Three fourths of chronic hepatitis C virus (HCV) infected adult patients in the United States (US) are born between 1945 and 1965, also known as baby boomers (BB). Prevalence of hepatocellular carcinoma (HCC) is raising in BB due to their advancing age and prolonged HCV infection. We evaluated inpatient hospitalization and mortality in BB associated with HCC.
Materials and methodsIt is a retrospective cohort study utilizing the Healthcare Utilization Project-National Inpatient Sample (HCUP-NIS) database. From 2003 to 2012, top five primary cancer related hospitalization and mortality among BB were studied.
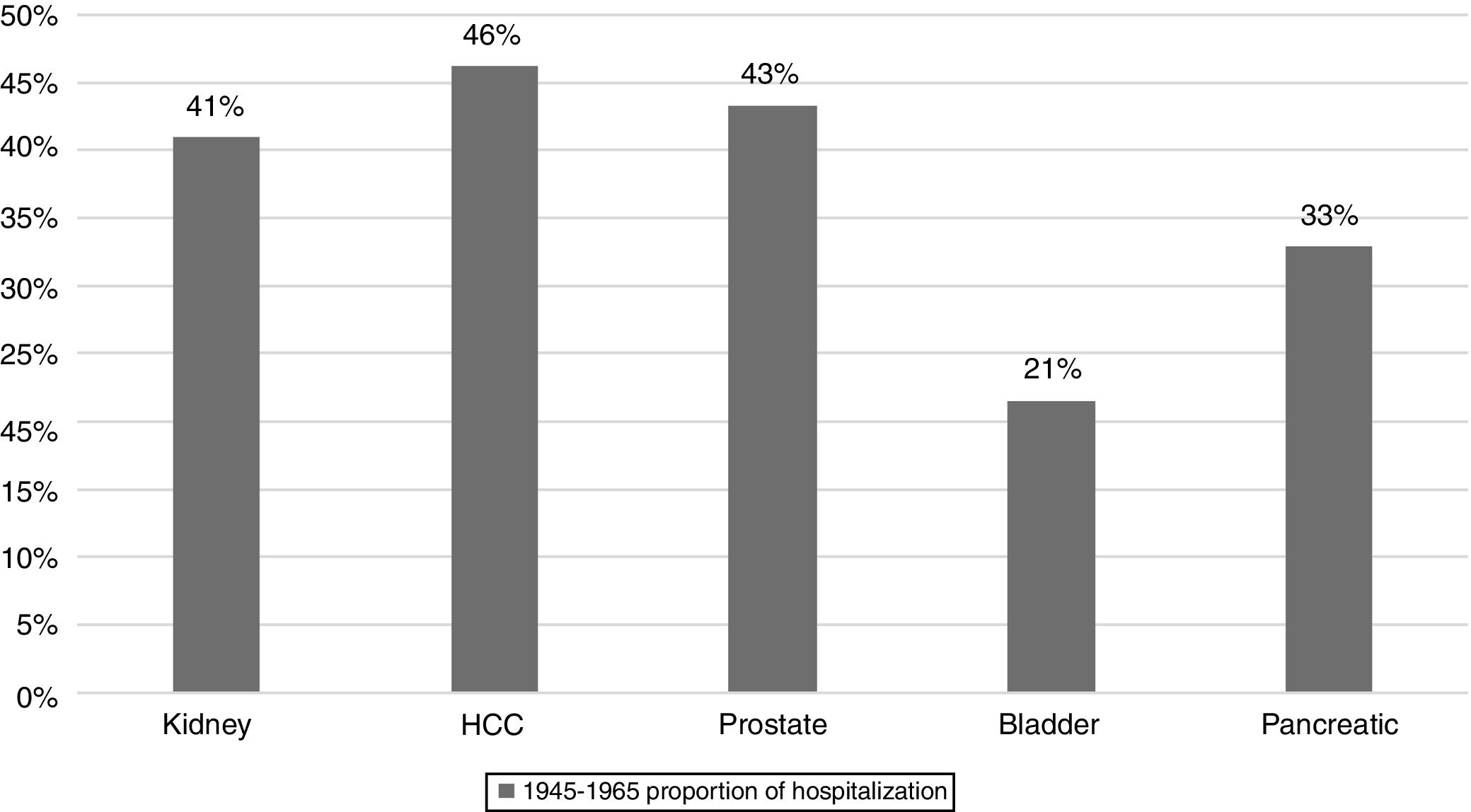
ResultsAmong 48,733 hospitalizations related to HCC in HCUP-NIS database from 2003 to 2012, BB accounted for 49.6% (24,210) whereas non-BB 50.4% (24,523). Within BB cohort, the top five cancers with the highest proportion of hospitalizations were HCC (46%), prostate (43%), kidney (41%), pancreas (33%), and bladder (21%). From 2003 to 2012, the proportion of HCC related hospitalizations represented by BB almost doubled (33.5 to 57.8%) whereas there was one-third reduction (66.4 to 42.1%) among non-BB. Similarly, HCC-related inpatient mortality in BB decreased by 28% (6.1 to 2.7 per 100,000 hospitalization) but it remained unchanged in non-BB (11.1 to 10.6). HCC accounted for 2nd highest mortality (4960 total deaths) among hospitalized BB behind pancreatic cancer. HCC related to HCV was disproportionately higher in BB compared to non-BB (50.6% vs. 19%; P<0.001).
