De novo malignancies represent an important cause of death for liver transplant recipients. Our aim was to analyze predictors of extra-hepatic non-skin cancer (ESNSC) and the impact of ESNSC on the long-term outcome.
PatientsWe examined data from patients transplanted between 2000 and 2005 and followed-up in five Italian transplant clinics with a retrospective observational cohort study. Cox Regression was performed to identify predictors of ESNSC. A 1:2 cohort sub-study was developed to analyze the impact of ESNSC on 10-year survival.
ResultsWe analyzed data from 367 subjects (median follow-up: 15 years). Patients with ESNSC (n = 47) more often developed post-LT diabetes mellitus (DM) (57.4% versus 35,9%, p = 0.004). At multivariate analysis, post-LT DM independently predicted ESNSC (HR 1.929, CI 1.029-3.616, p = 0.040). Recipients with ESNSC showed a lower 10-year survival than matched controls (46,8% versus 68,1%, p = 0.023).
ConclusionsPost-LT DM seems to be a relevant risk factor for post-LT ESNSC. ESNSC could have a noteworthy impact on the long-term survival of LT recipients.
Liver transplant (LT) is an efficient option for patients with acute liver failure, end-stage liver disease, and hepatocellular carcinoma (HCC) [1]. In the last years, the continuous improvement of surgical and clinical management together with the progressive eradication of hepatitis C virus (HCV) infection, led to a constant increase of LT recipients mean age [2]. The obvious consequence is a rise of age-related disorders such as malignancies and cardiovascular (CV) disorders [3,4].
LT recipients show a 11-fold major risk of malignancy in comparison with non-transplanted subjects [5]. Specifically, the occurrence of de novo cancer ranges from 3.1% to 14.4% at 5 years and from 10 to 14.6% at 10 years after LT [6,7]. The main reason of their higher risk of malignancy in respect to general population lies in their need of indefinitely immunosuppressive therapies that per se can increase the risk of cancer [2,[8]]. The onset of de novo malignancies in LT recipients has been also associated to aging and lifestyle, activation of oncogenic viruses and liver disorder that led to LT itself [9]. The most common post-LT cancers are the following: non-melanoma skin tumors, lymphoproliferative illnesses and solid-organ malignancies [3]. Notably, these malignancies are very different from a pathogenic, clinical and prognostic point of view. In fact, de novo HCC, post-transplant lymphoproliferative disorders, Kaposi's sarcoma and skin malignancies present specific risk factors such as recurrence of underlying liver disease, Epstein-Barr Virus, Human-Herpesvirus-8 and sun exposure while extra-hepatic solid non-skin cancer (ESNSC) do not show such a well-defined risk profile [10]. Moreover, from a clinical point of view, non-melanoma skin malignancies do not influence patient survival while other forms of de novo cancer lead to high mortality rates [11]. The chance of survival after de novo cancer onset markedly depends on the specific diagnosis and it is commonly poorer than non-transplanted subject with the same malignancy subtype [11].
Post-LT malignancies represent today one of the main clinical issues for the LT community and they are expected to quickly become the main cause of exitus [11–15]. Despite this, current guidelines on LT [2,16] do not suggest a well-defined screening and management of risk factors of de novo cancer, that is actually managed on individual basis with consequent unsuccessful prevention and late diagnosis [3]. Moreover, currently available data are still deficient in order to achieve an adequate risk stratification and a definitive clinical picture about the impact of de novo cancer on the LT recipient prognosis [3].
Our main aim was therefore, to detect the independent predictors of post-LT ESNSC development and to analyze their impact on patient survival. Moreover, we wanted to detect the main predictors of overall long-term survival.
2Material and methods2.1DesignThe present retrospective observational cohort study was developed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments and it was agreed by the Independent Ethics Committee of Tuscany region (“Comitato Etico Area Vasta Centro”; approval number: 19410).
The nature of the present study (retrospective analysis of old data) did not permit the collection of specific informed consent.
The reporting of this study conforms to STROBE guidelines [17].
In the present study we de-identified all patient details.
2.2SettingWe analyzed data of consecutively liver transplanted subjects (from 2000 to 2005) followed-up in 5 Italian transplant outpatient clinics (Transplant Center and Gastroenterology Outpatient Clinic, Modena; Hepatology Outpatient Clinic, Bologna; Gastroenterology Outpatient Clinic, Florence; Division of Gastroenterology, Bolzano; Liver Unit for Transplant Management, Caserta).
2.3PopulationIn the present study, we updated our published data [18] (from November 1st, 2019, to November 1st, 2020) of a cohort of patients transplanted from 2000 to 2005 and followed-up in 5 Italian transplant outpatient clinics.
2.4SamplingWe included adult patients (age >18 years) undergoing LT in the indicated period and followed-up by the involved Units. Death within 12 months after LT, combined transplants, Human Immunodeficiency Virus infection or co-infection, represented the exclusion criteria.
The sample size has been calculated based on studies previously conducted for the analysis of cancer incidence and predictors. Considering the 10-year post-transplant cancer incidence equal to 15% [19], hypothesizing a +/- 4% error, we had to enroll at least 306 subjects.
