Variceal bleeding is a dramatic complication of cirrhosis. Primary prophylaxis against variceal bleeding is indicated for patients with high-risk varices. In order for these patients to be identified, endoscopic screening for esophageal varices has been traditionally recommended at the time of the diagnosis of cirrhosis. Considering that many patients do not have esophageal varices in the early stages of cirrhosis and, therefore, are submitted to endoscopy unnecessarily, non-invasive methods for variceal screening have been studied. Among these non-invasive methods, the most extensively studied probably are platelet count/spleen diameter ratio, liver stiffness, spleen stiffness and an association between liver stiffness and platelet count, referred to as the Baveno VI criteria. The Baveno VI criteria has recently been recommended by different medical associations for variceal screening. This is a critical review on the non-invasive methods for variceal screening, in which the performances of the different methods are presented and the limitations of the existing evidence is discussed. Despite reasonable performances of some of these methods, especially platelet count/spleen diameter ratio and the association between liver stiffness and platelet count, we understand that the available evidence still has relevant limitations and that physicians should decide on screening cirrhotic patients for esophageal varices with endoscopy or non-invasive methods on a case-by-case basis.
Variceal bleeding is the one of most dramatic complications of cirrhosis, with a mortality rate of up to 20% in six weeks, despite all the undeniable advances verified in the last few decades. This is why it is mandatory to offer prophylactic measures against variceal rupture for patients at high risk for the first bleeding (primary prophylaxis). In order to identify those patients at higher risk, it is traditionally recommended that every patient undergo upper gastrointestinal endoscopy at the time of the diagnosis of cirrhosis [1]. Nevertheless, patients with cirrhosis must have clinically significant portal hypertension before they develop esophageal varices (EV), and while varices are present in around 70% of Child-Pugh B or C patients, they are present only in approximately 40% of Child-Pugh A patients [2].
Bearing in mind the abovementioned figures, it is obvious that a significant part of patients with a new diagnosis of cirrhosis will undergo endoscopy unnecessarily (they will not be diagnosed with EV). This became even more important in recent years, when cirrhosis is diagnosed earlier because of the availability of non-invasive methods for its diagnosis (such as liver stiffness measurement) [3]. Moreover, one should consider that endoscopy is an invasive procedure, associated to some risks (yet quite low), patient discomfort and high costs. Therefore, many researchers have been dedicated to find non-invasive methods to identify patients with cirrhosis who could safely spare endoscopy due to an extremely low chance of having EV in general or at least varices in which primary prophylaxis against bleeding would be recommended (high-risk varices). This paper aims at critically reviewing the best studied non-invasive methods to screen for EV in patients with cirrhosis.
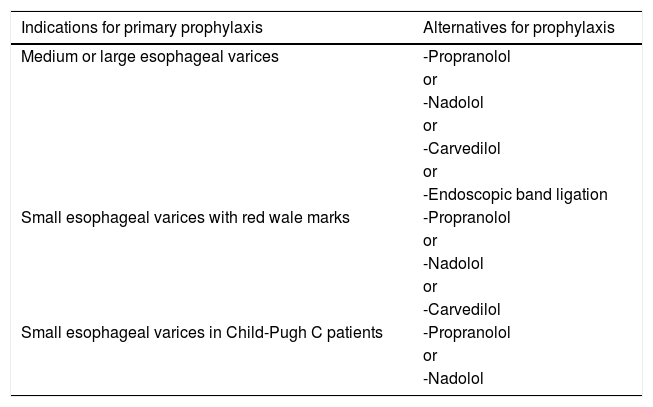
2Primary prophylaxis against variceal bleedingIn order to define which EV are clinically important to be detected, it is essential knowing when primary prophylaxis against variceal bleeding is indicated. According to the Baveno VI Consensus [1], as well as to the European Association for the Study of the Liver (EASL) Guideline [2] and to the American Association for the Study of Liver Diseases (AASLD) Guidance [4], primary prophylaxis should be recommended for patients with medium or large EV. Patients with small EV should also be submitted to primary prophylaxis if they are classified as Child-Pugh C or if their varices have red wale marks [1,4]. Table 1 summarizes the indications for primary prophylaxis, as well as the recommended alternatives for such prophylaxis.
