The role of hepatitis C virus (HCV) is well established in the development of chronic hepatitis, cirrhosis and hepatic carcinoma, as well as in mixed type II cryoglobulinemia, membranoproliferative glomerulonephritis (MPGN) and porphyria cutanea tarda (PCT). Increasing evidence has been reported of a close association of HCV infection with autoimmune and hematological processes, mainly cytopenias and lymphoproliferative disorders such as B cell non-Hodgkin’s lymphoma. We describe the demographic, clinical and histopathological findings of nine patients from the Mexican population with non-Hodgkin’s lymphoma and HCV infection.
The role of hepatitis C virus (HCV) has been well defined in the development of chronic C hepatitis, cirrhosis and hepatic carcinoma, as well as in mixed type II cryoglobulinemia. HCV is lymphotrophic and can stimulate clonal proliferation of type B lymphocytes. Moreover, some studies have shown an association between antihepatitis C virus antibodies and B cell non-Hodgkin’s lymphoma (NHL). Specific genomic sequences of the virus have also been identified in the lymph nodes of patients with NHL as well as in patients with hyperplasic lymphadenopathies.1,2
The aim of this study was to document the clinical and pathologic characteristics of nine patients with HCV infection and NHL, and to review the literature about this subject.
MethodsNine patients with diagnoses of hepatitis C and NHL were included. They were seen at three different institutions—Medica Sur Clinic & Foundation, Instituto Nacional de Ciencias Médicas y de la Nutrición Salvador Zubirán, and Centro Medico Nacional Siglo XXI, all located in Mexico City-between March 1998 and December 2005. Lymphoma localization and demographic and clinical findings are described. Laboratory findings as well as treatment and survival are also reported.
ResultsDemographic and clinical characteristicsThe clinical and demographic characteristics of the patients are shown in Table I. Nine patients (two men and seven women) with an average age of 59.1 years (range, 38-77 years) were included. All patients had a history of blood transfusion before the diagnosis of NHL and HCV infection. The average time between the transfusion and the diagnoses was 22 years. Four patients had cirrhosis, three patients had chronic hepatitis, and two were asymptomatic.
Clinical characteristics of patients with hepatitis C virus and non-Hodgkin’s lymphoma.
| Age (years) | Gender | Previous surgery | Previous blood transfusion | Hepatic disease | |
|---|---|---|---|---|---|
| Patient 1 | 53 | Female | Yes | Yes | Chronic hepatitis |
| Patient 2 | 67 | Female | Yes | Yes | Cirrhosis |
| Patient 3 | 38 | Male | Yes | Cirrhosis | |
| Patient 4 | 77 | Male | Yes | Yes | Cirrhosis |
| Patient 5 | 64 | Female | Yes | Yes | Chronic hepatitis |
| Patient 6 | 56 | Female | Yes | Yes | Hepatic steatosis EV I/IV |
| Patient 7 | 32 | Female | Yes | Yes | Hepatic steatosis |
| Patient 8 | 79 | Female | Yes | Yes | Chronic hepatitis |
| Patient 9 | 64 | Female | Yes | Yes | Chronic hepatitis |
EV = Esophageal varices
Six patients had abnormal liver function tests at the time of diagnosis of NHL. One patient had normal liver enzyme activities but a low albumin concentration. Two patients had increased liver enzyme activities after chemotherapy.
Main laboratory findings in patients with hepatitis C virus and non-Hodgkin’s lymphoma.