2.5Data collectionWe developed a retrospective examination of prospectively collected data. An analytic database was created to register clinical and demographic data comprising the following: age at the time of LT, biological gender, etiology of liver disorder, Model for End-Stage Liver Disease (MELD) score at the time of LT. Furthermore, the following data about the period before and after LT: HCC staging, body mass index (BMI) at the time of LT, DM, Metabolic syndrome (MetS), diagnosis of CV event and extra-hepatic malignancies. Post-LT BMI, DM and MetS were verified after 12 months from the transplant. We also recorded some donor patterns: age, steatosis (%), BMI, nature (living or deceased). Data on immunosuppressive regimen were documented too: prednisone with CNIs (CSA or TAC) represented the first choice. Mycophenolate mofetil (MMF) with CNIs or mTOR inhibitors (EVE or SIR) were the second therapeutic choice. According to the consolidated protocols, corticosteroids were stopped 6 months after LT.
2.6DefinitionsMetS was defined according to the American Heart Association [20]. Patients were considered affected by post-LT diabetes mellitus (DM) only if transient hyperglycemia associated to corticosteroids persists after drug withdrawal [21].
Diagnosis of malignancy was established because of clinical follow-up, histologically assessed, and recorded following the International Classification of Diseases and Related Health Problems, 10th revision (ICD-10).
2.7Data analysisData are reported as mean [±standard deviation (SD)] or median (with range, 25th and 75th percentile) whenever suitable. Confidence interval (CI) was reported when appropriate. Significance of modifications between variables was computed with non-parametric tests. Chi-Square/Fisher's exact test was used for categorical parameters and Kruskal Wallis’ for continuous variables.
Stepwise Cox regression analysis was used for detecting the independent predictors of ESNSC and overall mortality. Clinical and laboratory patterns before LT, the chief donor patterns and the main clinical events after LT were evaluated.
Firstly, we tested all variables individually at the Univariate analysis. Variables with P value < 0.1 were then evaluated together in the Multivariate analysis (Cox Regression) with significance that was achieved with P < 0.05.
Survival analysis was done with the Kaplan-Meier method and log-rank test was used to compare subgroups. A p value less than 0.05 was evaluated significant for all tests. We used the SPSS® software (IBM SPSS Statistics, version 20.0) for all the described analyses.
2.8Matched cohort sub studyWe then conducted a matched (1:2) cohort sub study to analyze the influence of ESNSC on the long-term outcome. Among the cohort members, patients who developed post-LT ESNSC after LT were classified as cases. For each case, two control patients were randomly selected from the entire cohort who did not show an ESNSC diagnosis. Controls were matched according to biological gender, age at LT (± 3 years), liver disorder indicating LT, pre-LT HCC. We appointed to each control the same index date (date of cancer diagnosis) as their matched case. For each subject, time at risk was estimated as the time passed from the date of malignancy diagnosis (or index date for controls) to the date of death, or to end of follow-up period. The registered time was cut at 10 years.
2.9ProcedureThe present study did not involve any procedure.
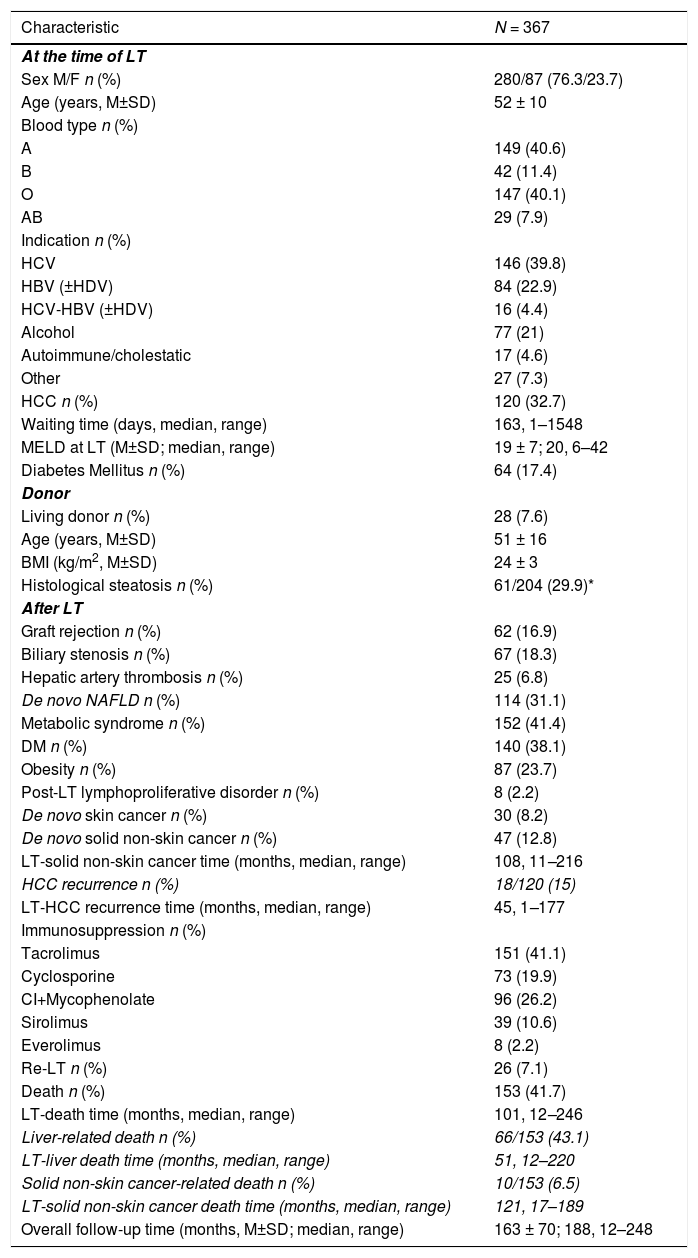
3Results3.1Study PopulationThe study includes 367 patients. Full patterns of the study population are described in Table 1. Pre-LT characteristics and donor patterns have been already presented in our previous study [18].
