Hepatitis C poses a substantial global health burden. Three to five percent of individuals with liver cirrhosis secondary to hepatitis C will develop hepatocellular carcinoma. The development of hepatocellular carcinoma is closely associated with cirrhosis in hepatitis C infection, whereas in hepatitis B virus infection, hepatocellular carcinoma may occur in the absence of cirrhosis. Although uncommon, hepatocellular carcinoma has been reported in hepatitis C patients without cirrhosis and, in very rare cases, in the absence of active viral replication. We report the case of a 51-year-old patient with hepatitis C who developed hepatocellular carcinoma in the absence of fibrosis and after having achieved sustained virological response with combination peginterferon and ribavirin therapy seven years prior. The patient successfully underwent surgical resection, and histopathological examination of the resected tissue demonstrated a poorly-differentiated hepatocellular carcinoma in an otherwise unremarkable liver. The patient continues to do well and has no evidence of tumor recurrence 18 months post-operatively. This case raises question regarding the carcino-genesis of hepatocellular carcinoma as a sequela of chronic hepatitis C in noncirrhotic liver and moreover after achieving sustained virological response.
Abbreviations: Alpha-fetoprotein (AFP), computerized tomography (CT), hepatitis B (HBV), hepatitis C (HCV), hepatocellular carcinoma (HCC), inferior vena cava (IVC), polymerase chain reaction (PCR), sustained virologic response (SVR)
Conflicts of interest, financial support, copyright transfer withholdings: none
IntroductionHepatitis C virus (HCV) poses a global health burden, with a worldwide prevalence of nearly 3%. It is estimated that 75% of exposed individuals will develop chronic HCV infection.1–3 Nearly 20% of those chronically infected will develop cirrhosis within 25 years. The estimated annual incidence for both hepatic decompensation and hepatocellular carcinoma (HCC) in this group is 3%.3,4 The most efficacious and standard treatment for chronic HCV infection is combination peginterferon and ribavirin, whereby sustained virological response (SVR), the primary treatment endpoint, can be achieved in 40%-45% of patients with HCV genotype 1 and approximately 80% of patients with genotype 2 or 3.5 Hepatocellular cancer rarely develops in the HCV patient unless there is underlying cirrhosis, although several reports have described the development of HCC in the absence of cirrhosis if there is underlying fibrosis.6–11 We describe a rare case of non-fibrolamellar HCC in a patient with chronic HCV who had no hepatic fibrosis and had achieved SVR for over seven years.
Case reportA 51 year-old Caucasian woman had no significant medical history except for genotype 2a HCV infection first diagnosed in 2000. She was presumed to have acquired HCV from a one-time use of intravenous drugs at 17 years of age. She had no other known risk factors for liver disease and no clinical evidence of cirrhosis. Her social history was significant only for rare alcohol intake. A biopsy performed in July 2000 demonstrated grade 1 inflammation and stage 0 fibrosis. In 2000, her HCV was successfully treated with 6 months of pegylated interferon and ribavirin. She achieved sustained virological response (SVR).
In 2007, the patient underwent abdominal ultrasonography for hepatomegaly detected on routine physical examination. A large right hepatic lobe mass was detected. Computerized tomography (CT) found this to be a hypodense, peripherally enhancing 12 cm right hepatic lobe mass. Magnetic resonance imaging of the abdomen with and without gadolinium similarly demonstrated a 12 cm mass with peripheral enhancement and no associated lymphadenopathy or portal vein thrombus. CT of the chest was negative for metastatic lesions.
On examination, vital signs were significant for blood pressure of 157/85 mmHg and weight of 232 pounds (body mass index 37.4 kg/m2). Abdominal exam showed mild hepatomegaly and no splenomegaly. There were no stigmata of cirrhosis or portal hypertension. Pertinent laboratory findings included hemoglobin 12.9 g/dL, white blood cells 7.3 × 103/mL, and platelets 325 × 103/mL. Liver tests included alanine aminotransferase 15 U/L, alkaline phosphatase 116 U/L, and albumin 4.4 g/dL. HCV RNA was undetectable (< 50 IU/mL) by serum polymerase chain reaction (PCR). She was HBV surface antigen and core antibody negative. Alpha-fetoprotein (AFP) was 36,000 IU/mL. Given the patient’s radiologic findings and highly elevated AFP, a diagnosis of HCC was presumed, and the patient was referred for resection.
Intra-operatively, there was no evidence of cirrhosis or portal hypertension. The mass occupied much of the right lobe and was found to invade the right hepatic vein and the vena cava Right hepatic lobectomy with resection and primary repair of a portion of inferior vena cava (IVC) was performed. Her post-operative course was complicated by thrombosis of the infrahe-patic IVC requiring surgical thrombectomy. She was discharged home on hospital day 24 and has returned to full activity but has moderate persistent lower extremity edema. Post-operative AFP level has dropped from 36,000 ng/mL pre-operatively to 18 ng/mL 6 months after surgery, and she remains free of any sign of recurrent or metastatic disease.
