Many hepatoprotective herbal preparations have been recommended in alternative systems of medicine for the treatment of hepatic disorders. No systematic study has been done on protective efficacy of Leucophyllum frutescens to treat hepatic diseases. Protective action of L. frutescens methanol extract (obtained by maceration) was evaluated in an animal model of hepatotoxicity induced by carbon tetrachloride (CCl4). Wistar albino rats were divided into five groups. Group I was normal control group; Groups II-V received CCl4. After inducing hepatic damage, Group II served as control CCl4; Group III was given silymarin as reference hepatoprotective; and Groups IV and V received different doses of plant extract. Liver marker enzymes were assayed in serum. Samples of livers were observed under microscope for the histopathological changes. Levels of marker enzymes such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were increased significantly in CCl4 treated rats (Group II). Groups IV and V intoxicated with CCl4 and treated with L. frutescens methanol extract significant decreased the activities of these two enzymes. Also these groups resulted in less pronounced destruction of the liver architecture, there is not fibrosis and have moderate inflammation compared with Group II. The present study scientifically validated the traditional use of L. frutescens for liver disorders. In conclusion the methanol extract of L. frutescens aerial parts could be an important source of hepatoprotective compounds.
Liver diseases such as cirrhosis, fatty liver, and chronic hepatitis are important world health issues. The effectiveness of the treatment with interferon, colchicine, penicillamine, and corticosteroids is inconsistent and had profound side effects.1 There is the need of effective therapeutic agents. Traditional herbal medicine has been established over thousands of years and is based on experience and practice. For these reasons, developing drugs for liver diseases from plants used in traditional medicine, may lead to improve therapies.2,3
There are approximately 1000 higher plants used in Mexican traditional medicine, and only 20% have been studied chemically and biologically.4Leucophyllum frutescens (Berl.) I.M. Johnst (Scrophulariaceae) is native of Texas, New México, and northern Mexico. It is now widely cultivated in Florida and Southeast Asia, where it flowers magnificently in steamy tropical weather. L. frutescens is used to alleviate fever, cough, asthma, and rheumatic pain. It is also used to treat liver and bladder disorders in México.5 To our knowledge there is only one phytochemical study of L. frutescens. This study revealed the presence of phytotoxic furofuran lignans called diayangambin, epiyangambin, diasesartemin, and epiashantin.6 In order to validate the use of this plant in the Mexican traditional medicine as hepatoprotector we evaluate the effect of L. frutescens methanolic extract against CCl4 induced hepatotoxicity in rats.
Experimental proceduresPlant materialThe aerial parts of the plant were collected at Lampazos de Naranjo, Nuevo León, Mexico in May 2003. The plant was authenticated by M.C. Mauricio González Ferrara. The reference number (24759) was given by Dr. Marcela González and a sample was deposited at the Herbarium UNL located in Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, México.
Plant extractThe plant material was air dried and pulverized in a Wiley Mill. One kg of powdered plant material was extracted exhaustively with methanol at room temperature. The extract was filtered and distillated under reduced pressure. The yield of the extract was 2.6 per cent w/w of powdered methanol extract, which was stored in refrigerator for further use.
AnimalsAdult male albino rats of the Wistar strain weighing 200 g were used in the experiments. The animals had free access to normal standard chow diet (Rodent Laboratory Chow 5001-PMI Purina) and tap water. Throughout the experiment, the animals were housed, three or four per cage, in laminar flow cages maintained at 22 ± 2 °C, 50-60% relative humidity, under a 12 h light-dark cycle. The animals were kept in these facilities for at least one week before the experiment. Animal care and treatment was carried out in accordance with the Guiding principles for the production, care and use of laboratory animals of the Mexican Official Norm N0M-062-Z00-1999 published in the Federal Official Diary on 22 August 2001. All protocols used in this study were approved by the ethics committee of Centro de Investigaciones Biomedicas del Noreste del Instituto Mexicano del Seguro Social.
Animal treatmentAnimals were randomly divided into 5 groups (I-V), each having 6 animals. All groups received 1 ml of corn oil orally (by a feeding needle) twice a week for 28 days. Group I served as normal control. For inducing hepatotoxicity (in vivo), animals of Groups II, III, IV and V were administered orally 2 ml/kg body weight of carbon tetrachloride (mixed with an equal volume of corn oil) twice a week for 50 days.7 After CCl4 intoxication, Group II served as control CCl4 Group III served as positive control and was given silymarin (20 mg/kg in water) three times a week for a period of 28 days. Group IV and Group V were administered orally the plant extract diluted in water at a dose of 100 and 200 mg/kg body weight, respectively, three times a week for a period of 28 days.