ConclusionHCC ranks number one among the top five cancers with highest proportion of inpatient burden. Future studies should focus on understanding the underlying reasons for this ominous trend.
According to the most recent estimates in 2015, hepatocellular carcinoma (HCC) is 6th most common cancer worldwide, 4th leading cause of overall cancer-related death and 2nd among men [1]. In the United States (US), HCC incidence and mortality have been rising at an alarming rate for past three decades, with majority cases (50–70%) attributed to chronic hepatitis C virus (HCV) infection [2]. Other notable risk factors for HCC include hepatitis B virus (HBV) infection, especially in immigrants from endemic areas, alcoholic liver disease (ALD), and non-alcoholic steatohepatitis (NASH) [3]. The Center for Disease Control and Prevention (CDC) estimates that the majority of 2.7–3.9 million people living in the US with chronic HCV infection are unaware of their infection and therefore not receiving appropriate medical care [4]. According to the 2015 Census, persons born between 1945 and 1965, referred to as baby boomers (BB), comprise of 25% of the US population, but account for 81% of chronic HCV infection and 73% of HCV-related mortality [4]. Currently, BB account for over 15 billion dollars in annual inpatient healthcare expenditure for the care of chronic HCV infection, and it is projected that more than 1 million BB will develop HCV-related cirrhosis, hepatic decompensation, or HCC by 2020 [5,6]. As BB age, the risk of HCC will continue to rise due to the increasing number of patients in this cohort with prolonged duration of chronic HCV infection resulting in cirrhosis. Therefore, to mitigate the risk of HCC in the aging BB in the era of escalating costs of healthcare, it is crucial to screen, diagnose, treat and survey this high-risk cohort. In this context, we seek to identify the most common primary cancers affecting the BB generation, evaluate inpatient hospitalization and mortality trends associated with HCC.
2Materials and methods2.1Study designThis is a nationally representative, retrospective, cohort study from 2003 to 2012 by utilizing data from the Healthcare Cost and Utilization Project (HCUP)-National Inpatient Sample (NIS) sponsored by the Agency for Healthcare Research and Quality (AHRQ). The HCUP-NIS contains individual discharge records from one fifths of all inpatient admissions from 1000 non-federal hospitals in 48 states within the US. Both community and academic hospitals are included in HCUP-NIS database, which are selected by stratified sampling strategy. All discharges from participating hospitals are included in HCUP-NIS database. Each individual record has a unique identifier, demographic data, hospital type, admission type, primary diagnosis, and up to 15 secondary diagnoses. Institutional review board (IRB) approval was not required for this study as HCUP-NIS data is publicly available without individual identifiers.
2.2Cohort selectionWe identified all adult (18-year-old and above) patients who were hospitalized for a primary or a secondary cancer diagnosis between 2003 and 2012. Using Clinical Classification Software (CCS), 30 primary-site cancers were classified including (1) head and neck cancer, (2) esophageal cancer, (3) stomach/gastric cancer, (4) colon cancer, (5) rectal/anal cancer, (6) liver cancer/HCC, (7) pancreatic cancer, (8) gastrointestinal carcinoid tumor, (9) lung cancer, (10) other respiratory cancer, (11) bone and connective tissue cancer, (12) skin melanoma, (13) non-melanoma skin cancer, (14) breast cancer, (15) uterine cancer, (16) cervical cancer, (17) ovarian cancer, (18) female genital cancer (non-cervical and non-ovarian), (19) prostate cancer, (20) testicular cancer, (21) male genital cancer (non-testicular), (22) bladder cancer, (23) kidney/renal cell carcinoma, (24) other urinary cancer, (25) brain cancer, (26) thyroid, (27) Hodgkin's lymphoma, (28) non-Hodgkin's lymphoma, (29) leukemia and (30) multiple myeloma.
2.3Birth-cohort specific populationAll adult patients who were hospitalized with a primary or a secondary cancer were divided into 3 cohorts: (1) baby boomers (BB), those born between 1945 and 1965, (2) Pre-BB, those born before 1945, and (3) Post-BB, those born after 1965. HCUP-NIS data provides race/ethnicity, which is utilized to evaluate the differences associated with following ethnic cohorts: Caucasians, African-Americans, Hispanics, and Asians.
2.4Primary outcomesOur primary objective was to determine temporal trends in hospitalization and mortality associated with primary cancers within birth-specific cohorts. Birth cohort specific trends in hospitalization and in-patient mortality rates associated with HCC were analyzed. Inpatient mortality was defined as a yes/no variable.