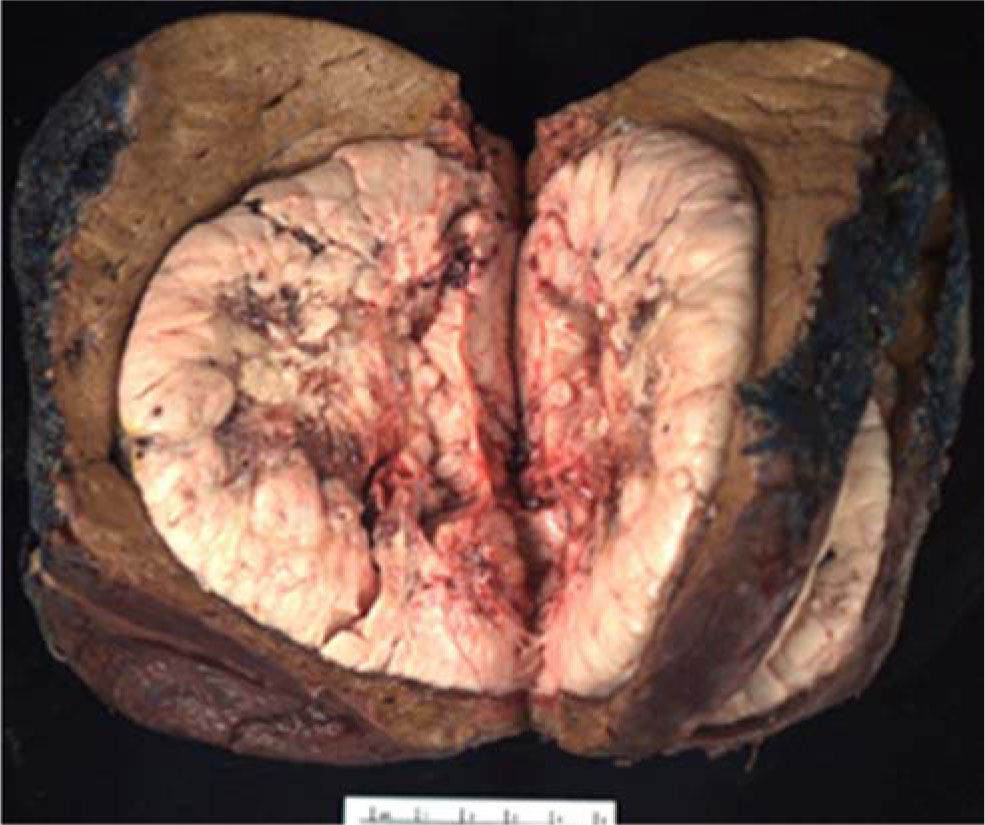
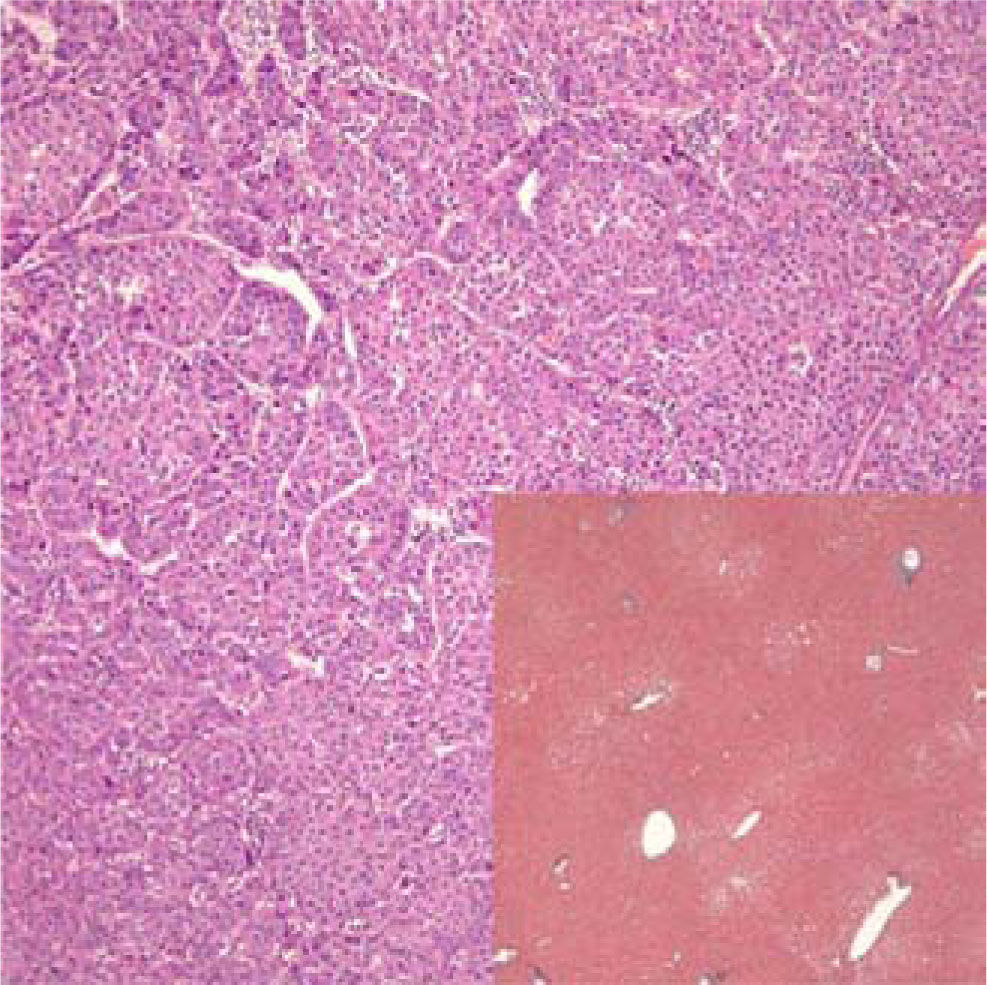
Histopathological examination demonstrated a poorly-differentiated, non-fibrolamellar HCC in an otherwise unremarkable liver without fibrosis or satellite lesions (Figures 1 and 2). There was no evidence of alcoholic injury, hemochromatosis, primary biliary cirrhosis, • •1 antitrypsin disease, steatohepatitis, or other entity that would be a risk factor for the development of HCC. HCV RNA in the tumorous and surrounding normal liver tissue was negative by polymerase chain reaction.
