We present the case of a patient referred to the gastroenterology service for investigation of abnormal liver function tests. She had been taking nitrofurantoin for 16 months as prophylaxis against urinary tract infections. CT scan showed evidence of lung pneumonitis and low attenuation in the liver parenchyma. Nitrofurantoin-in-duced pneumonitis and hepatotoxicity was diagnosed. The patient responded both clinically and biochemically to withdrawal of nitrofurantoin. This combination of adverse reaction to nitrofurantoin is rare.
A 57-year-old woman was referred to the gastroenterology service with abnormal liver function tests (LFTs). She was referred by the respiratory team who had seen her initially for a chronic, non-productive cough and abnormal chest radiograph. She had a background history of multiple sclerosis diagnosed 17 years previously and suffered recurrent urinary tract infections due to intermittent self catheterization. She had been taking Nitrofuran-toin 100 mg at night for 16 months as prophylaxis against urinary tract infections.
She was referred with ALT 424 IU/L (normal 0-40), AST 301 IU/L (normal 0-40), GGT 894 U/L (normal 055), ALP 282 U/L (normal 39-118) and Bilirubin 8 mmol/L (normal 0-17). She had no abdominal pain. There were no risk factors for chronic liver disease. Her other medications included: salbutamol inhaler, be-clathasone inhaler, amitriptyline, and lansoprazole. There was no family history of liver disease. She had never smoked and did not have any pets.
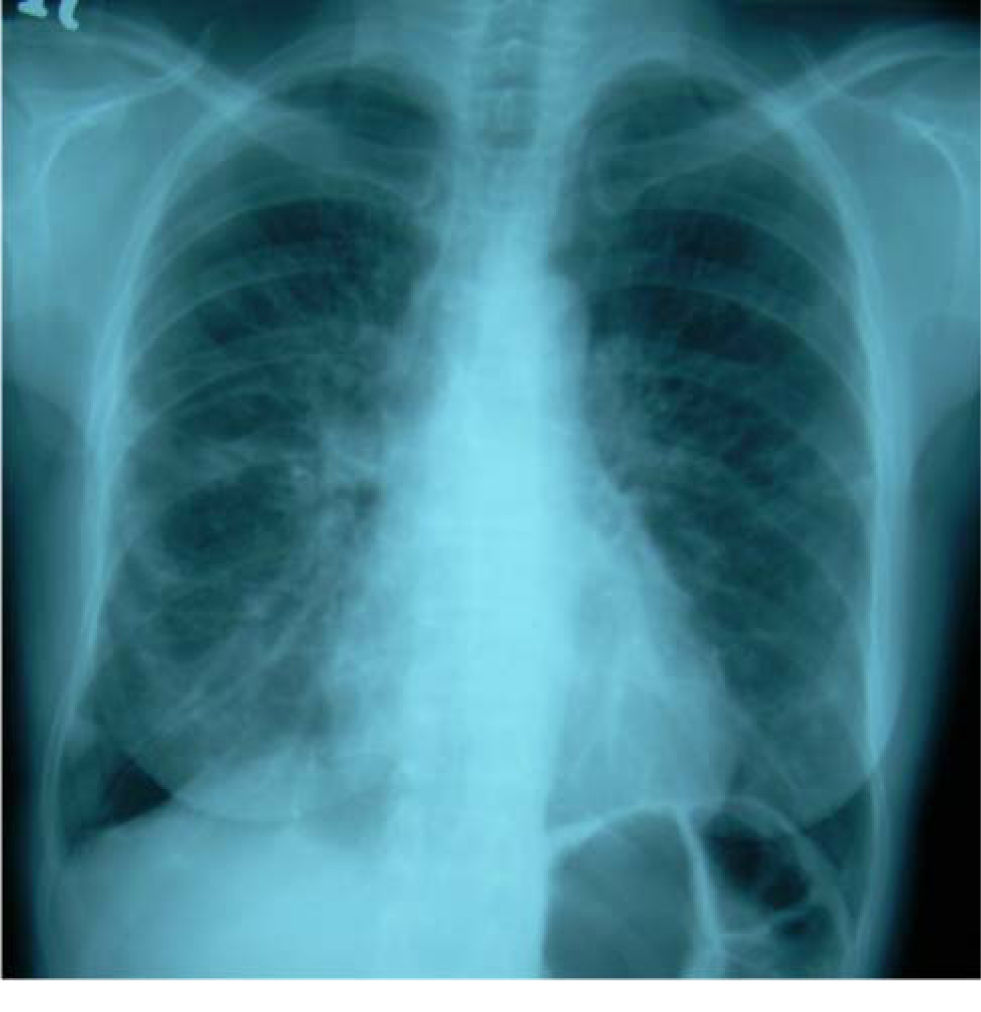
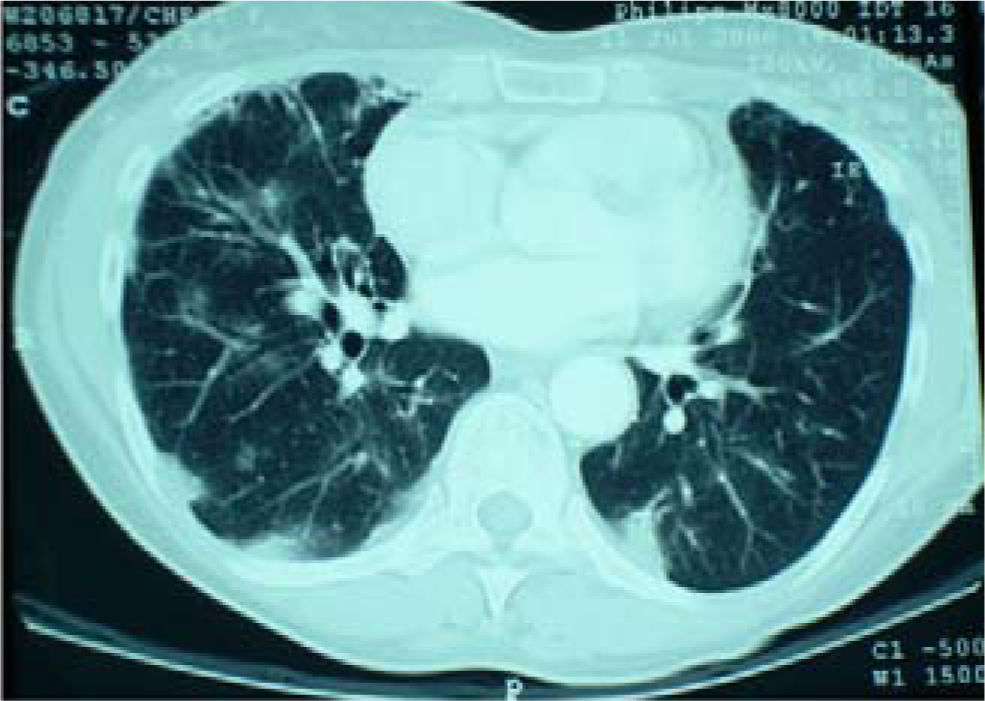
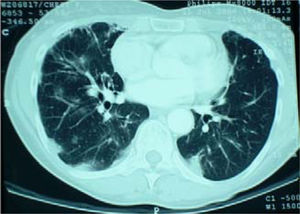
Her cough had been present for 8 months. A chest radiograph performed 5 months prior to referral had shown no abnormality. A repeat chest radiograph at the time of referral demonstrated multiple opacities in both lung fields (Figure 1). A CT scan of the chest was organized urgently. This revealed multiple areas of airspace shadowing in keeping with pneumonitis of uncertain origin (Figure 2). Spirometry testing showed a restrictive ventilatory pattern.
