Background. Eplerenone is a selective mineralocorticoid receptor (MR) antagonist, and its potential protective role in cardiovascular injury has been reported by several studies. However, whether and how this drug can ameliorate hepatic injury in rats is unknown.
Material and methods. The present study was conducted to investigate effect of eplerenone against liver injury induced by carbon tetrachloride (CCl4) in rats. The biochemical liver function tests and oxidative stress parameters including malondialdehyde (MDA), reactive oxygen species (ROS), in addition to the reduced glutathione (GSH) levels were evaluated. Moreover, serum tumor necrotic factors (TNF-α) level and histopathological changes were examined.
Results. Our results show that pre-treatment with eplerenone (4 mg/kg per day for 4 weeks) revealed attenuation in serum activities of alanine aminotransferase (ALT), aspartate aminotransferase, (AST), alkaline phosphatase (ALP) and bilirubin levels that were enhanced by CCl4. Further, pre-treatment with eplerenone inhibited the elevated hepatic MDA content and restored hepatic GSH to its normal level. The enhanced hepatic ROS production in CCl4-treated group was markedly decreased by eplerenone administration. Eple-renone pre-treatment significantly attenuated the inflammatory responses caused by CCl4 as evident by the decreased serum TNF-α level. Histopathological studies showed that eplerenone alleviated the liver damage and reduced the lesions caused by CCl4.
Conclusion. Collectively, the present study provides a proof to hepatoprotective actions of eplerenone via reducing oxidative stress and inflammatory responses in CCl4-induced liver damage in rat model.
Aldosterone is the main effector peptides of the renin-angiotensin-aldosterone system and is known to play an important role in the development of hepatic fibrosis and portal hypertension.1-3 Aldosterone can promote tissue inflammation and fibrosis and plays a crucial role in cell proliferation and apoptosis.4-6 In addition, oxidative stress and pro-inflammatory cytokines play essential roles in the development of cholestatic liver injury.7 It has been reported that induction of oxidative stress and mineralocorticoid receptor (MR)-dependent transcription of proinflam-matory genes are some of the mechanisms account for the injurious effects of aldosterone.8-10 Accumulating evidences indicate that MR also mediates inflammation and fibrosis through the proinflammatory transcription factor NF-kB activation in liver, heart, and glomerular mesangial cells.11-13 Although several reports stated that angiotensin ll type 1 receptor blockers and angiotensin-converting enzyme inhibitors have been reported to prevent the development of hepatic fibrosis in numerous animal14,15 and human,16-18 little is known regarding role of al-dosterone antagonist in liver injury.
Additionally, the results arising from these studies have shown to be controversial.2,19,20 While one report demonstrated that spironolactone prevented pig serum-induced hepatic fibrosis in rats,2 another study showed that spironolactone did not produce any anti-fibrotic effects during bile duct ligation-in-duced hepatic fibrosis.20
On the other hand, eplerenone has been shown to reveal a high specificity for MR.21,22 Several reports demonstrated the efficacy and safety of eplerenone in the treatment of hypertension.23-25 However, there is scanty information concerning both effect of eplerenone on liver injury and the mechanism (s) involved in this effect. The preventive action of CCl4-induced liver damage has been widely used as an indicator of liver protective activity of drugs.26 Therefore, the present study was undertaken to examine effect of eplerenone on liver injury induced by CCl4 in rat model and underline its mechanism (s) in this setting.
Material and MethodsDrugs and chemicalsEplerenone, Lucigenin, NADPH oxidase and SOD (Sigma-Aldrich, St. Louis, MO, USA); TNF-α kit (Koma Biotech. Inc., Korea); Carbon tetrachloride (BDH Chemicals, England). All other chemicals are of analytical grade.
AnimalsAdult male Sprague-Dawley rats (150-180 g) were used throughout the experiments. Rats were fed a standard diet of commercial rat chow and tap water ad libitum and left to acclimatize to the environment for at least one week prior to inclusion in the experiments. Experiments were conducted in accordance with the guidelines for animal care of the United States Naval Medical Research Centre, Unit No. 3, Abbaseya, Cairo, Egypt.
The animals were divided into 4 groups each of 8 rats:
- •
Group 1. Rats of this group were injected with normal saline, intraperitoneally and served as a control.
- •
Group 2. Rats were treated with (4.0 mg/kg, orally) of eplerenone for 4 weeks.27
- •
Group 3. Hepatotoxicity was induced in rats of this group by administration of CCl4 (1 mL/kg ip; diluted 1:4 in olive oil; twice per week for 4 weeks).
- •
Group 4. Rats were pretreated with (4.0 mg/kg, orally, 4 week) of eplerenone 1 h before CCl4 (1 mL/kg, ip) administration.
After 4 weeks, blood samples were collected via cardiac puncture from the rats under anesthesia.