Main patterns of the whole study cohort: pre-transplant data, donor patters, post-transplant features.
| Characteristic | N = 367 |
|---|---|
| At the time of LT | |
| Sex M/F n (%) | 280/87 (76.3/23.7) |
| Age (years, M±SD) | 52 ± 10 |
| Blood type n (%) | |
| A | 149 (40.6) |
| B | 42 (11.4) |
| O | 147 (40.1) |
| AB | 29 (7.9) |
| Indication n (%) | |
| HCV | 146 (39.8) |
| HBV (±HDV) | 84 (22.9) |
| HCV-HBV (±HDV) | 16 (4.4) |
| Alcohol | 77 (21) |
| Autoimmune/cholestatic | 17 (4.6) |
| Other | 27 (7.3) |
| HCC n (%) | 120 (32.7) |
| Waiting time (days, median, range) | 163, 1–1548 |
| MELD at LT (M±SD; median, range) | 19 ± 7; 20, 6–42 |
| Diabetes Mellitus n (%) | 64 (17.4) |
| Donor | |
| Living donor n (%) | 28 (7.6) |
| Age (years, M±SD) | 51 ± 16 |
| BMI (kg/m2, M±SD) | 24 ± 3 |
| Histological steatosis n (%) | 61/204 (29.9)* |
| After LT | |
| Graft rejection n (%) | 62 (16.9) |
| Biliary stenosis n (%) | 67 (18.3) |
| Hepatic artery thrombosis n (%) | 25 (6.8) |
| De novo NAFLD n (%) | 114 (31.1) |
| Metabolic syndrome n (%) | 152 (41.4) |
| DM n (%) | 140 (38.1) |
| Obesity n (%) | 87 (23.7) |
| Post-LT lymphoproliferative disorder n (%) | 8 (2.2) |
| De novo skin cancer n (%) | 30 (8.2) |
| De novo solid non-skin cancer n (%) | 47 (12.8) |
| LT-solid non-skin cancer time (months, median, range) | 108, 11–216 |
| HCC recurrence n (%) | 18/120 (15) |
| LT-HCC recurrence time (months, median, range) | 45, 1–177 |
| Immunosuppression n (%) | |
| Tacrolimus | 151 (41.1) |
| Cyclosporine | 73 (19.9) |
| CI+Mycophenolate | 96 (26.2) |
| Sirolimus | 39 (10.6) |
| Everolimus | 8 (2.2) |
| Re-LT n (%) | 26 (7.1) |
| Death n (%) | 153 (41.7) |
| LT-death time (months, median, range) | 101, 12–246 |
| Liver-related death n (%) | 66/153 (43.1) |
| LT-liver death time (months, median, range) | 51, 12–220 |
| Solid non-skin cancer-related death n (%) | 10/153 (6.5) |
| LT-solid non-skin cancer death time (months, median, range) | 121, 17–189 |
| Overall follow-up time (months, M±SD; median, range) | 163 ± 70; 188, 12–248 |
NAFLD, Non-Alcoholic Fatty Liver Disease; χ2, Chi-Square test; MW-U, Mann-Whitney U test; M/F, Males/Females; LT, Liver Transplant; M, mean; SD, standard deviation; HCV, hepatitis c virus; HBV, hepatitis B virus; HDV, hepatitis D virus; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; BMI, body mass index; NAFLD, non-alcoholic fatty liver disease; DM, diabetes mellitus; CI, calcineurin inhibitor.
