Background and aims: Hepatocellular carcinoma has been reported as a rare complication of autoimmune liver diseases. We describe herein two patients with this neoplasia associated with autoimmune hepatitis and primary biliary cirrhosis, and we also review the literature.
Cases report: The first case corresponds to a 49-year-old woman presented for evaluation of right upper abdominal pain. She had been diagnosed with autoimmune hepatitis 4 years before. Alpha-fetoprotein was markedly elevated and an abdominal MRI showed a 10 cm × 9.0 cm mass. She received transarterial chemoembolization, and currently the disease has progressed to the lungs and bones, and she is on supportive care. The second case corresponds to a 68-year-old woman presented for evaluation of a liver mass found in a screening ultrasound. She had been diagnosed with primary biliary cirrhosis 5 years previously. At admission alpha-fetoprotein was 1000 ng/mL and an abdominal MRI revealed a 4 cm × 3 cm liver tumor. She was treated with percutaneous radiofrequency ablation getting complete response, and currently she has no evidence of neoplastic disease. These two patients constitute the only cases of hepatocellular carcinoma associated to autoimmune liver diseases that have been attended in our Institute.
Conclusion: These cases highlight that hepatocellular carcinoma secondary to autoimmune hepatitis and primary biliary cirrhosis, although rare, can occur in the absence of coexistent viral hepatitis, or excessive alcohol consumption. The utility of screening for hepatocellular carcinoma in autoimmune liver diseases is still not defined.
The principal primary autoimmune liver diseases are autoimmune hepatitis (AIH) and primary biliary cirrhosis (PBC). AIH is a chronic, but fluctuating hepatitis that occurs in children and adults of all ages. The diagnosis is based on histologic abnormalities, characteristic clinical and biochemical findings, and abnormal levels of serum globulins, including autoantibodies.1 PBC is a slowly progressive disease of the liver that histopathologically is characterized by portal inflammation and immune-mediated destruction of the intrahepatic bile ducts. In PBC the loss of bile ducts leads to decreased bile secretion and the retention of toxic substances within the liver, resulting in further hepatic damage, fibrosis, cirrhosis, and eventually, liver failure.2
Hepatocellular carcinoma (HCC) is among the most lethal and prevalent cancers in the human population, since it is the fifth most common neoplasm and the third cause of cancer-related death in the world.3,4 However, HCC has been reported as unusual and sporadic complication of autoimmune liver diseases.5,6
The aims of this article are to report two cases of HCC associated with AIH and PBC, review the existing literature, and to discuss some diagnostic and therapeutic approaches for these patients.
Cases reportCase 1A 49-year-old woman presented for evaluation of right upper quadrant abdominal pain of few weeks duration.
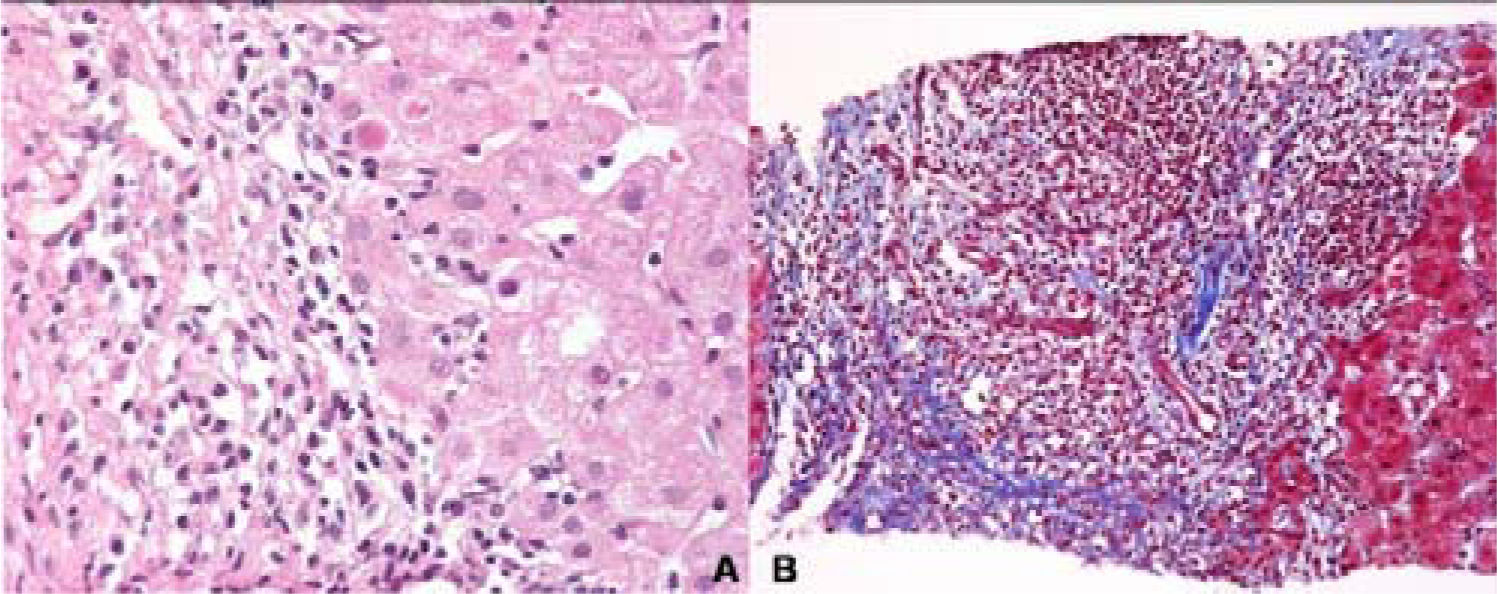