Then the rats were sacrificed by decapitation and liver tissues were isolated. The blood samples were centrifuged at 5,000 g for 10 min, and serum samples were collected for biochemical tests. All liver tissues were immediately dissected out and immediately dipped into liquid nitrogen. Other liver tissues were fixed in 10% neutral buffered formalin for subsequent sectioning and mounting on microscope slides. The remaining liver tissue and serum samples were stored at-80°C until studied for other parameters. Thereafter, the liver tissues were homogenized in 0.25 M sucrose, 10 mM Tris-HCl, 1 mM EDTA medium, pH 7.4, centri-fuged at 10,000 g for 10 min and supernatants used for biochemical analysis. Total protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (Pierce Chemicals, USA).
Assessment of serum aminotransferases, alkaline phosphatase and bilirubinLiver function tests in the serum such as AST, ALT were determined according to the method described by Reitman and Frankel.28 Serum ALP was determined according to the method of Eaton RH29 using colorimetric kit obtained from Diamond Co., Egypt. Total or direct serum bilirubin was determined spectrophotometrically according to the method of Merino, et al.30 using kits purchased from Ran-dox, Laboratory. Ltd., UK.
Determination of hepatic malondialdehyde and GSH levelsLipid peroxidation was determined in liver homo-genates as thiobarbituric acid reactive species (TBARS; sometimes referred to as MDA as a marker of oxidative stress according to the method described by Buege and Aust).31 Additionally, hepatic GSH was determined spectrophotomerically at 412 nm. Hepatic GSH values were expressed as nmol/mg protein.
Measurement of ROS production in liver homogenatesROS production of liver homogenates were measured using lucigenin-enhanced chemiluminescence assay.32 Briefly, liver homogenates were resuspen-ded (100 /μg protein/well) in modified HEPES buffer containing (mM) NaCl 140, KCl 5, MgCl2 0.8, CaCl2 1.8, Na2HPO4 1, HEPES and 1% glucose, pH 7.4. Immediately before recording chemiluminescence, NADPH (final concentration 100 μM) was added and dark-adapted lucigenin (5 μM) was added via an auto-dispenser. Light emission was recorded using a luminometer (Perkin-Elmer, Ltd.UK) and expressed as mean arbitrary light units/min over 20 min. Each experiment was performed in triplicate. To verify the specificity of chemiluminescence signals, some experiments using tissue homogenates were pre-incu-bated with superoxide dismutase (SOD, 200 U/mL) before measurement of chemiluminescence.
Determination of serum TNF-α levelSerum TNF-α level was assessed in this study using enzyme-linked immunosorbent assay (ELISA) using a microplate reader (Spectra lll, Austria). Results are expressed as pmol/mL.
Histopathological examinationParts of liver samples were kept in 10% buffered formaldehyde for histophathological examinations. Sections were cut on glass slides (4-6 thick) and stained with hematoxylin and eosin (H & E) for routine histomorphology. The extent of liver damage was assessed.
Statistical analysisAll results are expressed as mean ± SEM. Biochemical parameters of groups were compared by ANOVA for repeated measures followed by Bonferroni’s test. P value < 0.05 was accepted as statistically significant. Statistical analysis was performed by Graph pad Prism, version 5.
ResultsBiochemical serum parameters (ALT, AST, ALP and bilirubin)Biochemical results of serum ALT, AST, ALP, total and direct bilirubins are summarized in table 1.
Biochemical results of serum ALT, AST, ALP, total and direct bilirubin in rats treated with CCl4 in the presence or absence of eplerenone.
| Group/parameters | ALT (IU/L) | AST (IU/L) | ALP (IU/L) | Total-bilirubin (umol/L) | Direct-bilirubin (umol/L) |
|---|---|---|---|---|---|
| Control | 41 ± 3.8 | 102 ± 9 | 200 ± 12 | 0.65 ± 0.05 | 0.25 ± 0.01 |
| Eplerenone | 50 ± 4.3 | 115 ± 10 | 222 ± 15 | 0.74 ± 0.01 | 0.30 ± 0.02 |
| CCl4 | 190 ± 10** | 320 ± 22** | 334 ± 24** | 2.10 ± 0.09** | 0.71 ±.0.03* |
| Eplerenone + CCl4 | 70 ± 4.5* | 150 ± 8* | 257 ± 14* | 0.92 ± 0.04 | 0.35 ± 0.01* |
Each value represents the mean ± SEM, (n = 7-9/group). Data comparison was performed using ANOVA followed by Bonferroni’s test.
* P < 0.05;
** P < 0.01; compared with the initial value of control; *P < 0.05 compared with CCl4 group as indicated.
As major criteria for liver dysfunction, serum ALT and AST, ALP total and direct bilirubin levels in the serum of rats treated with CCl4 were found to be significantly higher than control. CCl4 produced dramatic changes in these parameters. However, pre-treatment with eplerenone reduced these values significantly compared to that of the control group (Table 1).