| Parameter | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 |
|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 15.8 | 12 | 14.2 | 11.4 | 14.2 | 15.5 | 13.3 | 15.4 | 12.1 |
| Leukocyte count (× 103/mm3) | 8.6 | 9.9 | 3.9 | 2.6 | 5.8 | 4.8 | 2.4 | 5.2 | 4.1 |
| Platelets (× 103/mm3) | 233 | 117 | 86 | 131 | 284 | 262 | 238 | 257 | 166 |
| Total bilirubin (g/dL) | 1.2 | 1.1 | 1.8 | 1.5 | 0.9 | 0.7 | 1.27 | 1.04 | 0.8 |
| Direct bilirubin (g/dL) | 0.2 | 0.2 | 0.4 | 0.4 | 0.4 | 0.2 | 0.37 | 0.8 | 0.2 |
| AST (U/L) | 100 | 33 | 63 | 215 | 76 | 75 | 301 | 65 | 107 |
| ALT (U/L) | 84 | 16 | 115 | 297 | 55 | 81 | 294 | 63 | 98 |
| FA (U/L) | 134 | 91 | 172 | 166 | 124 | 614 | 102 | 80 | 150 |
| Prothrombin time (%) | 82 | 52 | 65 | 72 | 88 | – | – | 88 | – |
| Albumin (g/dL) | 3.4 | 2.3 | 2.3 | 3.4 | 4.1 | 3.5 | 3.8 | 3.9 | 4.0 |
| ELISA anti-HCV test | + | + | + | + | + | + | + | + | |
| RIBA-2 antibody test | – | Positive | – | – | – | – | – | – | |
| HCV RNA test (UI/mL) | 50,887 | – | 66,000 | Positive | Positive | Positive | Positive | 11,100,000 | 532,000 |
In every patient, the diagnosis of HCV infection followed positive antibodies measurement by a third-generation ELISA test. In one patient, the diagnosis was confirmed by the RIBA (recombinant immunoblot assay) antibody test; in eight by HCV-RNA detection using the Roche Amplicor RT-PCR Test:, four with a quantitative test (lower limit of detection was 600 Ul/ml or 1,620 copies/mL) and four with a qualitative test (lower limit of detection 50 UI/mL).
Clinical presentation, localization and histological type of the non-Hodgkin’s lymphomaTable III shows lymphoma localization, histological type, treatment and survival of patients. Brief clinical histories are presented.
Localization and histological type of non-Hodgkin’s lymphoma in patients with hepatitis C virus.
| Patient | Clinical finding | Histological finding | Treatment | Survival |
|---|---|---|---|---|
| 1 | Weakness, hypoxia, hepatic mass | B cell lymphoma | Six cycles of CHOP chemotherapy | No activity at 29 months |
| 2 | Hematemesis and melena, gastric polyp | Large cell lymphoma | Six cycles of CHOP chemotherapy | No activity at 34 months |
| 3 | Pain, paresthesia and hypoesthesia, T1-T6 lesion | B cell lymphoma | Radiotherapy, CHOP | No activity at 38 months |
| 4 | Fever, adenomegaly, pulmonary infiltrates | Diffuse non-Hodgkin’s lymphoma | Six cycles CHOP of | No activity at 45 months chemotherapy |
| 5 | Refractory conjunctivitis | B cell lymphoma | Radiotherapy-23 sessions | No activity at 17 months |
| 6 | Asthenia, adynamia, splenic infiltration | Immunoblastic non-Hodgskin’s lymphoma | Six monts of CHOP chemotherapy | Activity |
| 7 | Activity in lymph nodes, spleen and kidney | Non-Hodgkin’s lymphoma | CHOP 4 cycles of chemotherapy | Activity |
| 8 | Cervical adenomegaly | B cell lymphoma | CHOP 4 cycles of chemotherapy | No activity since March 2006 |
