of the patients according to the response to SOC treatment before increasing the RBV dose.
| All patients (20) | NR (8) | PR (12) | p | |
|---|---|---|---|---|
| Age, years | 46 (25-68) | 43 (34-59) | 49 (25-68) | Ns |
| Male sex | 14 (70%) | 5 (62.5/) | 9 (75/) | Ns |
| ALT, U/l | 67.5 (28-218) | 76 (28-218) | 65 (35-125) | Ns |
| Platelet count, ×103/microl | 195 (64-299) | 147 (85-284) | 210 (64-299) | Ns |
| HCV-RNA log10, IU/mL | 5.8 (5.1-7.1) | 5.7 (5.1-6.4) | 5.9 (5.3-7.1) | Ns |
| HCV-RNA ≥ 600,000 IU/mL | 11 (55%) | 3 (37.5%) | 8 (66.7%) | Ns |
| Stage of fibrosis: | ||||
| No or minimal (F0-F1) | 11 (55%) | 3 (37.5%) | 8 (66.7%) | Ns |
| Portal fibrosis (F2) | 2 (10%) | 1 (12.5%) | 1 (8.3%) | Ns |
| Bridging fibrosis (F3) | 4 (20%) | 1 (12.5%) | 3 (25%) | Ns |
| Cirrhosis (F4) | 3 (15%) | 3 (37.5%) | 0 0.049 | |
| BMI, kg/m2 | 24.8 (21-33) | 25.1 (21-33) | 24.7 (23-32) | Ns |
| Overweight | 10 (50%) | 4 (50%) | 6 (50%) | Ns |
| Hypertension | 3 (15%) | 1 (12.5%) | 2 (16.7%) | Ns |
| Diabetes | 2 (10%) | 0 | 2 (16.7%) | Ns |
| Naive status | 7 (35%) | 2 (25%) | 5 (41.7%) | Ns |
| Use of Pegylated IFN a-2a | 7 (35%) | 2 (25%) | 5 (41.7%) | Ns |
| RS12979860 (18/20 available) | ||||
| CC | 1 (5.6%) | 1 (14.3%) | 0 | Ns |
| TC | 14 (77.8%) | 6 (85.7%) | 8 (72.7%) | Ns |
| TT | 3 (16.6%) | 0 | 3 (27.3%) | Ns |
Values are number (proportion) or median (range).
Antiviral therapy was carried out with PEG-IFN alpha-2a in 7 patients (35%) and with PEG-IFN alpha-2b in 13 patients (65%). The initial mean dose of RBV, which was administered according to body weight in all patients, was 990 mg/day (range, 800-1,200) and the mean [tRBV], calculated according to GFR, was 11.3 µmol/L (range, 7.3-16.7).
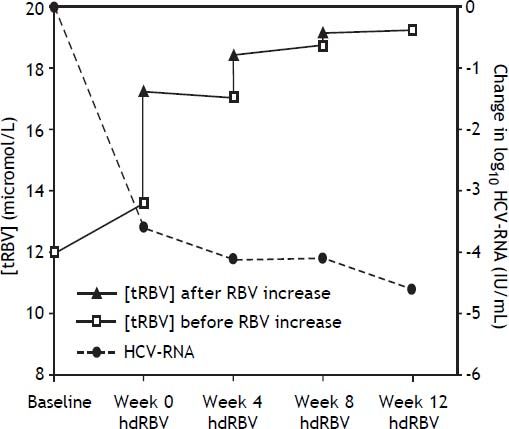
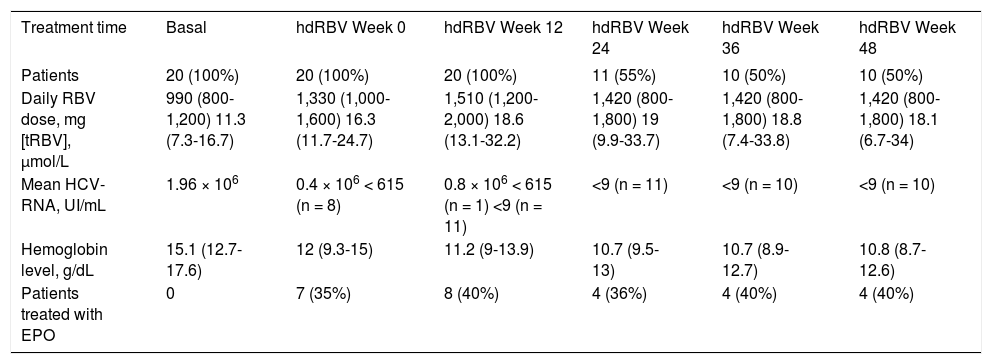
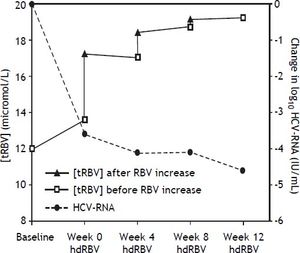