2.5Variables of interestUsing the International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) and CCS provided by HCUP-NIS, we analyzed our variables of interest. In addition, CCS classified certain diagnoses and procedures into unique single-level and multi-level hierarchal classification systems. Using ICD-9-CM and the HCUP-NIS comorbidity software, we identified secondary covariates of interest including demographic data (age, gender, ethnicity, health insurance type, household median income level), etiology of liver disease (HCV, ALD, HBV, or other), presence of cirrhosis, severity/extent of cancer (localized or metastatic disease), complications of portal hypertension (ascites, hepatic encephalopathy, hepatorenal syndrome [HRS], spontaneous bacterial peritonitis [SBP], esophageal variceal bleeding [EVB]), and comorbidities including diabetes, hypertension, renal failure, congestive heart failure [CHF], peripheral vascular disease [PVD], chronic obstructive pulmonary disease [COPD]/asthma, depression, psychosis, alcohol abuse, drug abuse. Severity of comorbid conditions was calculated using the Elixhauser Comorbidity Index (ECI) which produces a score for severity of disease (0, 1, 2 or ≥3) utilizing 30 weighted comorbidity indicators or ICD-9-CM diagnoses [7]. ECI has been validated to be statistically superior to other comorbidity indices including the Charlson comorbidity index in predicting various inpatient outcomes [8].
2.6Statistical analysesAll analyses were performed using SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC). Clinical and demographic characteristics were compared across birth cohorts and presented as frequency (N) and proportions (%) for categorical variables, and mean and standard deviation for continuous variables. Pearson Chi-square test was used for categorical variables and ANOVA test was used for continuous variables. P values less than 0.05 were deemed significant for all statistical analyses. For binary variables such as treatment and comorbidities, 1 indicates event and 0 indicates the opposite. All missing data were omitted. Multivariate logistic regressions were performed for analyzing mortality, and multivariate linear regressions with categorical variables were performed for estimating cost of care and patients’ LOS in the hospital. The covariates in all the regressions are demographic information, complications, comorbidities, and insurance payment methods. Mean values were used to characterize continuous data.
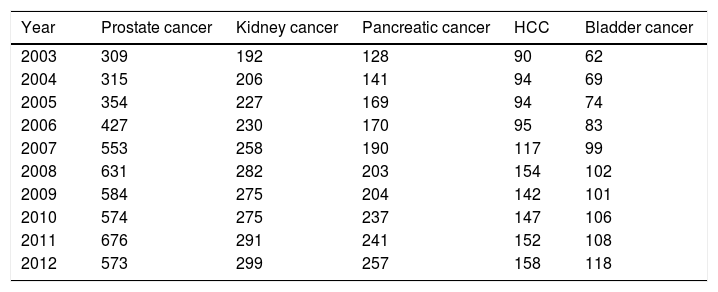
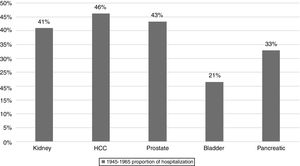
3Results3.1Top 5 cancers with the highest inpatient incidenceAmong 30 types of cancers evaluated, the top five cancers with highest inpatient incidence rates in the studied birth cohort, BB were as follows: HCC, prostate, kidney, pancreatic, and bladder cancers. Of these, HCC was associated with the highest inpatient proportion incidence (46%) compared to prostate (43%), kidney (41%), 33% pancreas (31%), and bladder (21%) (Fig. 1). From 2003 to 2012, inpatient incidence increased for all top five cancers (Table 1).
Top 5 cancers with rising inpatient incidence rates in the Baby Boomer cohort, HCUP-NIS 2003–2012.
| Year | Prostate cancer | Kidney cancer | Pancreatic cancer | HCC | Bladder cancer |
|---|---|---|---|---|---|
| 2003 | 309 | 192 | 128 | 90 | 62 |
| 2004 | 315 | 206 | 141 | 94 | 69 |
| 2005 | 354 | 227 | 169 | 94 | 74 |
| 2006 | 427 | 230 | 170 | 95 | 83 |
| 2007 | 553 | 258 | 190 | 117 | 99 |
| 2008 | 631 | 282 | 203 | 154 | 102 |
| 2009 | 584 | 275 | 204 | 142 | 101 |
| 2010 | 574 | 275 | 237 | 147 | 106 |
| 2011 | 676 | 291 | 241 | 152 | 108 |
| 2012 | 573 | 299 | 257 | 158 | 118 |
Incidence rate per 100,000 hospitalizations for baby boomers.
Baby Boomer cohort is comprised of those born between 1945 and 1965.
Abbreviation: HCUP-NIS, Healthcare Utilization Project-National Inpatient Sample; HCC, hepatocellular carcinoma.