| 9 | Fever, epistaxis | Non-Hodgkin’s lymphoma | Radiotherapy, CHOP | No activity since 1997 (year of diagnosis) |
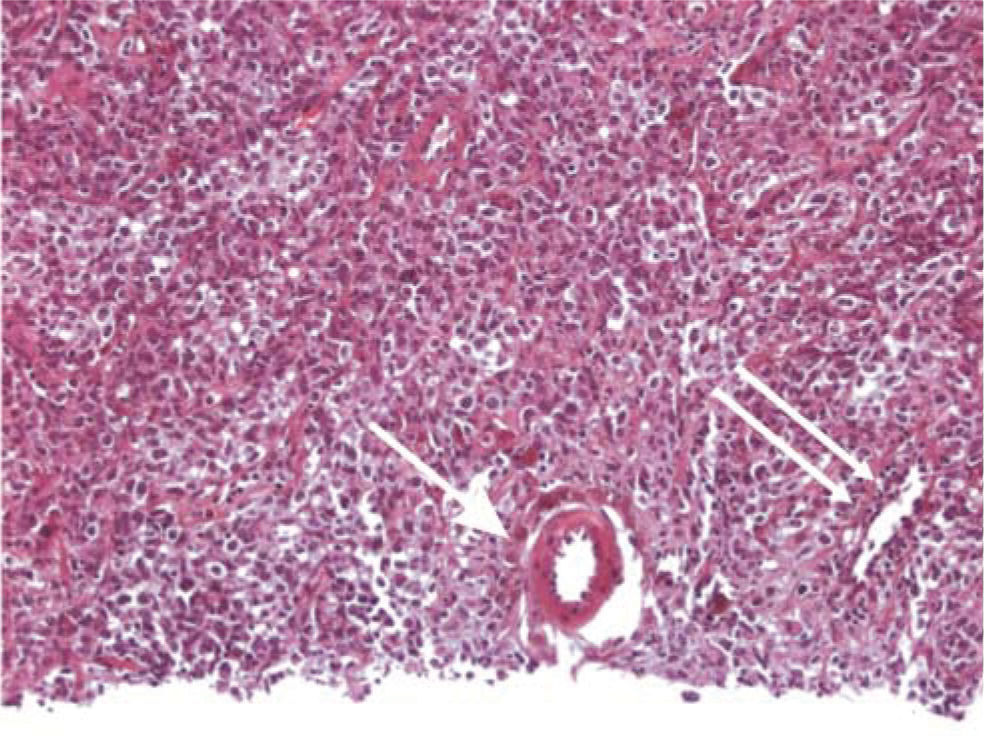
Patient 1: A 53-year-old woman with chronic HCV infection presented in July 1999 with weakness and anorexia. Hepatomegaly was detected, along with a firm epigastric mass. Liver aspiration biopsy showed an undifferentiated tumor, probably hepatic carcinoma. A segmentectomy was performed with resection of a 3 cm ovoid mass. Histopathological examination showed a type B diffuse large cell NHL (Figure 1). Nontumor tissue showed chronic hepatitis with grade III fibrosis, moderate portal inflammation, lobular inflammation and mild periportal necrosis. She received six sessions of CHOP chemotherapy. Thereafter, she was given interferon alpha-2b and ribavirin. Unfortunately, she did not tolerate a complete course, with reduced hemoglobin and platelets. HCV RNA testing was positive at week 12. Therefore, antiviral treatment was discontinued. In December 2004, her lymphoma continued to be in remission.
Patient 2: A 67-year-old woman with compensated HCV cirrhosis known to our clinic since 1994. HCV infection was diagnosed in 1990. In 1998, she developed upper gastrointestinal bleeding (hematemesis and melena). An endoscopy showed a 3 cm polypoid gastric lesion. The biopsy disclosed a large B cell NHL. A gastric tissue specimen was negative for Helicobacter pylori infection. A partial gastric resection was performed, followed by five cycles of CHOP chemotherapy. She was asymptomatic until December 2004.
Patient 3: A 38-year-old male patient with HCV-related cirrhosis complicated with ascites and treated with diuretics. In October 2000, he complained of burning posterior thoracic pain that extended to the lower limbs and was followed by paresthesia and diminished sensation. A magnetic resonance image of the spinal cord showed an extramedullary lesion extending from T1 to T6 compressing the medullary space. Surgical resection was performed with decompression and spinal cord fixation. Histological diagnosis revealed B cell NHL. The patient received chemotherapy and radiotherapy. He is now recovering movement of lower extremities and his lymphoma continued to be in remission until July 2004.
Patient 4: A 77-year-old man with a diagnosis of compensated cirrhosis secondary to HCV infection since 1995. In March 1998, he presented with fever, adynamia, cervical lymph node enlargement and hepatosplenomegaly. An X-ray showed multiple pulmonary infiltrates. Cervical lymph node biopsy showed large cell, diffuse NHL. He received six cycles of CHOP chemotherapy and achieved remission from the adenomegaly, visceromegalies and pulmonary infiltrates. During his follow-up, no tumor activity was detected until December 2001. Because of persistent thrombocytopenia, the patient did not receive treatment for HCV.