Indications and recommended alternatives for primary prophylaxis against variceal bleeding.
| Indications for primary prophylaxis | Alternatives for prophylaxis |
|---|---|
| Medium or large esophageal varices | -Propranolol |
| or | |
| -Nadolol | |
| or | |
| -Carvedilol | |
| or | |
| -Endoscopic band ligation | |
| Small esophageal varices with red wale marks | -Propranolol |
| or | |
| -Nadolol | |
| or | |
| -Carvedilol | |
| Small esophageal varices in Child-Pugh C patients | -Propranolol |
| or | |
| -Nadolol |
Many non-invasive methods have been investigated regarding screening for EV. Some simple parameters were identified as being associated to the presence of varices: platelet count, spleen diameter, portal vein diameter, the presence of ascites, the presence of telangiectasias, prothrombin activity, albumin, alanine aminotransferase, the Child-Pugh classification, among others [5–8]. Nevertheless, their performance in predicting the existence of EV was not sufficient.
Aspartate aminotransferase-to-platelet ratio index (APRI) was also evaluated in this context. APRI was first described for the prediction of liver fibrosis in patients with hepatitis C [9]. The fact of being a predictor of liver fibrosis, which is itself related to the genesis of portal hypertension in cirrhosis, and the fact of using platelet count on its denominator, a variable independently associated to the presence of EV, have led it to be studied as a screening method for EV.
The first study to evaluate APRI for this purpose demonstrated that it was associated to the presence of EV in the univariate analysis, but not in the multivariate one [10]. Berzigotti et al. [5] demonstrated an association between APRI and clinically significant portal hypertension, but not directly to the presence of EV. When evaluating patients with compensated cirrhosis related to hepatitis C, Castéra et al. [11] found that APRI had a sensitivity of only 68% for predicting EV. In a paper by Tafarel et al. [12], APRI was independently associated to the presence of EV, but its sensitivity to predict them was even lower (56.7%). Stefanescu et al. [13] found that APRI had a sensitivity of 66.24% for predicting EV, while Wang et al. [14] found a sensitivity of 71%. We have also studied APRI in patients with cirrhosis and found a sensitivity of 64.7% and a negative predictive value (NPV) of only 43.2% for predicting EV [15]. Considering the presented evidence, one can easily realize that APRI is not a suitable method for screening for EV.
Until recently, the most studied and promising non-invasive method for screening for EV was platelet count/spleen diameter ratio (PC/SD). It was proposed by Giannini et al. [16], who identified a NPV of 100% both in a training set of 145 cirrhotic patients and in a validation cohort of 121 patients, using a cut-off point of 909 for PC/SD. According to that study, using a PC/SD above 909 to predict the absence of EV would have saved 27.4% of endoscopies in the whole sample of 266 patients. The rationale for PC/SD is correcting thrombocytopenia, which has many causes associated to liver diseases, by spleen diameter, since splenomegaly is mostly associated to portal hypertension in these patients [17]. Moreover, aside from the impressive results of the first published papers on PC/SD, this method would have the advantage of not increasing costs of the assistance of patients with cirrhosis, since both platelet count and abdominal ultrasound are already part of the routine workup of these patients.
Posteriorly, Giannini et al. [18] suggested that PC/SD could be used to follow-up patients without varices over time and tested PC/SD in a multicenter validation cohort of 218 cirrhotic patients, but their results were not as impressive as in the original study (sensitivity of 91.5% and NPV of 87.0%) [19]. At that time, an editorial questioned if the results of the multicenter validation cohort study were not biased by a significant part of patients being enrolled by the centre where PC/SD had been developed [20]. We, therefore, tested PC/SD in a cohort of 164 cirrhotic patients from a centre completely independent from that where it had been developed. In our study [21], PC/SD had a sensitivity of 77.5% and a NPV of only 42.6%, and platelet count was the only variable independently associated to the presence of EV. Considering the importance of platelet count in this context and trying to increase its weight in the index, as well as considering the possible benefit of including APRI in the formula, we also evaluated the platelet count squared/spleen diameter-aspartate aminotransferase ratio, which had a sensitivity of 95.8%, but a NPV of only 66.7% [22].