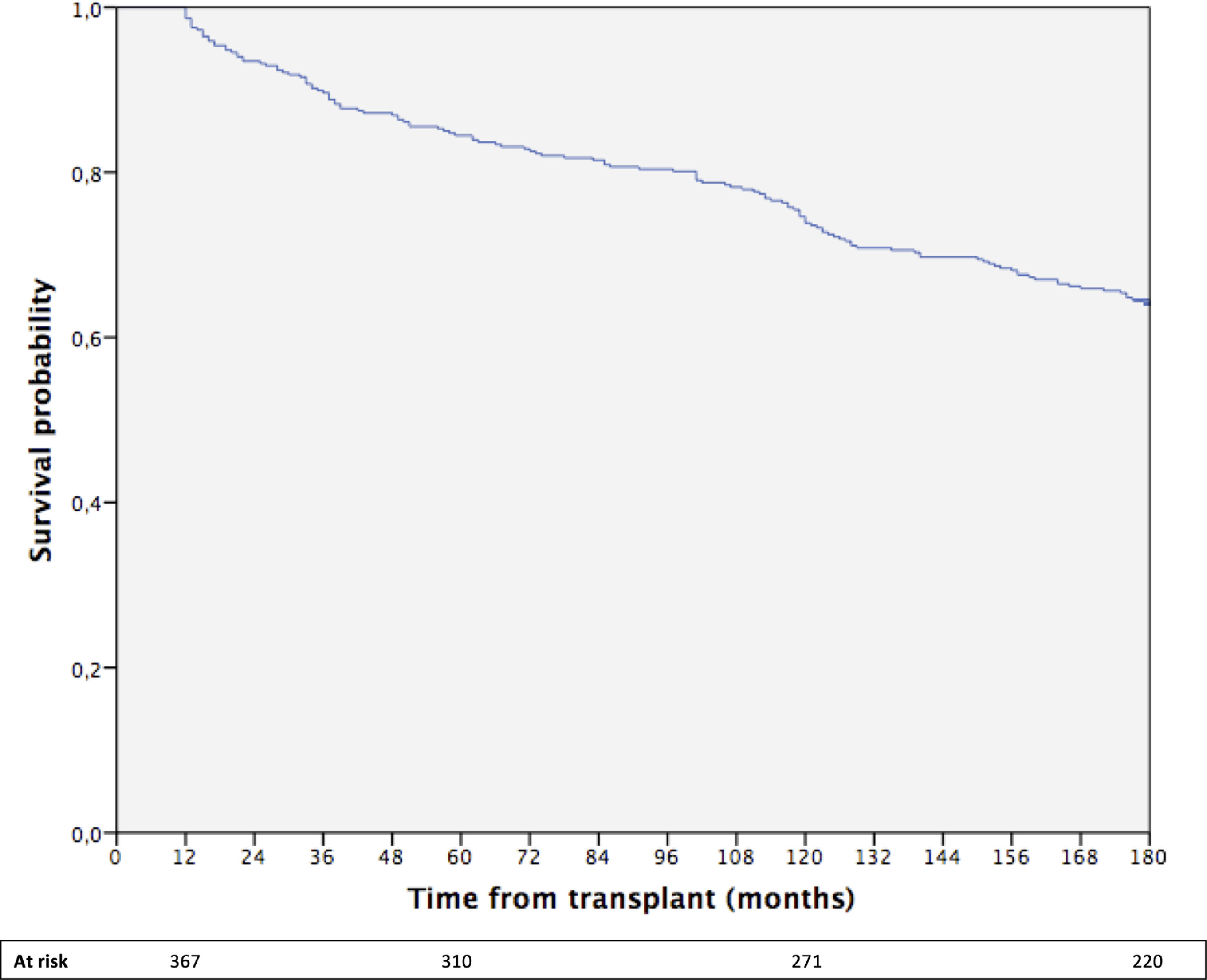
The median follow-up time was 188 months (range 12–248; 25th percentile 119 month, 75th percentile 215 months). During follow-up, 47 patients developed ESNSC equally distributed during the follow-up time and described in detail in the Supplementary Table 1. During the overall observation, 153 patients died, approximately half of whom for liver-related causes and 6.5% due to ESNSC.
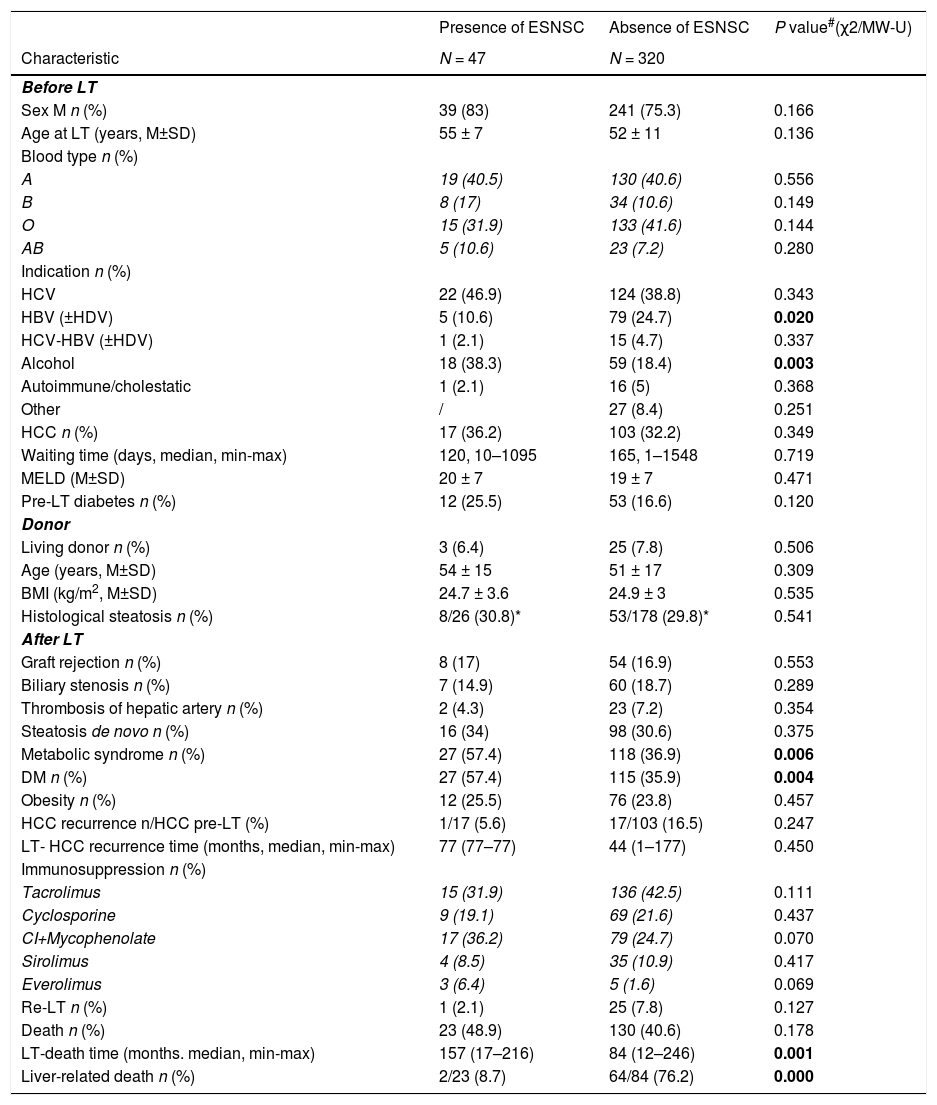
3.2Comparison between patients with and without post-LT ESNSCMain patterns of recipients with and without post-LT ESNSC are summarized in the Table 2. Patients with diagnosis of ESNSC had more frequently an alcohol liver disease as pre-LT disorder indicating LT (38.3% versus 18.4%, p = 0.003) and developed more often a Mets and a DM after LT (57.4% and 57.4% versus 36.9 and 35.9%, p = 0.006 and p = 0.004, respectively).