Patient 5: A 64-year-old female patient with compensated cirrhosis secondary to HCV infection since 1995. In July 2000, she presented with bilateral, persistent conjunctivitis, mainly in the left eye, which was refractory to treatment. A biopsy was done, and a diffuse, large, type B, MALT-cell lymphoma was diagnosed (Figure 2). She received 23 sessions of radiotherapy. She was free of disease until December 2001.
Patient 6: A 56-year-old female patient with a history of blood transfusion in 1990 was treated in 1999 for immunoblastic NHL localized to the spleen with high doses of methotrexate, cyclophosphamide, vincristine, bleomycin and etoposide, with which she achieved complete remission. She presented with liver enzyme elevation accompanied with asthenia and adynamia. An abdominal ultrasound showed hepatic steatosis and a simple right lobe hepatic cyst, without gallbladder or spleen abnormalities. An endoscopic study revealed esophageal varices grade I, and mild portal gastropathy. The serologic test was positive for HCV infection.
Patient 7: A 32-year-old woman, previously asymptomatic but positive for HCV, was diagnosed with NHL in 2001, with tumor activity detected in the neck, pelvis, spleen and kidney. She received four cycles of chemotherapy with cyclophosphamide, vincristine, mitoxantrone and prednisone. In April 2002, she had abnormal liver function tests. A percutaneous hepatic biopsy was done, and the result was indeterminate, suggesting drug liver injury. Therefore, chemotherapy was suspended.
Patient 8: A 67-year-old woman diagnosed with HCV infection by a screening test in February 2003. She received a blood transfusion in 1968 because of upper gastrointestinal bleeding secondary to a gastric ulcer. She reported fatigue, and her aminotransferase activities were slightly elevated. A liver biopsy showed chronic hepatitis with mild activity. Unfortunately, she had no response to PEG-Interferon and ribavirin treatment, and HCV RNA was positive at the 24th week. Therefore, antiviral treatment was discontinued. In January 2005, three submaxillary lymph nodes were detected and a biopsy showed B cell NHL. She received four cycles of CHOP chemotherapy. The patient was asymptomatic until March 2006.
Patient 9: A 64-year-old woman diagnosed with nasal NHL in February 1997. She received CHOP chemotherapy and radiotherapy until October 1997. She has been in remission since then. In 2004, she was diagnosed with chronic hepatitis C after a screening test suggested by a friend because of easy bruising. She had a history of blood transfusions in 1973 and 1975 because of epistaxis. Aminotransferase activities were elevated, and a liver biopsy showed chronic hepatitis with a Knodell activity score of 8/22. She began PEG-Interferon and ribavirin treatment in 2005 with adequate tolerance.
DiscussionThe viruses are the most important infectious agents associated with cancer development. The prevalence of viral markers in patients with cancer is always higher than the presence of cancer in patients with viral infections. It is well known that the expression of viral proteins can be associated with cancer development. There are other factors (genetics, race, hormonal and immunologic factors) required for malignant transformation. Some oncogenic viruses, such as Epstein Barr virus, type 6 and 8 herpes virus, and type 1 and 2 associated with human T lymphoma virus (HTLV-1, HTLV-2), begin a complex process of carcinogenesis by the expression of viral proteins that block some host proteins that are protective against cancer development. Conformational changes occur many years after the infection is acquired. During this latent period, the metabolic and immunologic factors that act as inducers of malignant transformation are added.3
The association between HCV and lymphoproliferative diseases has previously been documented. Its prevalence has been reported in Italy and other countries (Table IV). In addition, an association between HCV infection and mixed cryoglobulinemia, a proliferative disease considered an expression of low-grade NHL, has been shown. HCV RNA can be detected in patients with chronic hepatitis C infection. This persistent viremia represents a chronic stimulation of B lymphocytes, causing clonal expansion of these immunoglobulin-producing cells and originating malignant B-cell lymphoproliferative disease. Based on this hypothesis, and as was stated above, chronic infection by HCV alone or in combination with other factors can promote the development of B cell NHL.6