Assessment of liver damageAfter completing the treatment, rats were anesthetized with pentobarbital (50 mg/kg, ip), the abdomen of each was cut opened and the blood was collected from the aorta artery. Blood samples were kept at room temperature for 30 min and centrifuged at 2000 rev min-1 for 15 min to obtain serum, which was kept at -20 °C until further assay. Liver damage was assessed by the estimation of serum activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using commercially available test kits from Johnson-Johnson. The livers were preserved in neutral buffered formalin and were processed for paraffin embedding, following the standard microtechnique. Five micron sections of livers, stained with haematoxylin and eosin as well as trichromic Mason’s technique were observed under microscope for the histopathological changes.
Statistical analysisAll values are expressed as means ± SEM. Comparison between any two groups was performed using Student´s t- test. Statistically significant differences between groups were defined as P< 0.05. Calculations were performed with the Microsoft Excel TM program.
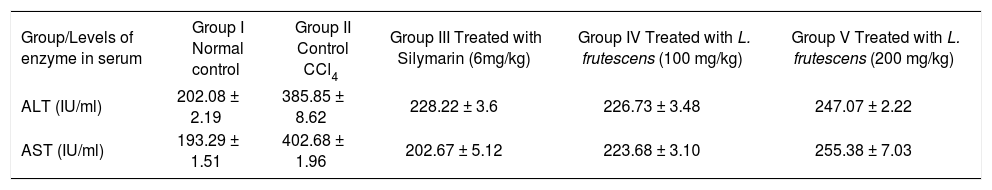
ResultsResults in Table I revealed a significant elevation of serum AST and ALT activity in CCl4 treated group as compared to normal controls (p < 0.05), indicating that CCl4 induced damage to the hepatic cells. A significant (p < 0.05) reduction was observed in AST and ALT in the groups treated with methanol extract of L. frutescens in comparison with those observed in the control CCl4 treated group, though the decrease was maximum (p < 0.05) in group rats which received a dose of 100 mg/kg of the methanol extract of L. frutescens. These results suggested the possibility of the extract to give protection against liver injury upon CCl4 induction.
Effect of L. frutescens methanol extract on the activity of serum enzymes in rats.
| Group/Levels of enzyme in serum | Group I Normal control | Group II Control CCl4 | Group III Treated with Silymarin (6mg/kg) | Group IV Treated with L. frutescens (100 mg/kg) | Group V Treated with L. frutescens (200 mg/kg) |
|---|---|---|---|---|---|
| ALT (IU/ml) | 202.08 ± 2.19 | 385.85 ± 8.62 | 228.22 ± 3.6 | 226.73 ± 3.48 | 247.07 ± 2.22 |
| AST (IU/ml) | 193.29 ± 1.51 | 402.68 ± 1.96 | 202.67 ± 5.12 | 223.68 ± 3.10 | 255.38 ± 7.03 |
n = 6 rats in each group. Results given as mean ± SEM. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
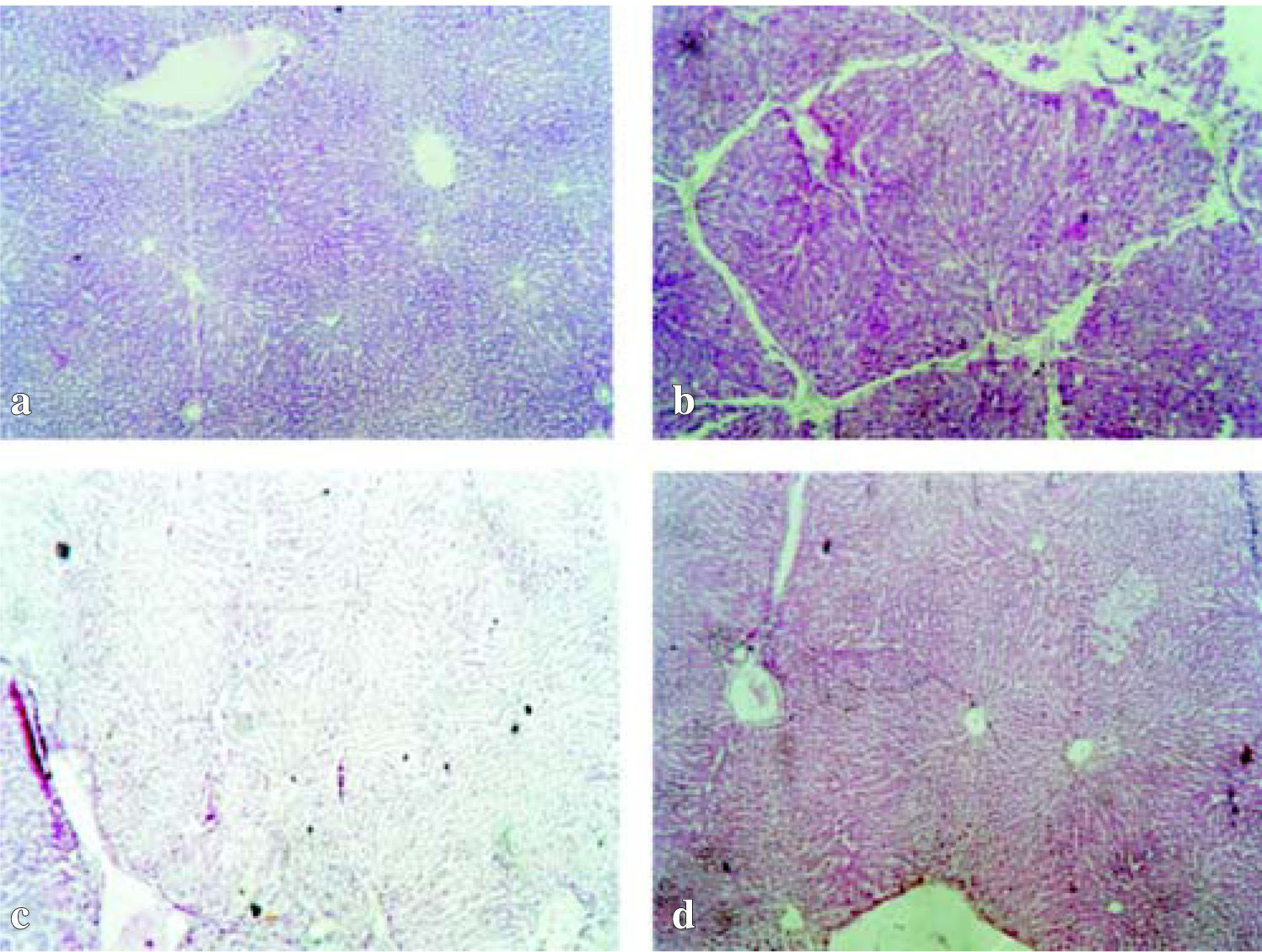