Main pre-transplant data, donor patters, post-transplant features according to the onset of extra-hepatic solid non-skin cancer.
| Presence of ESNSC | Absence of ESNSC | P value#(χ2/MW-U) | |
|---|---|---|---|
| Characteristic | N = 47 | N = 320 | |
| Before LT | |||
| Sex M n (%) | 39 (83) | 241 (75.3) | 0.166 |
| Age at LT (years, M±SD) | 55 ± 7 | 52 ± 11 | 0.136 |
| Blood type n (%) | |||
| A | 19 (40.5) | 130 (40.6) | 0.556 |
| B | 8 (17) | 34 (10.6) | 0.149 |
| O | 15 (31.9) | 133 (41.6) | 0.144 |
| AB | 5 (10.6) | 23 (7.2) | 0.280 |
| Indication n (%) | |||
| HCV | 22 (46.9) | 124 (38.8) | 0.343 |
| HBV (±HDV) | 5 (10.6) | 79 (24.7) | 0.020 |
| HCV-HBV (±HDV) | 1 (2.1) | 15 (4.7) | 0.337 |
| Alcohol | 18 (38.3) | 59 (18.4) | 0.003 |
| Autoimmune/cholestatic | 1 (2.1) | 16 (5) | 0.368 |
| Other | / | 27 (8.4) | 0.251 |
| HCC n (%) | 17 (36.2) | 103 (32.2) | 0.349 |
| Waiting time (days, median, min-max) | 120, 10–1095 | 165, 1–1548 | 0.719 |
| MELD (M±SD) | 20 ± 7 | 19 ± 7 | 0.471 |
| Pre-LT diabetes n (%) | 12 (25.5) | 53 (16.6) | 0.120 |
| Donor | |||
| Living donor n (%) | 3 (6.4) | 25 (7.8) | 0.506 |
| Age (years, M±SD) | 54 ± 15 | 51 ± 17 | 0.309 |
| BMI (kg/m2, M±SD) | 24.7 ± 3.6 | 24.9 ± 3 | 0.535 |
| Histological steatosis n (%) | 8/26 (30.8)* | 53/178 (29.8)* | 0.541 |
| After LT | |||
| Graft rejection n (%) | 8 (17) | 54 (16.9) | 0.553 |
| Biliary stenosis n (%) | 7 (14.9) | 60 (18.7) | 0.289 |
| Thrombosis of hepatic artery n (%) | 2 (4.3) | 23 (7.2) | 0.354 |
| Steatosis de novo n (%) | 16 (34) | 98 (30.6) | 0.375 |
| Metabolic syndrome n (%) | 27 (57.4) | 118 (36.9) | 0.006 |
| DM n (%) | 27 (57.4) | 115 (35.9) | 0.004 |
| Obesity n (%) | 12 (25.5) | 76 (23.8) | 0.457 |
| HCC recurrence n/HCC pre-LT (%) | 1/17 (5.6) | 17/103 (16.5) | 0.247 |
| LT- HCC recurrence time (months, median, min-max) | 77 (77–77) | 44 (1–177) | 0.450 |
| Immunosuppression n (%) | |||
| Tacrolimus | 15 (31.9) | 136 (42.5) | 0.111 |
| Cyclosporine | 9 (19.1) | 69 (21.6) | 0.437 |
| CI+Mycophenolate | 17 (36.2) | 79 (24.7) | 0.070 |
| Sirolimus | 4 (8.5) | 35 (10.9) | 0.417 |
| Everolimus | 3 (6.4) | 5 (1.6) | 0.069 |
| Re-LT n (%) | 1 (2.1) | 25 (7.8) | 0.127 |
| Death n (%) | 23 (48.9) | 130 (40.6) | 0.178 |
| LT-death time (months. median, min-max) | 157 (17–216) | 84 (12–246) | 0.001 |
| Liver-related death n (%) | 2/23 (8.7) | 64/84 (76.2) | 0.000 |
ESNSC, extra-hepatic solid non-skin cancer; LT, liver transplant; χ2, Chi-Square test; MW-U, Mann-Whitney U test; M/F, Males/Females; M, mean; SD, standard deviation; HCV, hepatitis C virus; HBV, hepatitis B virus; HDV, hepatitis D virus; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; BMI, body mass index; DM, diabetes mellitus; CI, calcineurin inhibitor.