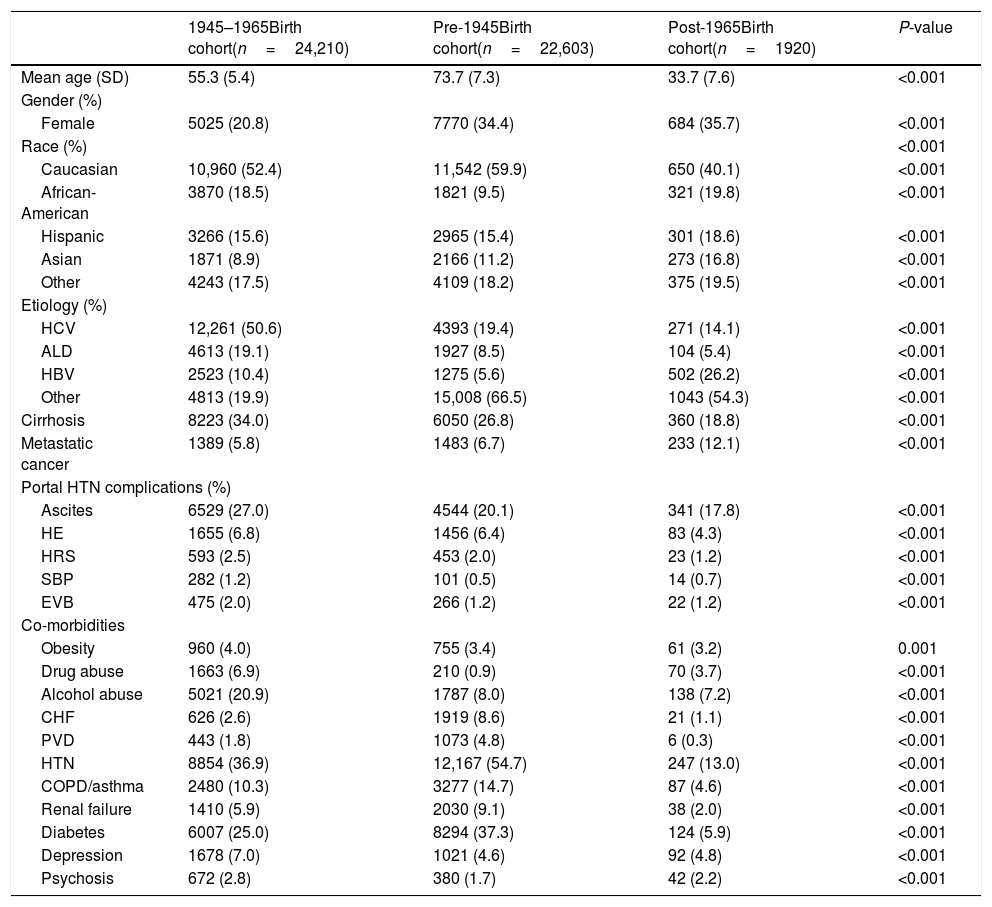
From 2003 to 2012, there were a total of 19,237,419 hospitalizations. Among those, 48,733 hospitalizations were related to HCC, 24,210 in BB cohort and 24,523 in non-BB cohort (22,603 born pre-1945 and 1920 born post-1965). Males and Caucasians represented a majority of HCC patients among all birth cohorts (Table 2). Post-1965 cohort has significantly higher proportion of Asians and lower proportion of Caucasians compared to pre-1945 and BB cohorts. As expected, at the time of cancer diagnosis, HCC patients in pre-1945 cohort were significantly older than BB cohort whereas those in post-1965 cohort were younger. This observation corresponds with our study design which is based on birth-specific cohorts. BB cohort has significantly higher proportion of HCV as the etiology of liver disease compared to pre-1945 and post-1965 cohorts (50.6% vs. 19.4% vs. 14.1%). BB cohort had higher proportion of ALD compared to other cohorts (19.1% vs. 8.5% vs. 5.4%). Interestingly, post-1965 cohort had higher proportion of HBV compared to BB or pre-1945 cohorts (26.2% vs.10.4% vs. 5.6%). Similarly, post-1965 cohort had higher proportion of metastatic HCC compared to BB cohort or pre-1945 cohorts (12.1% vs. 5.8% vs. 6.7%). HCC patients in BB-cohort had higher rates of cirrhosis compared to HCC patients in pre-BB and post-BB cohorts (34% vs. 26.8% vs. 18.8%) and portal hypertension complications such as ascites, HE, HRS, SBP and EVB. BB-cohort had significantly higher prevalence of alcohol and drug abuse compared to other cohorts. Among all HCC patients, pre-1945 cohort had higher proportion of major comorbidities including CHF, hypertension, diabetes, PVD, renal failure, and COPD. Table 2 summarizes the demographic and clinical characteristics of hospitalized patients with the diagnosis of HCC categorized into three birth cohorts.
Birth cohort-specific demographics and clinical characteristic of patients hospitalized for HCC, HCUP-NIS 2003–2012.