She denied use of alcohol, acetaminophen, herbal medication; and she had never has a blood transfusion. She had been diagnosed with type 1 AIH 4 years before with antinuclear antibodies titer 1:640 with cytoplasmic pattern, and negative serologic test that included hepatitis B surface antigen, hepatitis B core antibody, hepatitis C ELISA and qualitative PCR. A liver biopsy reveled interface hepatitis, with lymphoplasmacytic infiltrate and extensive fibrosis of the portal space (Figure 1A & B). Her pre-treatment International Autoimmune Hepatitis score was 18 point (pre-treatment diagnosis 15 point), and she started treatment with prednisone 30 mg daily tapering 5 mg weekly until reach 10 mg daily, and azathioprine 50 mg daily until the day of presentation.
The physical examination revealed right upper abdominal tenderness. Laboratory evaluation showed alanine aminotransferase, 96 UI/L; aspartate aminotransferase, 141 UI/L; alkaline phosphate, 342 UI/L; albumin, 3.9 g/dL; and total bilirubin, 1.5 mg/dL. Alphafetoprotein was markedly elevated (3,000 ng/mL, normal < 10 ng/mL). Abdominal MRI showed a 10 cm × 9.0 cm mass tumor within the inferior posterior portion of the liver (Figure 2A). She received transarterial chemoembolization getting stabilization of the tumor for 1 year (Figure 2B). Currently the disease has progressed to the lungs and bones, and she is on supportive care only.
Case 2A 68-year-old woman was referred to our department for further management of an asymptomatic liver mass. She had been diagnosed with PBC 5 years previously, when she presented upper bleeding of esophageal varices, and positive antimitochondrial antibodies by ELISA technique (3 units, normal < 0.1). She went under surgery to treat portal hypertension (mesocaval derivation) and a liver biopsy was taken, that showed lymphocytic infiltration of portal space, ductal damage, and moderate and fibrosis confined to portal and periportal areas (Stage II of histological classification for PBC; Figure 3). She started treatment with ursodeoxycholic acid 15 mg/kg per day until the day of presentation.
On admission, physical examination showed mild hepatomegaly but no ascites. Laboratory evaluation showed alanine aminotransferase, 59 UI/L; aspartate aminotransferase, 67 UI/L; alkaline phosphate, 892 UI/L; gamma glutamyl transpeptidase, 346 IU/L; albumin, 3 g/ dL; and total bilirubin, 1.0 mg/dL. She had negative serologic results for hepatitis B surface antigen, hepatitis B core antibody, and qualitative PCR for hepatitis C virus.
Alpha-fetoprotein was elevated (1,000 ng/mL, normal < 10 ng/dL). Abdominal MRI showed a 4 cm × 3 cm mass tumor within the superior anterior portion of the liver (Figure 4A & B). She was treated with percutaneous radiofrequency ablation getting complete response, and currently she does not have evidence of neoplastic disease and the last alpha-fetoprotein determination was 5 ng/mL.
DiscussionAutoimmune liver diseases are disorder of unknown etiology, in which progressive destruction of the hepatic parenchyma occurs, and in more severe cases often progresses to cirrhosis and carries a high mortality. This report described two cases of HCC arising in patients with type 1 AIH and PBC.