The trends of [tRBV] and HCV-RNA after starting hdRBV are showed in the figure 2, while the values of RBV doses and corresponding concentrations, HCV-RNA and hemoglobin levels and EPO use during hdRBV treatment are reported in table 2. The new assessed mean [tRBV] after the hdRBV beginning was 16.3 µmol/L (range 11.7-24.7). After a gradual 4 week dose escalation, the mean daily dose of RBV at treatment week 12 was 1,510 mg/day (range, 1,200-2,000) and the calculated mean [tRBV] further raised to 18.6 µmol/L (range, 13.1-32.2) (Figure 2 and Table 2). As reported in table 2, only one patient did not reach the target [tRBV] of 15 µmol/L. In this patient, since HCV-RNA was un-detectable at week 8, a further increase of RBV dose was considered not indicated. Moreover, it is of note that the [tRBV] of this patient at baseline was as low as 7.3 µmol/L.
RBV doses and corresponding theoretical serum concentrations, viral load, hemoglobin levels and erythropoietin treatment before and after RBV dose increase.
| Treatment time | Basal | hdRBV Week 0 | hdRBV Week 12 | hdRBV Week 24 | hdRBV Week 36 | hdRBV Week 48 |
|---|---|---|---|---|---|---|
| Patients | 20 (100%) | 20 (100%) | 20 (100%) | 11 (55%) | 10 (50%) | 10 (50%) |
| Daily RBV dose, mg [tRBV], µmol/L | 990 (800-1,200) 11.3 (7.3-16.7) | 1,330 (1,000-1,600) 16.3 (11.7-24.7) | 1,510 (1,200-2,000) 18.6 (13.1-32.2) | 1,420 (800-1,800) 19 (9.9-33.7) | 1,420 (800-1,800) 18.8 (7.4-33.8) | 1,420 (800-1,800) 18.1 (6.7-34) |
| Mean HCV-RNA, UI/mL | 1.96 × 106 | 0.4 × 106 < 615 (n = 8) | 0.8 × 106 < 615 (n = 1) <9 (n = 11) | <9 (n = 11) | <9 (n = 10) | <9 (n = 10) |
| Hemoglobin level, g/dL | 15.1 (12.7-17.6) | 12 (9.3-15) | 11.2 (9-13.9) | 10.7 (9.5-13) | 10.7 (8.9-12.7) | 10.8 (8.7-12.6) |
| Patients treated with EPO | 0 | 7 (35%) | 8 (40%) | 4 (36%) | 4 (40%) | 4 (40%) |
Values are number (proportion) or mean (range).
After 12 weeks from hdRBV initiation, 11 (55%) patients were HCV-RNA negative. The remaining 9 patients were HCV-RNA positive and discontinued the treatment. In the PR group a 1 log10 reduction of HCV-RNA was observed after 12 weeks of hdRBV (Figure 1).