Some systematic reviews have evaluated PC/SD. In 2012, the two first meta-analyses on this issue were published and they presented contradictory conclusions [23,24]. Ying et al. [23] performed a meta-analysis of 20 studies (3063 patients), in which PC/SD had pooled sensitivity of 92%. Despite the presence of significant heterogeneity among the included studies, the authors concluded that endoscopy could be avoided according to PC/SD. On the other hand, Chawla et al. [24] only included seven studies in their meta-analysis (1169 patients), reaching a pooled sensitivity of 89%, but the evidence was considered to be of low quality and there was heterogeneity among included studies, and authors concluded that PC/SD still could not replace endoscopy for screening for EV in patients with cirrhosis. As previously highlighted, a difference between these systematic reviews that might explain the disparity in the number of included studies is that the former included papers published both in English and in Chinese, while the latter did not include articles published in Chinese [25,26].
More recently, a systematic review including 67 studies on adult patients evaluated the performance of platelet count and spleen length individually, as well as that of PC/SD for the prediction of EV. Platelet count, for a cut-off value around 120,000/mm3 had a pooled sensitivity of 77% for the prediction of any varices. Spleen length, for a cut-off value around 110mm had a pooled sensitivity of 85% for the prediction of any varices. PC/SD, for a cut-off value of 909 had a pooled sensitivity of 93% for the prediction of any varices. The hierarchical summary of receiver operating characteristic model was used to compare the three non-invasive methods of screening for EV, an PC/SD had higher overall accuracy than both other parameters used individually (p<0.001). The performance of the three methods was also evaluated regarding prediction of high-risk varices, and PC/SD performed better than the others also in this context (p<0.01), with a pooled sensitivity of 85%. Authors concluded that, considering high-risk EV, none of the studied non-invasive methods was accurate enough to replace endoscopy. On the other hand, they suggested that future assessments of new non-invasive methods for screening for varices should include a comparison with PC/SD [27].
3.2Elastographic methodsElastography both of liver and spleen has been studied for screening for EV. Regarding liver stiffness, Castéra et al. [11] prospectively evaluated 70 patients with hepatitis C-related cirrhosis and identified that liver stiffness by transient elastography had a sensitivity of 76% for screening for EV. Stefanescu et al. [13] evaluated 231 cirrhotic patients, identifying a sensitivity of 84.00% for liver stiffness measured by transient elastography and 76.16% for the Lok score. Authors suggested combining elastography and the Lok score, reaching a sensitivity of 91.42% for EV. In the study by Wang et al. [14], the sensitivity of liver stiffness by transient elastography to predict EV in 126 patients with hepatitis B-related cirrhosis was only 67%. When it was combined with APRI, the sensitivity increased to 77%. On the other hand, when considering only high-risk EV, the sensitivity of the combination reached 92%. In another study, Berzigotti et al. [28] used transient elastography to determine liver stiffness in 117 cirrhotic patients and evaluated the performance of varices risk score (VRS) and liver stiffness x spleen size/platelet count (LSPS) regarding prediction of EV. VRS had a sensitivity of 80.0%–91.9%, while LSPS had a sensitivity of 80.8%–91.9%, according to different cut-off points. As we have previously highlighted though, the authors did not present their results in an intention-to-diagnose manner, which might have influenced their results, especially considering that over 10% of patients were excluded from the training set due to failure of transient elastography [29]. In yet another study, Augustin et al. [30] evaluated 49 patients with cirrhosis and demonstrated that liver stiffness by transient elastography had a sensitivity of only 80.0%. Nevertheless, the authors highlighted that patients with liver stiffness under 25kPa, platelet count over 150,000/mm3 and normal abdominal ultrasound had very low risk of having EV.