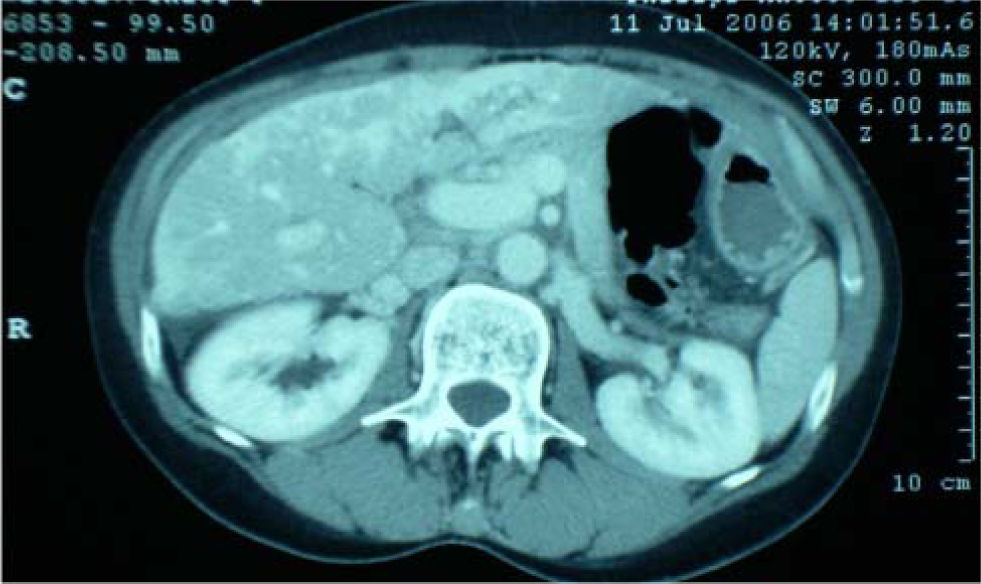
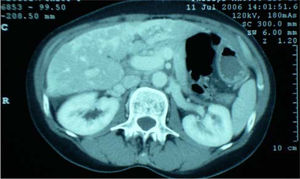
Further investigation revealed normoglycaemia, normal thyroid function, an erythrocyte sedimentation rate (ESR) of 24 mm/h (normal 0-8) and no coagulopathy. She tested negative for hepatitis viruses A, B and C and negative for autoantibodies; ferritin and alpha feta protein were also normal. CT scan of abdomen revealed gallstones but no dilation of the common bile duct. The liver was noted to be abnormal with most of the parenchyma demonstrating abnormal low attenuation areas -in patchy distribution-of uncertain cause (Figure 3). Doppler ultrasound showed no evidence of portal or hepatic vein occlusion.
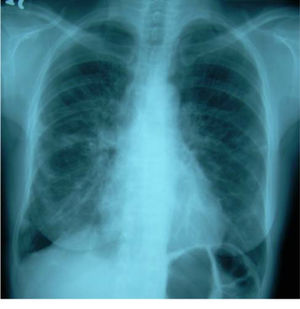
Nitrofurantoin-induced hepatitis and pneumonitis was suspected. The nitrofurantoin was discontinued and the patient was commenced on a course of oral prednisolone. She noted a gradual but steady improvement in her respiratory symptoms. Her LFTs improved and are almost back to baseline at 4 months follow up. Repeat spirome-try testing showed a normal ventilatory pattern.
DiscussionNitrofurantoin is a lipid-soluble, synthetic nitrofuran and a weak acid. It is metabolized by the liver and is excreted renally.1 It is primarily used for the treatment and prevention of urinary tract infections.
Adverse reactions to nitrofurantoin have been reported in the medical literature since its widespread use in the treatment of urinary tract infections. Pulmonary hy-persensitivity, pneumonitis and fibrosis caused by nitro-furantoin are well described. There are a wide variety of clinical presentations. Symptoms and signs may include cough, dyspnoea, pleuritic chest pain, fatigue, pyrexia, urticarial rash and peripheral neuropathy. Blood investigation may reveal elevated (ESR) and peripheral eosino-philia.2-4 Pulmonary function tests (PFTs) may show a restrictive pattern with reduced carbon monoxide diffusion capacity (DLCO). A diffuse, bilateral interstitial fibrosis pattern may be evident on the chest radiograph [1].
Withdrawal of nitrofurantoin and treatment with oral corticosteroids usually results in both an improvement in the clinical picture and in a resolution of radiographical changes.
Hepatoxicity caused by nitrofurantoin is rare with an estimated incidence of 0.3 per 100,000 therapeutic courses.5 The severity of liver injury may vary from salient liver enzyme disturbance to severe, symptomatic chronic active hepatitis and liver failure necessitating liver transplantation.2 It is believed that cytotoxic T-cells play a pivotal role in the pathogenesis of nitrofurantoin-in-duced liver injury. It has been hypothesized that a breakdown product of the drug or the drug itself, complexed to an endogenous peptide, is presented by the class 1 HLA antigen on the hepatocyte cell membrane; this induces cytotoxic T-cell activation and subsequent hepatocyte death.6
Combined pulmonary and hepatic toxicity induced by nitrofurantoin is rare with very few published cases.1,2,6-10 Nitrofurantoin is metabolized in the liver by the glutathione S-reductase system but the aminofuran-toin derivative accounts for only a small proportion of drug elimination. Approximately 50-55% of the drug is eliminated unchanged through renal tubular secretion and glomerular filtration.1,11 Nitrofurantoin is contrain-dicated in patients with a creatinine clearance of < 60 mL/min as renal excretion of nitrofurantoin correlates directly with creatinine clearance.1 Use in renal impairment increases the risk of adverse or toxic effects of the drug. As with pulmonary toxicity, the prognosis of the acute hepatic reaction is generally good as they tend to resolve quickly with drug withdrawal or prednisolone therapy.