| 1945–1965Birth cohort(n=24,210) | Pre-1945Birth cohort(n=22,603) | Post-1965Birth cohort(n=1920) | P-value | |
|---|---|---|---|---|
| Mean age (SD) | 55.3 (5.4) | 73.7 (7.3) | 33.7 (7.6) | <0.001 |
| Gender (%) | ||||
| Female | 5025 (20.8) | 7770 (34.4) | 684 (35.7) | <0.001 |
| Race (%) | <0.001 | |||
| Caucasian | 10,960 (52.4) | 11,542 (59.9) | 650 (40.1) | <0.001 |
| African-American | 3870 (18.5) | 1821 (9.5) | 321 (19.8) | <0.001 |
| Hispanic | 3266 (15.6) | 2965 (15.4) | 301 (18.6) | <0.001 |
| Asian | 1871 (8.9) | 2166 (11.2) | 273 (16.8) | <0.001 |
| Other | 4243 (17.5) | 4109 (18.2) | 375 (19.5) | <0.001 |
| Etiology (%) | ||||
| HCV | 12,261 (50.6) | 4393 (19.4) | 271 (14.1) | <0.001 |
| ALD | 4613 (19.1) | 1927 (8.5) | 104 (5.4) | <0.001 |
| HBV | 2523 (10.4) | 1275 (5.6) | 502 (26.2) | <0.001 |
| Other | 4813 (19.9) | 15,008 (66.5) | 1043 (54.3) | <0.001 |
| Cirrhosis | 8223 (34.0) | 6050 (26.8) | 360 (18.8) | <0.001 |
| Metastatic cancer | 1389 (5.8) | 1483 (6.7) | 233 (12.1) | <0.001 |
| Portal HTN complications (%) | ||||
| Ascites | 6529 (27.0) | 4544 (20.1) | 341 (17.8) | <0.001 |
| HE | 1655 (6.8) | 1456 (6.4) | 83 (4.3) | <0.001 |
| HRS | 593 (2.5) | 453 (2.0) | 23 (1.2) | <0.001 |
| SBP | 282 (1.2) | 101 (0.5) | 14 (0.7) | <0.001 |
| EVB | 475 (2.0) | 266 (1.2) | 22 (1.2) | <0.001 |
| Co-morbidities | ||||
| Obesity | 960 (4.0) | 755 (3.4) | 61 (3.2) | 0.001 |
| Drug abuse | 1663 (6.9) | 210 (0.9) | 70 (3.7) | <0.001 |
| Alcohol abuse | 5021 (20.9) | 1787 (8.0) | 138 (7.2) | <0.001 |
| CHF | 626 (2.6) | 1919 (8.6) | 21 (1.1) | <0.001 |
| PVD | 443 (1.8) | 1073 (4.8) | 6 (0.3) | <0.001 |
| HTN | 8854 (36.9) | 12,167 (54.7) | 247 (13.0) | <0.001 |
| COPD/asthma | 2480 (10.3) | 3277 (14.7) | 87 (4.6) | <0.001 |
| Renal failure | 1410 (5.9) | 2030 (9.1) | 38 (2.0) | <0.001 |
| Diabetes | 6007 (25.0) | 8294 (37.3) | 124 (5.9) | <0.001 |
| Depression | 1678 (7.0) | 1021 (4.6) | 92 (4.8) | <0.001 |
| Psychosis | 672 (2.8) | 380 (1.7) | 42 (2.2) | <0.001 |
Abbreviations: HCC, hepatocellular carcinoma; HCUP-NIS, Healthcare Utilization Project-National Inpatient Sample; HCV, hepatitis C virus; ALD, alcoholic liver disease; HBV, hepatitis B virus; HE, hepatic encephalopathy; HRS, hepatorenal syndrome; SBP, spontaneous bacterial peritonitis; EVB, esophageal variceal bleeding; CHF, congestive heart failure; PVD, peripheral vascular disease; HTN, hypertension; COPD, chronic obstructive pulmonary disease.
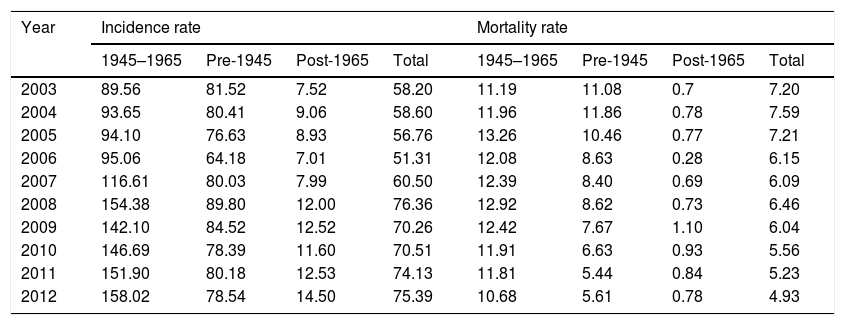
Overall HCC related hospitalizations increased by 18.4%, from 4643 in 2003 to 5501 in 2012, During this period, proportion of HCC represented by BB cohort increased by 72.5% (from n=1560/4643 in 2003 to n=3181/5501 in 2012. In 2012, overall HCC represented by BB cohort rose to 57.8% from 33.5% in 2003. In contrast, during the same period, HCC represented by non-BB cohort decreased by 36.6% (n=3083/4643 in 2003 to n=2320/5501 in 2012). The inpatient incidence of HCC among BB cohort increased from 89.5 per 100,000 BB hospitalizations per year in 2003 to 158 per 100,000 BB hospitalizations per year in 2012 (Table 3), whereas non-BB inpatient incidence decreased from 49.4 per 100,000 non-BB hospitalizations per year to 43.9 during the same period. Among non-BB patients from 2003 to 2012, HCC inpatient incidence in pre-1945 birth cohort decreased from 81.5 per to 78.5 per 100,000hospitalizations/year, and post-1965 birth cohort experienced increase from 7.5 to 14.5 per 100,000hospitalizations/year (Table 3).