Prevalence of HCV infection in series of patients with non-Hodgkin’s lymphoma22
| Reference | Country | Number of patients | Vhc (%) |
|---|---|---|---|
| Ferri, 1994 | Italy | 50 | 32 |
| Silvestri, 1996 | Italy | 311 | 9 |
| Luppi, 1998 | Italy | 157 | 22 |
| Pioltelli, 1996 | Italy | 126 | 21 |
| De Vita, 1997 | Italy | 162 | 22 |
| De Rosa, 1997 | Italy | 263 | 22 |
| Mazzaro, 1996 | Italy | 199 | 28 |
| Vallisa, 1999 | Italy | 175 | 37 |
| Pioltelli, 2000 | Italy | 300 | 16 |
| Germanidis, 1999 | France | 201 | 2 |
| Bauduer, 1999 | France | 136 | 8 |
| Hausfater, 2000 | France | 1,485 | 2.5 |
| Ellenrieder, 1998 | Germany | 69 | 4 |
| Thalen, 1997 | Netherlands | 115 | 0 |
| Cuculanu, 1999 | Romania | 68 | 30 |
| Zucca, 2000 | Switzerland | 180 | 9 |
| Paydas, 1999 | Turkey | 98 | 9 |
| Timuraglu, 1999 | Turkey | 48 | 9 |
| Hanley, 1996 | England | 38 | 0 |
| Brind, 1996 | England | 63 | 0 |
| McColl, 1997 | England | 72 | 0 |
| Collier, 1999 | Canada | 100 | 0 |
| Shariff, 1999 | Canada | 88 | 2 |
| King, 1998 | United States | 73 | 1 |
| Kashyap,1996 | United States | 312 | 7 |
| Zuckerman, 1997 | United States | 120 | 22 |
| Yoshikawa,1997 | Japan | 55 | 16 |
| Izumi, 1997 | Japan | 25 | 16 |
| Mizorogi, 2000 | Japan | 100 | 17 |
This article reports the association between HCV infection and NHL in nine patients: one with primary liver lymphoma, one with gastric lymphoma, one with central nervous system lymphoma, two with systemic lymphomas, one with lymphoma of the conjunctiva, two with splenic lymphomas, and one with nasal lymphoma. In our population, the frequency of HCV infection in patients with lymphoma is unknown. However, Italian studies have reported a high prevalence of between 9% and 32% of HCV infection in patients with B cell NHL.7 Studies from different authors have reported a 28% prevalence of HCV infection in patients with NHL compared with 2.9% in the general population and 3.1% in a group of patients with other malignancies (Table II)It has been proved that the prevalence of low-grade lymphomas is particularly high (15.2%). It seems that HCV plays an important role in the development of low-grade NHL.8 In spite of the strong association between HCV infection and NHL reported in Italy, Asia and the United States, other European studies have not confirmed this association, probably because of a particular geographic distribution, as it can be observed in some Japanese studies with high prevalence of HCV infection in patients with NHL. Duberg et al. reported the association between HCV infection and NHL in a Swedish cohort of 27,150 HCV-infected persons from 1990 to 2000, and there were 50 NHL cases diagnosed. The risk of NHL is significantly increased among patients with more than 15 years of infection (SIR 1.89 (95% CI, 1.10-3.03)).21
As previously published, in our study the frequency was higher in older women.6,9 It has been proposed that the association between age and the development of NHL can be explained by an accumulative risk of HCV infection and the long period that the virus needs to cause proliferation of lymphoid cells.
Lymphoplasmacytoid lymphoma/immunocytoma is the most frequent histological type among HCV-related lymphomas, with the infection being found in 26% to 49% of cases according to reports of more than 100 cases.11 Other types of lymphoma that have been associated with HCV are the B cell monocytoid lymphoma and the diffuse large B cell lymphoma. Other histological types include the follicular central lymphoma, the marginal cell lymphoma and the immunoblastic NHL.6,12 The latter is a high-grade lymphoma classified as an immunoblastic sarcoma. It is a very aggressive and generally extranodal lymphoma, which appears mainly in immunocompromised patients. Lymphomas associated with HCV infection have been mostly detected in advanced stages of the disease, suggesting a direct viral promotion of tumor dissemination and probable interference with immune system control. Both Hodgkin’s lymphoma and T-cell NHL consistently show no association with HCV.