One PR patient with undetectable HCV-RNA, discontinued treatment after 14 weeks because of severe asthenia mainly related to PEG-IFN administration. At the EOT the HCV-RNA was undetectable in 10 patients. Five patients (25% of the entire study population) achieved an SVR (4 naïve and 1 experienced). All patients who achieved SVR were PR to PEG-IFN/RBV and all presented a non-CC IL28B polymorphism.
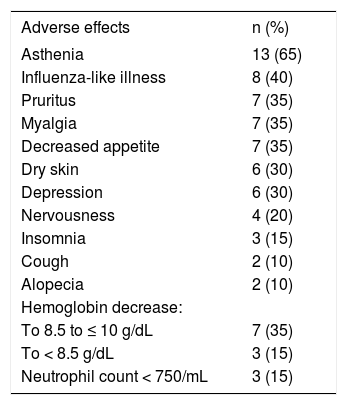
Side effectsAll patients presented at least one adverse event during the treatment. Table 3 shows the most frequent (> 10%) adverse events. The incidence of non-hematological side effects was similar to that observed during standard PEG-IFN/RBV treatment. Only a slight decrease in hemoglobin levels was observed during the treatment, as shown in table 2. EPO supplementation (range 10,000-40,000 UI/week) was required in 7 (35%) patients after the beginning of PEG-IFN/RBV. In only one patient, hemoglobin values fell after the hdRBV introduction requiring EPO supplementation. Overall, after EPO introduction, hemoglobin values remained stable despite the hdRBV, and RBV dose reduction was only required in one patient whose anemia did not respond to EPO and who refused to receive blood transfusion. No other patient needed blood transfusion. No patient needed PEG-IFN dose reduction.
Incidence of the most common adverse events during high dose RBV treatment.
| Adverse effects | n (%) |
|---|---|
| Asthenia | 13 (65) |
| Influenza-like illness | 8 (40) |
| Pruritus | 7 (35) |
| Myalgia | 7 (35) |
| Decreased appetite | 7 (35) |
| Dry skin | 6 (30) |
| Depression | 6 (30) |
| Nervousness | 4 (20) |
| Insomnia | 3 (15) |
| Cough | 2 (10) |
| Alopecia | 2 (10) |
| Hemoglobin decrease: | |
| To 8.5 to ≤ 10 g/dL | 7 (35) |
| To < 8.5 g/dL | 3 (15) |
| Neutrophil count < 750/mL | 3 (15) |
Despite the introduction of PI and triple therapy regimens, the treatment of patients with chronic hepatitis C genotype 1 remains challenging, since still a significant proportion of patients fails to achieve SVR. This seems to be a consequence of both insensitivity to PEG-IFN/RBV and drop-outs due to side effects. The management of anemia, the principal adverse event induced by RBV, mostly depends on RBV dose adjustment,18 apart from erythropoiesis stimulating agents and blood transfusion, especially during triple antiviral therapy, although this seems not to influence the SVR rate.19 It was shown that anemia is mainly associated with RBV plasma concentration, rather than with the administered dose, thus suggesting that RBV dose should be adjusted according to renal function.12
It should be of note also that we still do not have prompt re-treatment options for non-genotype 1 patients with a previous non-response or relapse to the PEG-IFN/RBV treatment. Moreover, there is a quote of genotype 1 patients presenting exclusion criteria to the treatment with PI.
Thus,
- •
The risk of resistant viral strains appearance, potentially compromising the future therapeutic options.
- •
The need of optimizing the management of anemia during the treatment schedules including RBV, and
- •
The still existing population of patients not candidates PEG-IFN/RBV, all justify a careful further exploration of the therapeutic potential of dual therapy, especially in the countries where the new antiviral drugs are still unavailable.