Inpatient hospitalization incidence rate and mortality trends related to HCC among different birth cohorts, HCUP-NIS 2003–2012.
| Year | Incidence rate | Mortality rate | ||||||
|---|---|---|---|---|---|---|---|---|
| 1945–1965 | Pre-1945 | Post-1965 | Total | 1945–1965 | Pre-1945 | Post-1965 | Total | |
| 2003 | 89.56 | 81.52 | 7.52 | 58.20 | 11.19 | 11.08 | 0.7 | 7.20 |
| 2004 | 93.65 | 80.41 | 9.06 | 58.60 | 11.96 | 11.86 | 0.78 | 7.59 |
| 2005 | 94.10 | 76.63 | 8.93 | 56.76 | 13.26 | 10.46 | 0.77 | 7.21 |
| 2006 | 95.06 | 64.18 | 7.01 | 51.31 | 12.08 | 8.63 | 0.28 | 6.15 |
| 2007 | 116.61 | 80.03 | 7.99 | 60.50 | 12.39 | 8.40 | 0.69 | 6.09 |
| 2008 | 154.38 | 89.80 | 12.00 | 76.36 | 12.92 | 8.62 | 0.73 | 6.46 |
| 2009 | 142.10 | 84.52 | 12.52 | 70.26 | 12.42 | 7.67 | 1.10 | 6.04 |
| 2010 | 146.69 | 78.39 | 11.60 | 70.51 | 11.91 | 6.63 | 0.93 | 5.56 |
| 2011 | 151.90 | 80.18 | 12.53 | 74.13 | 11.81 | 5.44 | 0.84 | 5.23 |
| 2012 | 158.02 | 78.54 | 14.50 | 75.39 | 10.68 | 5.61 | 0.78 | 4.93 |
Rate per 100,000 hospitalizations.
Abbreviations: HCC, hepatocellular carcinoma; HCUP-NIS, Healthcare Utilization Project-National Inpatient Sample.
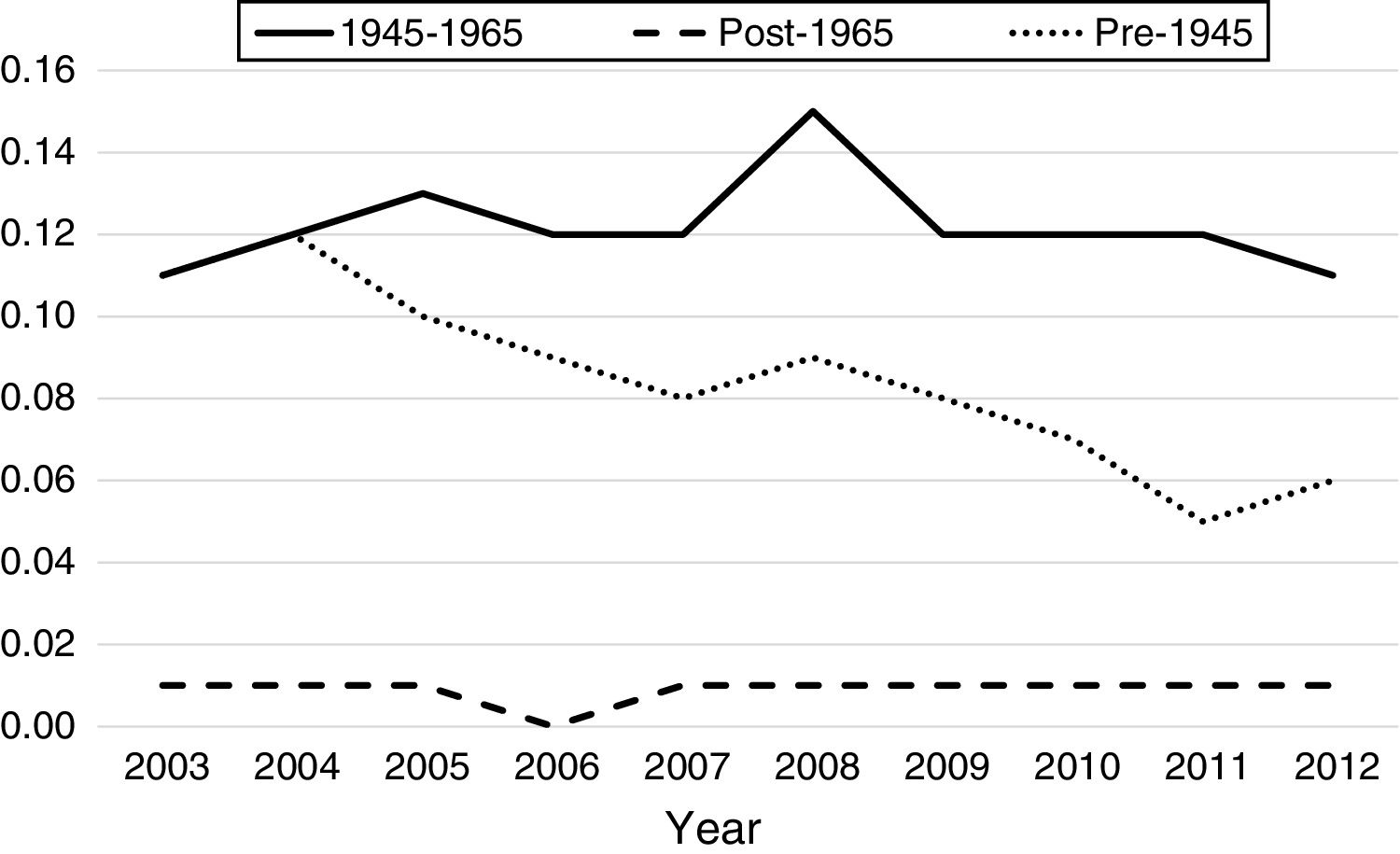
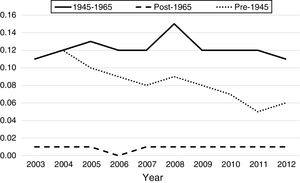
From 2003 to 2012, there were a total of 4960 inpatient deaths related to HCC. Overall inpatient mortality associated with HCC among all patients declined by 32% from 7.2 per 100,000 hospitalizations in 2003 to 4.9 in 2012 (Table 3). During this period, HCC related inpatient mortality among non-BB cohort declined by 28% from 6.1 per 100,000non-BBhospitalizations/year in 2003 to 2.7 per 100,000non-BBhospitalizations/year in 2012, whereas HCC-related mortality among BB cohort did not change significantly (11.1 in 2003 to 10.6 per 100,000hospitalizations/year in 2012). Of non-BB related HCC mortality, pre-1945 cohort experienced greater decline from 11 per 100,000hospitalizations/year in 2003 to 5.61 per 100,000hospitalizations/year in 2012, whereas post-1965 cohort experienced insignificant increase from 0.7 to 0.78 per 100,000 hospitalizations during same period. Table 3 summarizes the HCC-related inpatient hospitalization and mortality trends from 2003 to 2012 among different birth cohorts.