Concerning spleen stiffness, Takuma et al. [31] measured it with acoustic radiation force impulse imaging (ARFI) and evaluated it for variceal screening in 340 patients with cirrhosis. They described a sensitivity of 98.5% for the method regarding any varices and a sensitivity of 98.9% concerning high-risk varices. In that study, spleen stiffness had higher sensitivity (p<0.001) and accuracy (p=0.025) than PC/SD. When compared to liver stiffness, spleen stiffness had similar sensitivity (p=0.564), but better accuracy (p<0.001). Singh et al. [32] published a meta-analysis evaluating spleen stiffness for variceal screening and found a pooled sensitivity of 78%, which was considered suboptimal. Regarding this systematic review, we have previously highlighted that we believe it was not appropriate to meta-analyze data from studies evaluating spleen stiffness measured by different techniques, such as transient elastography, ARFI, real-time tissue elastography and virtual touch tissue quantification. We also showed concerns regarding the use of spleen stiffness as a screening method for EV, because studies report failure rates for spleen stiffness measurement of 4.5% with ARFI [31] and 11.5% [33] or 13% [34] with transient elastography, and because it is not widely available and is expensive [35].
Some studies evaluated both liver and spleen stiffness. Colecchia et al. [33] evaluated transient elastography in 113 patients with cirrhosis caused by hepatitis C, but elastography was inconclusive in 13 individuals, and, therefore, only 100 patients were analyzed. Sensitivity for variceal screening was 96.2% for liver stiffness, 98.1% for spleen stiffness, 98.1% for LSPS and 98.1% for PC/SD.
In a similar study, Sharma et al. [34] evaluated transient elastography for predicting EV in 174 cirrhotic patients. They described sensitivities of 91%, 94%, 89%, 76% and 90% for liver stiffness, spleen stiffness, LSPS, PC/SD and the combination of liver and spleen stiffness respectively.
Liver and spleen stiffness measured by magnetic resonance elastography have also been studied. In a retrospective study of 139 patients with cirrhosis, liver stiffness had a sensitivity of 85.9% and an area under the receiver operating characteristic curve (AUROC) of 0.821 for predicting EV. On the other hand, spleen stiffness had a sensitivity of 84.6% and an AUROC of 0.833 [36]. Aside from a suboptimal performance, we believe that magnetic resonance elastography is not an ideal substitute for endoscopy because, despite being non-invasive, it is uncomfortable, time-consuming, expensive and not widely available.
Combining liver stiffness, spleen stiffness and the Lok score was evaluated in a training set of 90 compensated cirrhotic patients regarding prediction of high-risk varices. While the sensitivity of each individual parameter was 60%, 89% and 84% respectively, the sensitivity of the algorithm combining all three parameters reached 94%. The algorithm was further tested in a validation set composed by 46 other patients and had a sensitivity of 100% for high-risk varices [37].
In a pragmatic non-inferiority randomized trial, universal endoscopic screening for clinically significant esophageal or gastric varices was compared to a strategy in which endoscopy was performed only in patients who had liver stiffness above 12.5kPa or spleen stiffness above 41.3kPa. Five hundred and forty-eight patients were randomized to one of the two groups. Among patients submitted to endoscopy only according to elastography, 4.0% were diagnosed with clinically significant varices, while 5.8% of patients in the control-group were diagnosed with clinically significant varices. Even though the difference between groups did not cross the preset non-inferiority margin, the authors considered that the absolute difference in the number of clinically significant varices detected was substantially lower in the elastography-group [38]. Another issue to be considered concerning this study is that patients with liver stiffness under 12.5kPa probably did not even have cirrhosis and, therefore, would not be candidates for variceal screening. Moreover, it is noteworthy that transient elastography failed in over 10% of patients.
A systematic review aimed at evaluating liver stiffness, spleen stiffness and LSPS for variceal screening. Forty-five studies fulfilled the inclusion criteria. In this meta-analysis, log diagnostic odds ratios for screening for any varices were 3.24, 3.35 and 2.26 for spleen stiffness, LSPS and liver stiffness, and the AUROCs were 0.899, 0.851 and 0.817 respectively. Sensitivities for screening for any varices were 90.00%, 91.00% and 85.00% for spleen stiffness, LSPS and liver stiffness respectively. Sensitivities were worse when screening only for high-risk varices, and authors concluded that neither of the methods were appropriate for this purpose [39].