Histopathological studies also provided supportive evidence for the biochemical analysis. Normal control group showed a normal liver architecture, hepatocytes very well arranged, central and portal veins without alterations (Figure 1a). The livers of rats treated with CCl4 for 50 days showed extensive accumulation of connective tissue resulting in formation of continuous fibrotic septa, nodules of regeneration, fatty changes, noticeable alterations in the central vein and pronounced inflammation compared to the normal control (Figure 1b). The group intoxicated with CCl4 and treated either with 100 mg/kg or 200 mg/kg of L. frutescens resulted in less pronounced destruction of the liver architecture without fibrosis and moderate inflammation (Figure 1c, 1d).
DiscussionLiver is an important organ actively involved in metabolic functions and is a frequent target of number of toxicants. Carbon tetrachloride has been widely used for inducing experimental hepatic damage due to free radical formation during its metabolism by hepatic microsome, leading to lipid peroxidation, and consequently, liver damage. The resulting hepatic injury is characterized by leakage of cellular enzymes into the blood stream and by necrosis and fibrosis.8-10
The efficacy of any hepatoprotective drug is essentially dependent on its ability in reducing the harmful effects or maintaining the normal hepatic physiology that has been disturbed by a hepatotoxin. In the present study, we observed that administration of 100 or 200 mg/Kg of L. frutescens methanolic extract to rats decreased the CCl4 induced elevated enzyme levels in treated groups (IV and V). This suggests the maintenance of structural integrity of hepatocytic cell membrane or regeneration of damage liver cells by the extract which was observed in the histopathological study. In conclusion, the present study demonstrated that methanol extract of L. frutescens has hepatoprotective effect in CCl4 induced liver damage. However, it is necessary to determine other parameters such as oxidative stress markers and molecular biology assays to confirm our findings. Also, we need to isolate and purify the active principle involve in the hepatoprotective activity of this plant and to determine its mechanism of action.
Acknowledgments:We greatly appreciate the assistance of Dr. Ricado Fuentes-Pensamiento, Laboratorio de Morfologia e Histologia, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León for histopathological analysis of the livers.