Previously, few cases of HCC complicating type 1 AIH have been reported;7-14 and it seems that improved survival with cirrhosis may result in an increased incidence of HCC. The initial Mayo Clinic experience,15 demonstrated that HCC occurs in 7% of patients with AIH and cirrhosis of at least five years duration, with an incidence of 1 per 350 patients-year; however, this study antedated the technological resources to exclude hepatitis C virus infection; and subsequently, they found only one case of HCC in 212 patients (0.5%) with AIH in absence of viral infection.5
Factors contributing to the development of HCC in AIH have yet to be fully elucidated; however, a recent study showed that persistent elevation of serum alanine aminotransferase levels during the follow-up, rather than factors existing prior to medical treatment are considered to be an important prognostic factor, and increase the risk of HCC.16 Some hypotheses have been proposed to explain the low occurrence of HCC in AIH; such as that corticosteroid-therapy attenuates the inflammatory reaction that is important in promoting continuous liver cell injury and malignant transformation on a background of cirrhosis; alternatively, the sporadic occurrence of HCC may reflect serendipitous exposure to another as yet undetected virus or carcinogen that is inadequately cleared by the cirrhotic liver; finally, improved survival in patients with AIH using corticosteroids or other immunosuppressives may allow for the development of HCC simply as a factor of longevity. Contrary, long-term corticosteroid use in these patients might contribute to increased tumor susceptibility by impairing immunologically mediated host defense mechanisms.11 Nevertheless, other authors consider that the relevancy of cirrhosis itself more than corticosteroid therapy, should be considered in the carcinogenesis of HCC in AIH, since HCC has been described in patients with no steroid treatment.9,10
In another hand, the association of PBC and HCC is unclear to date because epidemiological studies on this issue are limited and sometimes with contradictory results. Some studies have described that PBC does not increase the risk of developing HCC,17 although others showed that the incidence of HCC is high in patients with PBC.18-20 The latter studies indicated that male gender, advanced-stage PBC, hepatitis C virus superinfection, and a history of blood transfusion were associated with the development of HCC. Indeed, most cases of HCC were reported in patients with histopathological stage IV of PBC. Thus, dissimilar to our second case, most HCC arise in the advanced cirrhotic stage of PBC and there have been only a few cases of HCC arising in the non-cirrhotic stage of PBC;21,22 however, is important to mention that the liver biopsy taken in our patient was done five years before the diagnosis of HCC.
Some mechanisms have been proposed to explain the HCC occurrence in patients with PBC, such as the association of ductal proliferation with the presence of oval cells which are believed to arise from stem cells, and two sorts of evidence support the notion that oval cells are the troublemakers in HCC. Oval cells transfected with activated oncogene or a drop of tumor suppressor gene can give rise to HCC and c-Myc overexpression during hepatocarcinogenesis; and c-Myc inactivation resulted in HCC cells differentiating into hepatocytes and biliary cells forming bile duct structures.23 Moreover, the retention of proapoptotic bile acids in cholestasis produces apoptotic pressure, and promotes the formation of cancer-prone alterations in cellular gene expression.24
There are no specific treatment guidelines for HCC in patients with autoimmune liver diseases, so the decision of treatment should be similar to HCC arising from viral hepatitis, excessive alcohol consumption, or other etiologies; and principal considerations to take in account regarding therapy are performance status, liver function and tumor stage. Is important to select suitable treatment, without undue surgical stress whenever the diagnosis of HCC has been established.
The role of surveillance programs in autoimmune liver disease is not well defined. For many years autoimmune liver diseases were not considered risks factor for HCC; however, recent studies have shown that the risk of developing HCC in patients with PBC and older age, male sex, history of blood transfusion, any signs of portal hypertension or cirrhosis may be similar to that in patients with viral-related cirrhosis.19,25 Therefore, it is recommended that patients with advanced-stage of PBC and any of these factors should be submitted to regular screening for detection of HCC.20,25-27 Moreover, few cases of HCC have been reported in AIH, so it does not seem reasonable, based on conventional economic basis, to apply a screening policy for early detection of HCC in this liver disease.11,27 In our experience, these two patients are the only cases of HCC associated to autoimmune liver diseases attended in our Institute, from a cohort of 148 patients with HCC (update has not been published) from the year 1991 to 2001.28
In summary these cases highlight that HCC, although rare, can occur in the absence of coexistent viral hepatitis, or excessive alcohol consumption. The utility of screening for HCC in autoimmune liver diseases is still not defined.