The originality of our study in respect to the previous ones exploring the re-treatment options,20–24 is given by the novel design scheduling the introduction of hdRBV in the very time point that indicates a stopping rule due to a null or a partial response, according to the international guidelines.4
This pilot study, which started before the PI availability, strongly suggests that treatment discontinuation according to the current response-guided approach might not always be the best option in PR. Indeed, our PR study population attained 1 log decrease of HCV-RNA from hdRBV beginning, 47.4% reached an EOT virological response and 41.6% achieved the SVR. Eighty percent of the patients who finally achieved the SVR were naïve to prior antiviral therapies.
The same treatment schedule was applied to the NR, but none achieved SVR. Even though other factors are also involved to explain such a result, still it is worth mentioning that the patients with cirrhosis enrolled in our study were all included in the NR group. The distribution of the IL-28 genotype in the study population was homogeneous. Only 1 patient (NR) had the favorable IL-28 genotype (CC) and, despite this, he did not show a virological response following hdRBV.
It is important to remark that the SVR rate among PR group was surprisingly elevated, even when compared with the results obtained in PR populations included in the phase III clinical trials on telaprevir (59% and 54% with and without lead-in phase, respectively) and boceprevir (40% and 52% for the response-guided therapy group and the group treated for 44 weeks, respectively).6,8 This may implicate that the lead-in phase duration of 4 weeks in triple regimens is too short. Rather, a 12 weeks lead-in phase with PEG-IFN and with RBV administered at doses calculated according to the renal function would be of great advantage in triple antiviral schedules, since major reduction of the viremia and minor incidence of viral resistances could be obtained.
The type and the incidence of adverse effects observed in this study did not differ from those reported in other studies with PEG-IFN/RBV. In particular, gradual and monitored RBV dose increase according to renal function to reach the minimum RBV blood concentration of 15 µmol/L did not increase the occurrence of anemia.
It is interesting to note that the range of [tRBV] both at the beginning of hdRBV schedule and at the 12th week of treatment was quite wide. This finding indicates that the body weight is not sufficient per se to determine the adequate RBV dose, which is presumably better defined according both to the body weight and the renal function.
The main limitations of this study are the small number of patients and the unavailability of the plasma concentration of RBV (the concentration curves reported in figure 2 are only theoretical). Still, the measurement of the RBV concentration might not be always feasible in the real clinical practice, e.g. in the primary/secondary hepatologic centers, even more since the dosing should be ideally performed on erythrocytes rather than on plasma samples. Lindahl, et al.15 explored the safety and tolerance to hdRBV in combination with PEG-IFN in previously untreated patients and in this study the real plasma RBV concentrations were monitored, after setting the RBV individualized dose calculated from the above mentioned pharmacokinetic formula.11 Our study is to be considered a proof of principle study exploring the hdRBV based antiviral schedule in a treatment-experienced population.
However, the most important message emerging from our data is that dual therapy with PEG-IFN/ RBV of PR patients can be further optimized by adjusting the RBV dose according to renal function, likely without increasing the incidence of anemia. Such an optimization could help to reappraise not only the response-guided approach in the dual antiviral treatment, with a greater number of patients achieving SVR, but also PI-based schedules, especially those with a lead-in phase. This could help to avoid unnecessary and therefore inappropriate triple therapies, since more expensive and burdened with more side effects.
Considering that our results are referred specifically to the cohort of PR, this is particularly applicable in the population of relapsers to the standard treatment.
Disclosure StatementThe authors declare no competing interest.
Abbreviations- •
ALT: alanine transaminase.
- •
BMI: body mass index.
- •
CHC: chronic hepatitis C.
- •
EOT: end of treatment.
- •
EPO: erythropoietin.
- •
GFR: glomerular filtration rate.
- •
HCV: hepatitis C virus.
- •
hdRBV: high doses of RBV.
- •
NR: null responders.
- •
PCR: polymerase chain reaction.
- •
PEG-IFN: pegylated interferon.
- •
PI: protease inhibitors.
- •
PR: partial responders.
- •
RBV: ribavirin.
- •
SVR: sustained virological response.
- •
tRBV: target theoretical ribavirin concentration.