4DiscussionUsing data from a large, nationally representative registry of hospitalized patients in the US, we evaluated the trends of cancer-related hospitalizations of top five primary cancers among different birth-specific cohorts from 2003 to 2012, with a focus on persons born between 1945 and 1965. Of the top five cancers with highest hospitalization rates among BB, HCC accounted for the highest proportion of inpatient incidence. HCC was 2.8 times more common in hospitalized BB compared to non-BB. Although the overall HCC inpatient incidence increased by 18.4% between 2003 and 2012, BB cohort experienced disproportionate increase of 72.5% while non-BB cohort experienced a decline in incidence. The proportion of HCC-related hospitalizations represented by BB cohort increased from 33.5% in 2003 to 57.8% in 2012. The inpatient incidence and hospitalization trends in BB with HCC observed in our study mirrors the overall incidence of HCC in BB ( combined outpatient and inpatient). For example, Yan et al. used the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) data to show that the BB cohort had a 64% increase in HCC incidence from 2003 to 2011, with 57.4% of all HCC represented by BB cohort in 2011 [9].
HCC is the fastest growing cause of cancer-related death in the US [10]. In our study, HCC was associated with a higher disease severity and 2nd highest inpatient mortality, trailing pancreatic cancer. Unlike other top five cancers, HCC has a modifiable risk factor, HCV infection that is both highly prevalent in BB population and is amenable to cure. In our study, HCV was the leading etiology of liver disease, present in nearly 50% of BB patients who were hospitalized with HCC, a proportion that is substantially higher than pre-1945 and post-1965 cohorts (19.4% and 14.1% respectively). HCV is the leading cause of HCC in the US, with annual incidence rate of 3% in cirrhotics with untreated HCV-infection [2]. It is estimated that, compared to non-HCV counterparts, the HCV-infected patients have 15–20-fold increased risk of HCC with most of the excess risk represented in patients with advanced fibrosis [2]. Importantly, HCV-related cirrhosis accounts for nearly 50–70% of new cases of HCC in the US. According to the joint report on cancer incidence in the US by Center for Disease Control (CDC), American Cancer Society and National Cancer Institute, HCC incidence has sharply increased and is only next to thyroid cancer [10]. It is evident that overall HCC related disease burden is growing, but it is not equally distributed – the aging population of BB continue to share disproportionately a higher burden of HCV-related liver cirrhosis and HCC. Mainly for this reason, CDC and the United States Preventive Services Task Force (USPSTF) recommends that all persons born between 1945 and 1965 be screened for HCV infection at least once with a goal to treat and eradicate before progression to cirrhosis and cirrhosis-related complications, including portal hypertension and HCC [4,11,12]. However, studies have reported that a large proportion of BB have not been screened [13–15]. One proposed method to help HCV screening and contain the burden of undiagnosed HCV is a greater emphasis on primary care provider-directed HCV screening of BB regardless of presence or absence of past high-risk behaviors, and facilitating linkage to care [16]. Linkage to care does not merely imply a passive referral process but rather involves an establishment of care with providers (such as hepatologists, gastroenterologists, etc.) who specialize in comprehensive care of patients with liver disease. It is estimated that within the next decade, most of the patients with chronic HCV infection encountered in the clinical setting would be cured from HCV and attain sustained virological response (SVR) [17]. Although there have been concerns regarding the potential increase in HCC incidence following treatment with direct-acting antiviral (DAA) agents, recent studies have shown that HCC incidence substantially declines after achieving SVR following the DAA use [18].