In 2015, a guideline published by EASL and the Asociación Latinoamericana para el Estudio del Hígado [40] stated that endoscopy could not be replaced by non-invasive methods at that moment. Nevertheless, in the same year, the Baveno VI Consensus [1] recommended that all patients were submitted to endoscopy at the time of the diagnosis of cirrhosis, unless they had a liver stiffness measurement under 20kPa and a platelet count over 150,000/mm3, a situation in which patients could spare endoscopy due to the very low risk of having varices requiring prophylaxis. According to this Consensus, this recommendation was directed to patients with virus-related compensated cirrhosis and required that patients had two elastographies performed in different days. Moreover, patients were to be followed up with elastography and platelet count every year until liver stiffness increased or platelet count decreased, which would lead to an endoscopy. In 2017, AASLD [4] endorsed this recommendation.
Some studies have addressed the association of liver stiffness and platelet count for variceal screening. The Anticipate Study [41] was a multicenter cross-sectional study, which evaluated 542 patients with compensated advanced chronic liver disease, defined by a liver stiffness measurement over 10kPa. In this study, for screening for varices in general, the AUROCs for liver stiffness, liver stiffness associated to platelet count, LSPS and PC/SD were 0.71, 0.76, 0.79 and 0.70 respectively. For high-risk varices, these values were 0.67, 0.73, 0.79 and 0.74 respectively. The sensitivities of the methods were not provided in this study. We understand the abovementioned results are insufficient to support a recommendation of using one of these tools in order to identify patients who could avoid endoscopy. Moreover, we believe that the presented data must be carefully interpreted, since not all patients with liver stiffness over 10kPa actually have cirrhosis (this cut-off point includes both patients with advanced fibrosis and patients with cirrhosis). Considering that screening endoscopy is only recommended for patients with cirrhosis, those with advanced fibrosis, but not with cirrhosis, would not be submitted to endoscopy in the first place, which would decrease the number of endoscopies saved by the proposed criteria. Moreover, including patients before they develop cirrhosis probably reduced the prevalence of EV in the studied sample, which could have falsely increased the NPV of the evaluated non-invasive criteria.
Maurice et al. [42] described a similar cross-sectional study, in which 310 patients with liver stiffness over 10kPa were enrolled. Liver stiffness had sensitivity of 67%, NPV of 97% and AUROC of 0.686 for high-risk varices. Platelet count had sensitivity of 60%, NPV of 96% and AUROC of 0.599. The criteria proposed by the Baveno VI Consensus had sensitivity of 87%, NPV of 98% and AUROC of 0.746, but still missed 13% of patients with high-risk varices. As we have previously highlighted, in this study too, non-cirrhotic patients were probably included, given that the cut-off point of liver stiffness of 10kPa identifies patients with advanced fibrosis and not exclusively those with cirrhosis [43]. The authors of the study recognized that this was a limitation and could probably explain the high NPVs found [42]. Another limitation of this study according to our understanding is that authors did not consider small varices with red wale marks as high-risk varices, which impairs the interpretation of its results, since patients with such varices actually should be submitted to primary prophylaxis [43].
Ding et al. [44] also evaluated the role of liver stiffness and platelet count for ruling out EV. They enrolled 71 patients, but all of them had liver stiffness over 13.6kPa, which seems much more adequate, since this cut-off refers to cirrhosis, at least considering hepatitis C, which comprised most of their sample. Only patients classified as Child-Pugh A were included. The association of liver stiffness and platelet count had an AUROC of 0.87 for high-risk varices and 0.73 for any varices. Differently from the Baveno VI recommendation, the optimal threshold for excluding high-risk varices in this study consisted of a combination of liver stiffness of 25kPa or less and platelet count over 100,000/mm3. This strategy was validated in further 200 patients in the same study, but the AUROC was somewhat smaller.
In another study in which the Baveno VI recommendation for non-invasive screening for EV was evaluated, 161 American patients and 101 Italian patients with liver stiffness over 10kPa were analyzed. Considering the prediction of high-risk varices, the Baveno VI criteria had sensitivity of 100% and NPV of 100% in the American cohort, in which 25.5% of endoscopies would have been avoided. Regarding the Italian cohort, sensitivity and NPV were also 100%, but only 15.8% of endoscopies would have been avoided. Then, authors suggested another strategy to screen for high-risk varices, using platelet count over 150,000/mm3 or a Model for End-stage Liver Disease (MELD) score of 6. In the American cohort, these criteria had sensitivity and NPV of 100% and led to the avoidance of endoscopy in 54% of patients. In the Italian group, sensitivity was 94%, NPV was 97%, but only 30% of patients would have avoided endoscopy. Such criteria were further validated in another set of 146 patients, who had been diagnosed with compensated cirrhosis on clinical grounds (not with elastography). In this group, the platelet/MELD criteria had sensitivity of 97% and NPV of 98%, leading to the avoidance of endoscopy in 27% of cases [45].