χ2, chi square test; MW-U, Mann-Whitney U test
Among all donors, 204 underwent to liver biopsy before transplant.
Liver-related deaths were significantly less common among subjects with ESNSC than the others (8.7% versus 76.2%, p < 0.0001).
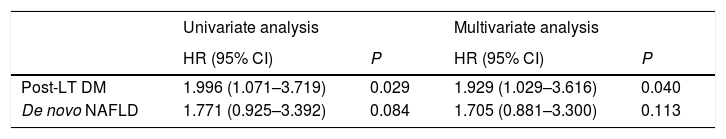
3.3Predictors of post-LT ESNSCAt univariate analysis, post-LT DM, and de novo non-alcoholic fatty liver disease, emerged as potential predictors of post-LT ESNSC. At multivariate test, only post-LT DM was independently related to ESNSC conferring a two-fold higher risk of developing ESNSC (Table 3).
Analysis of predictors of de novo solid non-skin cancer.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Post-LT DM | 1.996 (1.071–3.719) | 0.029 | 1.929 (1.029–3.616) | 0.040 |
| De novo NAFLD | 1.771 (0.925–3.392) | 0.084 | 1.705 (0.881–3.300) | 0.113 |
Only factors significant at univariate analysis are reported (p value<0.1).
HR, hazard ratio; CI, confidence interval; LT, Liver Transplant; DM, diabetes mellitus; NAFLD, non-alcoholic fatty liver disease.
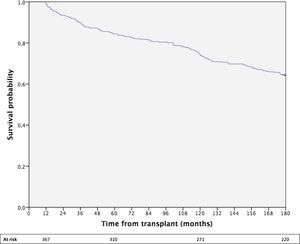
The cumulative 15-year survival of the whole cohort was 64% (Fig. 1).
At univariate test, age at LT, living donor, HCV-RNA positivity, HCC recurrence after LT, hepatic artery thrombosis, emerged as potential predictors of overall survival. Among them, living donor, HCV-RNA positivity, and HCC recurrence was found to be independent predictors of survival at the multivariate analysis (Supplementary Table 2).
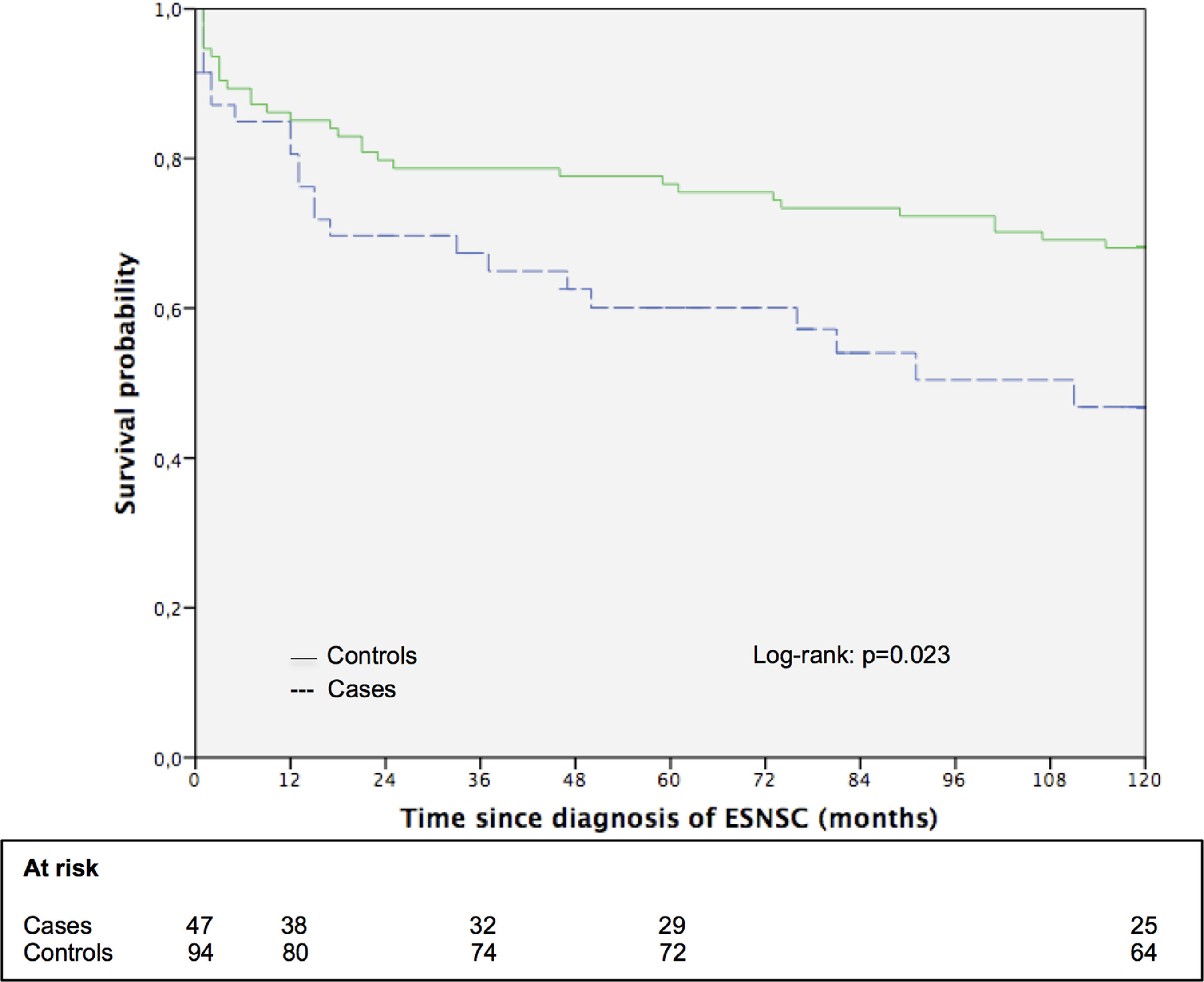
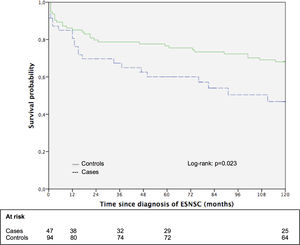
3.5Impact of ESNSC on the long-term survival: the matched cohort sub studySupplementary Table 3 shows the principal patters of 47 patients with ESNSC (cases) and corresponding 94 matched controls. Case and controls were perfectly matched for biological gender, pre-LT liver disease, pre-LT HCC and showed comparable mean age. Cases were followed up after malignancy diagnosis for a median of 58 months (range 1–211; 25th percentile 13 months, 75th percentile 118 months) while controls after index date for 185 months (range 1–236; 25th percentile 70 months, 75th percentile 208 months). During follow-up, 23 (48.9%) cases and 42 (44.7%) controls died Fig. 2. reports the 10-year survival of cases (after cancer diagnosis) and matched controls. Controls showed a longer overall survival compared to cases (the 5- and 10-year survival probabilities were 76.6% and 68.1% for controls; 60.1% and 46.8% for cases; p = 0.051 and 0.023, respectively).
4DiscussionTo our knowledge, this is the first study that demonstrates with a long median follow-up (188 months) that post-transplant development of DM seems to be associated with the onset of ESNSC. LT recipients who developed a post-LT ESNSC had almost a twofold higher prevalence of post-LT DM compared to subjects without ESNSC. In the general population, type 2 DM (and not type 1) significantly increases the risk of many malignancies, with strong evidence in liver, pancreas, and endometrium [22]. DM can favor cancer through many mechanisms such as insulin resistance, chronic inflammation, reduced antioxidant capacity, increase of adipokine production, and altered hormonal concentrations [23].