In our study, there is significantly higher proportion of males hospitalized with HCC within BB group compared to those in the pre-1945 or post-1965 cohorts. Similarly, BB also have substantially higher percentage of those with alcohol and drug abuse compared to the other cohorts. The prevalence of alcohol and drug abuse, and higher percentage of ALD-related cirrhosis in the BB correlates linearly with the higher proportion of cirrhosis and portal hypertension in this cohort (Table 2). Despite the higher prevalence of cirrhosis, BB had the lowest percentage of metastatic HCC; the post-1965 cohort had highest percentage of metastatic HCC. A possible explanation for the higher proportion of metastatic HCC in this relatively younger cohort (mean age 33.7±7.6 years) is that the post-1965 cohort is comprised of higher proportion of Asians and higher prevalence of HBV infection. We suspect that there may be a component of reduced perception of HCC risk in this younger group, which can lead to underestimating the risk of underlying cirrhosis and inadequate screening practices for HCC, increasing the risk for metastatic potential. On the otherhand, the higher prevalence of cirrhosis and cirrhosis-related complications in BB, along with increased awareness for screening for HCV infection in BB may have contributed to diagnosis of HCC at earlier stages, thus decreasing the risk for metastatic HCC. Between 2003 and 2012, the overall HCC-related inpatient mortality among all patients decreased by 32%, and by 26% in non-BB cohorts. Importantly, HCC-related inpatient mortality did not change in the BB despite the lower proportion of metastatic HCC (Fig. 2). Granular data regarding the receipt of treatment for HCC (including loco-regional therapy, surgical resection, and liver transplantation for eligible patients) among various cohorts would help understand the observed trends in the mortality. These data were not available in the HCUP-NIS database. Studies suggest that only 46% cases of HCC are diagnosed at an early stage and majority of those with HCC are not receiving curative therapy [19]. Additionally, recent studies have shown that the BB cohort, despite the earlier stages of HCC at diagnosis, do not undergo more surgical resection than other groups [9]. Surgical resection is a curative option in the setting of compensated cirrhosis or non-cirrhotic livers with resectable tumors, but patients with advanced cirrhosis and portal hypertension or hepatic decompensation are not optimal surgical candidates due to increased mortality risk in the absence of adequate hepatic reserve. Liver transplantation is a definitive and effective treatment option for HCC, but there are strict eligibility criteria for the liver transplantation waitlist. Studies have shown that liver transplantation rates for HCC have doubled in the US over the past decade [20]. According to a study using the United Network for Organ Sharing (UNOS) data from 2003 to 2014, Cholankeril et al. noted that while overall liver transplantation rates for HCV-related HCC have more than doubled, liver transplantation rates have tripled in BB, constituting 80.1% of all HCC-related liver transplants in the US [20].
Our retrospective cohort study using the HCUP-NIS database has several limitations. Due to limitations of the database, we are unable to identify how HCC was diagnosed (surveillance programs or based on symptoms), which could impact the survival outcomes. Details regarding the receipt of therapy for HCC including loco-regional therapy, resection and liver transplantation are unavailable in this database and are important to understand the reasons for lack of improvement in mortality in BB. Amongst the BB there is a higher rate of cirrhosis and cirrhosis-related complications, but the HCUP-NIS registry does not provide granular data to understand the relative contribution of specific factors on overall mortality in patients with concomitant HCC. The database also does not have the Milan criteria and University California at San Francisco (UCSF) criteria-based classification of HCC, which is utilized for determining candidacy for liver transplantation in the US. Other important clinical parameters such as Model for End-stage Liver Disease (MELD) score, Child–Turcotte–Pugh (CTP) score, and presence of portal vein thrombosis (PVT) are unavailable in the HCUP-NIS database. The decline in inpatient HCC incidence and mortality in pre-1945 cohort is perhaps due to attrition of this sub-cohort due to aging. Furthermore, the nature of our study design which is based on birth-specific cohort may have created a bias. Lastly, our study is limited by its retrospective design.
In conclusion, our analyses highlights existing challenges and opportunities associated with HCC-related disease burden among the BB. HCC is the leading cancer with the highest proportion of inpatient incidence in 1945–1965 birth cohort, and HCV remains the most common underlying etiology of HCC. Although BB demonstrate early and favorable staging of HCC compared to other cohorts, inpatient mortality has not improved in the BB, which may be due to higher prevalence of alcohol and substance use. While screening for HCV infection followed by DAA therapy is an important first step, other measures such as active linkage to care, referral to counseling and rehabilitation for substance abuse, screening for HCC followed by ongoing surveillance in patients with advanced fibrosis, early diagnosis of HCC and initiation of appropriate therapy must be integrated into the comprehensive management plan.AbbreviationsALD alcoholic liver disease baby boomers hepatitis B virus hepatitis C virus hepatocellular carcinoma National Inpatient Sample-Healthcare Utilization Project
Chiranjeevi Gadiparthi.
FundingThere was no financial or grant support for this study.
Author contributionsChiranjeevi Gadiparthi, Eric R. Yoo, Vijay Are, Paris Charilaou and George Cholankeril were responsible for study concept and design, acquisition of the data, analysis and interpretation of the data, and drafting and approval of the final manuscript.
Donghee Kim was responsible for drafting, critical revision, and approval of the final manuscript.
Capecomorin Pitchumoni and Aijaz Ahmed were responsible for the interpretation of the data, study supervision, drafting, critical revision and approval of the final manuscript.
All authors were involved in the final approval of the version of the manuscript submitted and have agreed to be accountable for all aspects of the work.
Conflict of interestAll authors declare no conflict of interest in the preparation of this manuscript, including financial, consultant, institutional, and other relationships that might lead to bias.