Lymphomas associated with HCV infection more frequently present as primary extranodal lymphomas, especially in liver, spleen and salivary glands. In our series of patients, there was a primary liver lymphoma and two lymphomas of the spleen. It is important to point out that other sites for NHL such as stomach (MALT),13 medulla and conjunctiva have also been reported, showing the ability of the virus to infect these structures. The gastric lymphoma reported in this series of patients was detected from a polypoid lesion that corresponded to a large B cell lymphoma. The search for Helicobacter pylori was negative; thus, it is not known if this lesion had an association with a mucosa lymphoma. Other studies have not found a relationship between MALT lymphomas and HCV infection; thus, infection with hepatitis C is less common in these types of lymphoma. Finally, a new type of diffuse cell lymphoma known as hepatosplenic lymphoma has been associated with HCV in 71.4% of cases in Japan and the United States.14
Treatment for patients with NHL and HCV infection is similar to the treatment of those without infection of HCV, with a very similar response in both groups. In some studies, it was demonstrated that during treatment with chemotherapy for NHL, chronic HCV infected patients had more adverse reactions than patients without HCV. Commonly, there is a mild increase in liver enzymes during treatment.6 However, cases of reported hepatotoxicity are less than two among 110 patients.
In our series, all patients received a multidrug chemotherapy scheme based on cyclophosphamide, vincristine, prednisone, and doxorubicin (CHOP) without any complications, except for one patient who had abnormal liver function tests. A percutaneous hepatic biopsy done at that time suggested drug-induced liver injury; thus, chemotherapy was suspended. The patients with conjunctival lymphoma and nasal NHL received radiotherapy, which was adequately tolerated.
Treatment in this group of patients has not been well established. Regression of mononuclear cell B expansion and elimination of the HCV has been demonstrated in patients with NHL treated with interferon.15,16 Regression has also been achieved in MALT tumors in patients in whom Helicobacter pylori was eradicated, although this has not been demonstrated in patients with hepatitis C. Interferon may have direct antiproliferative activity against B cells, and it has been suggested that it can have an important role in the treatment of patients with NHL, mainly in low-grade lymphomas through the elimination of HCV. In our series of patients, only two received treatment with interferon. One patient was given a reduced dose because of severe anemia and neutropenia associated with its administration. Interferon might be an attractive therapy for management of low-grade NHL, although more studies are required.
After a follow up of at least 30 months, there was no difference in survival among patients with or without infection with HCV, with similar results between low-and highgrade lymphomas. The three-year survival rate is 86% in patients without viral infection and 83% in patients with infection. In high-grade lymphomas, the three-year survival rate was 60% in patients without infection and 57% in patients with viral infection. In this study, 100% of patients were alive after 32 months. However, it is necessary to continue with close follow-up to evaluate the real survival.
It is important to point out that survival shows no variability in patients with lymphoma and HCV infection, but their quality of life is poorer.12,17,18
Coinfection of patients with HCV infection with the flavivirus known as GB type C virus or hepatitis G virus must be considered and ruled out. Recent studies have shown a high incidence of past infection with hepatitis G virus in patients with B cell lymphoma; however, this is controversial, and more studies are required to clarify the role of this virus in the development of lymphoma.19
ConclusionThere is a close relationship between HCV infection and NHL. The biological explanation is still unknown, although one hypothesis indicates that some HCV sequences cannot be integrated into the host genome, and the virus acts as an external stimulus, inducing clonal proliferation of B cells.10 Although it was originally assumed that perihepatic lymphadenopathy was a direct result of liver inflammation, there is the possibility that viral infection may play a direct role in perihepatic lymph node hyperplasia, possibly contributing to HCV associated NHL and other B-cell lymphoproliferative disorders.23 New studies will help in understanding the physiopathology of the association between the hepatitis C virus and non-Hodgkin’s lymphoma as well as the most efficient treatment regimen for this lymphoproliferative disease associated with HCV according to its natural history.