After LT, most patients develop a body weight increase and one or more metabolic disorders, due to an increase of caloric intake and negative metabolic effects of immunosuppressive drugs [24]. This metabolic weakening is one of the main causes of CV disorder [11–15] and, according to our data, a chief risk factor for ESNSC. Our data confirm, indeed, that the metabolic impairment occurring in many LT recipients represents the main driver of morbidity and mortality.
In addition to post-LT DM, we identified other potential ESNSC risk factors. In fact, subjects with post-LT ESNSC were considerably older at the time of LT (mean age 55 versus 52 years, p > 0.05) and more often males (83% versus 75.3%, p > 0.05). Neither older age nor male biological gender were independent predictor of ESNSC at multivariate analysis, but the reported tendencies are in line with available data from Scientific Registry of Transplant Recipients that indicated them as relevant cancer risk factors [5].
Our data are in line with Literature also regarding etiology of liver disease indicating LT. Therefore, subjects with post-LT ESNSC displayed more frequently alcoholic liver disease as indication for LT (38.3% versus 18.4%, p = 0.003) [5]. Notably, it is well known that a relevant increase of cancer risk (particularly in head-neck district) can be registered in patients transplanted for alcohol-related especially if they show a post-LT recurrence of alcohol use [25].
Despite immunosuppressive therapy (mainly calcineurin inhibitors alone or in association with m-TOR inhibitors) represents the ideal link between cancer and metabolism since it increases per se the malignancy risk and impairs both glucose and lipid profiles [8], we found, in line with the available literature [2], no definitive data related to immunosuppressive drugs as transplant-specific risk factors. Available data suggest that m-TOR inhibitors (like everolimus that is approved for LT) could be useful in primary or secondary prophylaxis of de novo cancer since this class of immunosuppressive drug shows an anti-angiogenic activity [26]. However, there are no strong evidence for solid conclusions and recommendations [26]. Finally, as expected, we did not detect any donor pattern as potential predictor of long-term ESNCS.
Our study is unique as we separately analyzed cancers without specific risk factors (like ESNSC) from those with already known risk factors like de novo HCC, lymphoproliferative disorders, Kaposi's sarcoma, and skin malignancies that present specific risk factors such as recurrence of underlying liver disease, Epstein-Barr Virus, Human-Herpesvirus-8 and sun exposure, respectively [10]. Many studies carried out so far exploring risk factors for post-LT cancer put together any type of de novo malignancies [3] or lymphoproliferative disorder with solid cancer [5]. However, the presence of so specific risk factors and the differences in the outcome, make de novo HCC, lymphoproliferative disorders, Kaposi's sarcoma and skin malignancies completely different from the ESNSC. Therefore, ESNSC should be analyzed separately as we did in the present study. Moreover, as the scenario about cancers their relative risk factors rapidly changes overtime, we decided to analyze data from a longer follow-up after LT than the one reported in the available studies about post-LT malignancies [10], most of them also carried out more than 10 years ago.