Augustin et al. [46] also studied the Baveno VI criteria for the screening of EV and they tried to expand these criteria. First, the authors retrospectively evaluated 499 patients from the cohort of the previously cited Anticipate Study and demonstrated that the Baveno VI criteria would miss 3% of high-risk varices and would allow sparing 14% of endoscopies. On the other hand, if endoscopy were avoided in patients with platelet count over 110,000/mm3 and liver stiffness under 25kPa (Expanded-Baveno VI criteria), 1.9% of high-risk varices would be missed and 32% of patients would spare endoscopy. Posteriorly, they validated their findings in two other cohorts from Barcelona (117 patients) and London (309 patients). Considering the whole sample of 925 patients, the Expanded-Baveno VI criteria would miss 1.6% of high-risk varices and would allow to avoid 40% of endoscopies. Authors also evaluated adding MELD=6 criterium to both Baveno VI and Expanded-Baveno VI criteria, demonstrating a slight improvement in the performances of both methods.
In a post hoc analysis of yet another study, the Baveno VI recommendation was also evaluated. In 127 cirrhotic patients, the criteria had sensitivity of 90.9% and NPV of 95.5% for the prediction of high-risk varices [38].
In a quite recent publication, Thabut et al. [3] evaluated 891 patients with virus-related compensated cirrhosis (diagnosed by liver biopsy) regarding the role of the Baveno VI criteria in predicting EV. Among the 221 patients who fulfilled Baveno VI criteria, 92.8% did not have EV, 5.9% had small EV and 1.3% had medium or large varices. On the other hand, among the 670 who did not fulfil the Baveno VI criteria, 69.6% did not have EV, 20.1% had small EV and 10.3% had medium or large varices (p<0.001, when compared to patients fulfilling the Baveno VI criteria). Similar findings were presented when the analysis was restricted to patients who had viral suppression, which was an original contribution of the study.
A systematic review with meta-analysis also evaluated the association of liver stiffness and platelet count for predicting EV. Fifteen studies were included, but not all of them used the cut-off points recommended by the Baveno VI Consensus. Patients with lower liver stiffness and higher platelet count had lower odds of having high-risk varices than those with higher liver stiffness and lower platelet count (odds ratio of 0.22, p<0.001). The same was true for varices of any kind (odds ratio of 0.23, p<0.001). According to this meta-analysis, the pooled estimate rates for high-risk varices and for any varices among patients with low liver stiffness and normal platelet count was 4% and 15% respectively [47].
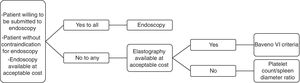
4ConclusionConsidering all that was reviewed, we see a pressing need for noninvasive methods for variceal screening in cirrhotic patients, but we believe that the available evidence still has relevant limitations. Moreover, elastographic methods are not as widely available and inexpensive in many countries as an ideal screening strategy should be. Therefore, we understand that physicians should decide on screening cirrhotic patients for EV with endoscopy or non-invasive methods on a case-by-case basis, considering the preferences of each patient as well as regional particularities. In this context, we propose an algorithm which could contribute with decision-making (Fig. 1).AbbreviationsEV
esophageal varices
EASLEuropean Association for the Study of the Liver
AASLDAmerican Association for the Study of Liver Diseases
APRIaspartate aminotransferase-to-platelet ratio index
NPVnegative predictive value (NPV)
PC/SDplatelet count/spleen diameter ratio
VRSvarices risk score
LSPSliver stiffness x spleen size/platelet count
ARFIacoustic radiation force impulse imaging
AUROCarea under the receiver operating characteristic curve
MELDModel for End-stage Liver Disease
This paper did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict-of-interestThere are no conflicts of interest.