Photomicrograph of moderately differentiated hepa-tocellular carcinoma with exaggerated trabecular and solid patterns (Hematoxylin-eosin 100x). Inlay: non-tumorous liver demonstrating mild macrovesicular steatosis, but otherwise no features of chronic hepatitis, fibrosis, or other significant change (Trichrome 4x).
In approximately 90% of cases, HCC develops in a background of cirrhosis.12,13 The incidence of HCC in patients with cirrhosis depends on the underlying cause of cirrhosis and geographic origin of the patient, varying from a cumulative 5-year incidence of 30% in Japanese patients with HCV to 4% or less in patients with biliary cirrhosis.13 The mechanism of hepatocarcinogenesis in cirrhosis has been postulated to be related to chronic repetitive hepatic injury and resulting hepatocyte proliferation and genetic alteration.14 HBV and HCV are major epidemiological predisposing factors for HCC, accounting for approximately 80% of cases.13,14 It has been repeatedly shown that HBV can cause HCC even in the absence of cirrhosis.15 In contrast, HCV-related HCC has previously been thought to occur only in patients with cirrhosis. Several recent reports, however, indicate that HCC may also occur in a small fraction of noncirrhotic HCV patients who have developed fibrosis.6–11 Noncirrhotic HCC has also been described in association with several non-virogenic conditions, including three cases in primary biliary cirrhosis, 11 cases in hereditary hemo-chromatosis, one case in autoimmune hepatitis, and over 200 idiopathic cases.6–23 In the majority of these non-virogenic cases, the HCC was not specified as being either typical or fibrolamellar variant HCC. The pathogenesis of HCC has not been well studied in any of these HCV-related and non-virogenic cases.24
To our knowledge, this is the first reported case of a patient with HCV who developed HCC not only in the absence of fibrosis but also with a history of SVR. SVR, in addition to reducing inflammation and fibrosis scores, has been shown in several large studies to drastically reduce the incidence of HCC in HCV patients across all levels of fibrosis.10,11,25 Two prior studies have documented the development of HCC in small numbers of HCV patients with SVR in the absence of cirrhosis, but these patients had at least mild fibrosis.10,11 Further, the possibility of past HBV exposure was not investigated in these studies, which is important given some HBV-exposed patients may develop HCC despite being HBsAg-negative.26 In both these studies, male gender and advanced age were associated with the development of HCC after SVR.
Our patient was a relatively young female who had an estimated 21 years of chronic HCV based on her risk factor history, followed by 7 years of SVR prior to developing HCC. In this scenario, the inflammatory mileau over the previous 7 years should have been minimal, and in fact the nontumor liver at the time of liver resection did not have typical inflammatory changes of chronic hepatitis C (Figure 2). It is conceivable that despite the SVR and absence of HCV RNA and inflammation in the liver, HCV played a role in developing HCC by one of several mechanisms including: 1) unknown cumulative effects of chronic inflammation prior to HCV treatment; 2) genomic alterations attributable to prior HCV exposure which have not been elucidated; or 3) the persistent presence of intrahepatic viral proteins that cannot be detected with the current technology.2,27
This report adds to a small but growing body of literature that HCV infection is associated with an increased risk of HCC even years after viral clearance and even if there has been no evidence of permanent liver damage. The patient presented here had no evidence of hepatic fibrosis and had demonstrated SVR for 7 years prior to presenting with an 11 cm poorly differentiated HCC. While this body of literature is not strong enough to recommend HCC surveillance for all non-cirrhotic HCV patients, it is important for medical practitioners to be aware of a possible association between HCC and any prior HCV infection.