We also analyzed the impact of ESNSC on patient survival finding a significantly lower 10-year survival from diagnosis of cancer in cases (46.8% versus 68.1% p = 0.023). This is not a completely new finding, even though it has been achieved through a distinctive methodology. In fact, studies developed in the setting of LT already reported that the higher tumor burden among LT recipients might considerably impair their overall survival [11,27], but most of these studies have been conducted through external comparisons with general population [28,29].
Since LT recipients have already been shown to have a higher risk of death compared to the general population [30], they represent the ideal control group to evaluate the impact of de novo cancer post LT on survival. Following this concept, we developed a matched (1:2) cohort sub study, with a stronger matching and a better-defined type of cancer (we included only ESNSC excluding de novo HCC, lymphoproliferative disorders, Kaposi's sarcoma, and skin malignancies) than similar studies conducted in this setting [31] finding comparable 5-year and 10-year survival rates.
Regarding the overall survival, with the present study we can confirm many well-known independent predictors of mortality such as living donor, HCV active infection and HCC recurrence [2].
After LT, patients with cancer can be strongly disadvantaged than the non-transplanted counterpart since the use of immunosuppressive drugs and the weakness of treatment options might alter the outcome [3]. Nevertheless, we expect a continuous rise of de novo cancer for the increase of transplanted mean age and for the increase of metabolic disorders in pre- and post-LT time due to the incessant upsurge of non-alcoholic steatohepatitis as indication for LT [3].
Some relevant assets of our study can be emphasized. The median follow-up was about 15 years, much longer than the one reported in many other studies evaluating cancer risk in the context of LT. Moreover, we used a well-established definition of cancer considering only ESNSC. The multicenter design of the study with enrollment involving different Italian areas represents another strength point, making the results more robust.
However, the retrospective design is the most relevant limitation of the present study, although a prospective multicenter study with such a long period of observation is not practical. We do not have complete data about family history of cancer and smoking that, in the general population context, represent two consolidated risk factors for several malignancies [32]. Nevertheless, neither familiarity nor smoking habit has been stated as a post-LT risk factor [5]. A possible reason for this discrepancy between transplant setting and general population, could be that LT recipients are usually accurately pre-screened since cancer is one of the main contraindications for LT itself [2]. Therefore, subjects with consolidated cancer risk factors such as smoking habits are more likely to be discouraged during the pre-transplant screening.
The lack of an exact analysis of tacrolimus exposure by the measurement of serum concentration within a certain period or by time-dependent cumulative exposure represents another limitation of the study. In fact, it is known that tacrolimus can represent a strong risk factor for both post-LT diabetes and cancer [33,34]. Further studies could better investigate the role of tacrolimus (and other immunosuppressive drugs) as metabolic and cancer risk factor. Furthermore, data about time-dependent variables such as post-LT diabetes or de novo NAFLD should be interpreted with caution. In fact, it would be relevant to analyze not only the appearance of these conditions but also the timing with respect to the LT and therefore the length of the patient's exposure to that risk factor.
We did not stratify cancer according to the stage at diagnosis while it might be relevant. Merchea et al [35]. analyzed the incidence of colorectal cancer after transplant (liver and kidney) demonstrating that a noteworthy rate of recipients (one fourth) showed a stage IV cancer at diagnosis. Authors demonstrated an important impact of the stage on the prognosis. At five years, none patients in stage 4 was alive; on the other hand, five-year survival rate for stage 1, 2, and 3 patients was 77%, 50%, and 42%.
Notably, the overall number of enrolled patients is relatively low. Indeed, all data, but especially the survival analysis, should be considered with caution. We are evaluating to extend the enrollment period from 2000–2005 to 2000–2010 to broaden the study cohort. In general, further studies with more consistent number of enrolled subjects should be developed to confirm our suggestions and to develop solid guidelines. In fact, as reported by Acuna et al [36]. and more recently by Dharia et al [37]., the available guidelines show some relevant limitations and new specific studies are mandatory to create strong evidence-based cancer screening recommendations.
5ConclusionsWith the rise of non-alcoholic steatohepatitis and the consolidation of alcohol use disorder as main indications for LT and the contemporaneous decrease of hepatitis B and C, we have a new scenario. In fact, both non-alcoholic steatohepatitis and alcohol disease favor the onset of metabolic impairment that shows a chief role in affecting the medium and long-term outcome of LT recipients. We are firstly reporting that post-LT DM might lead to an increased risk of cancer, and it seems that de novo cancer itself can impair the 10-year patient survival. The present study might offer information that could be used in developing new management strategies after LT as personalized nutritional programs. In general, calcineurin inhibitors should be minimized as more as possible [38] and both alcohol and smoking should be powerfully discouraged. Further larger studies with strong and well-defined criteria about the type of cancer classification are mandatory to reinforce our ability of risk stratification to improve patient outcome.
Source of fundingNone declared.
Ethics approvalThe present study was approved by the Independent Ethics Committee of Tuscany region (“Comitato Etico Area Vasta Centro”; approval number: 19410).
We kindly thank Salvatore De Masi, Epidemiologist, Head of Clinical Trial Center of University Hospital Careggi, Florence, Italy, for the accurate revision of the study's statistical analysis.