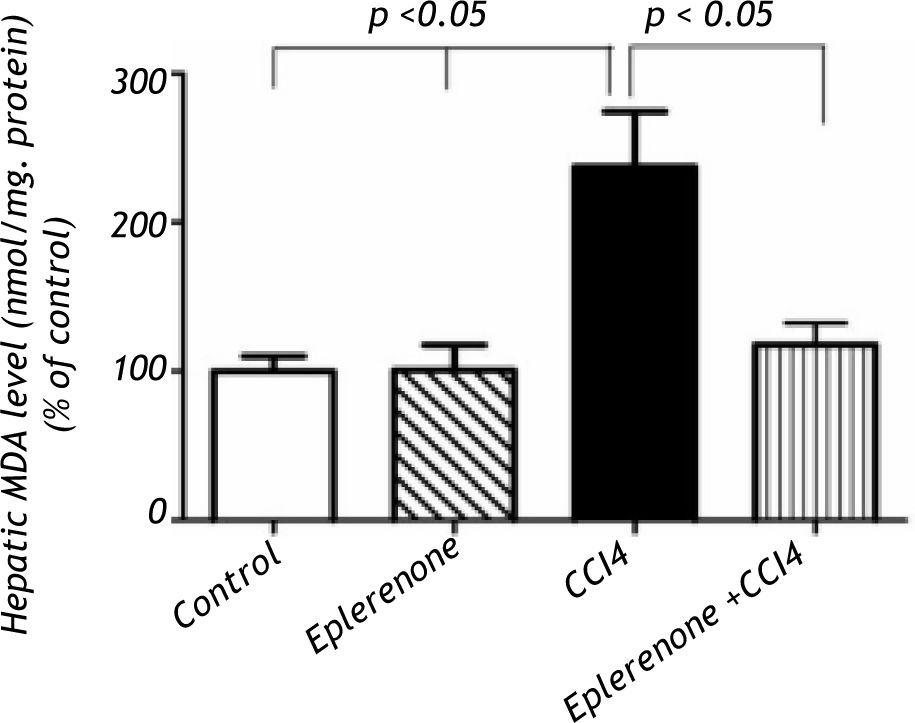
Effects of eplerenone on oxidative stress biomarkers: MDA, GSH contents and ROS production in liver homogenatesThe biochemical marker of oxidative stress; hepatic MDA contents were significantly increased following to CCl4 treatment (P < 0.05) and pre-treatment of rats with eplerenone markedly (P < 0.05) decreased these levels to that of control (Figure 1).
Hepatic MDA levels in the study groups: control and CCl4-treated groups, in the absence or presence of eple-renone. Values of each bar represent the mean ± SEM. The hepatic MDA contents were significantly increased following to CCl4 treatment (P < 0.05) and pre-treatment of rats with eplerenone significantly (P < 0.05) decreased these levels to that of control, *p < 0.05 compared with control value.
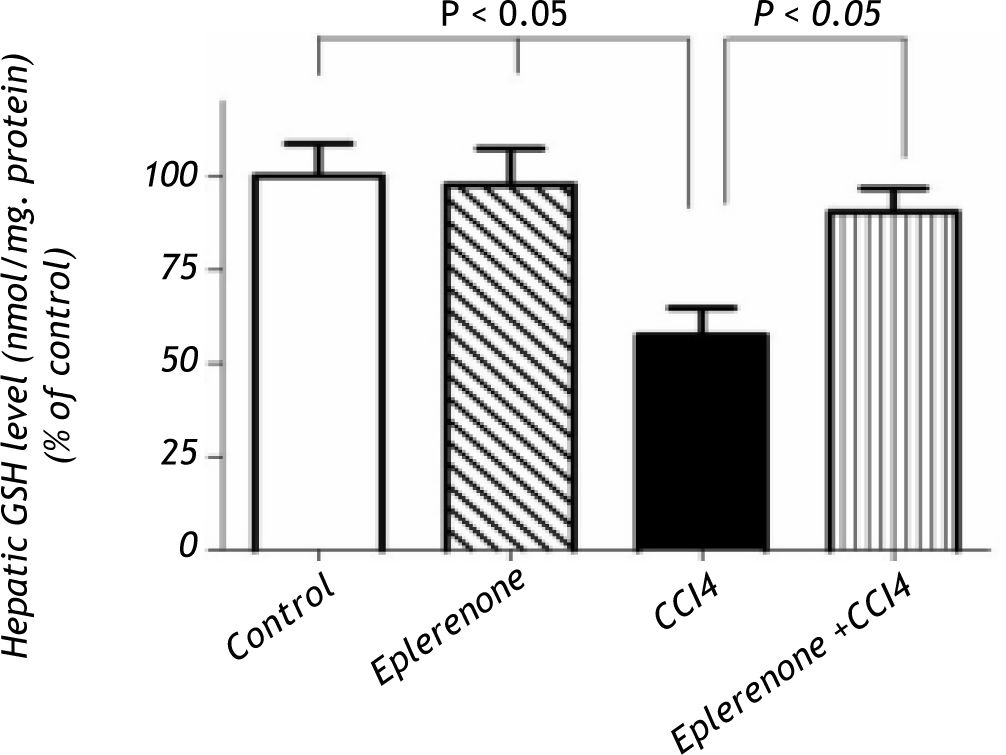
In addition, CCl4 showed a significant decrease in the hepatic GSH levels; however, eplerenone restored GSH almost to its control level (Figure 2).
Hepatic GSH content determination: control and CCl4-treated groups, in the absence or presence of eplerenone. Values of each bar represent the mean ± SEM. CCl4 showed a significant decrease in the hepatic GSH levels and eplerenone restored GSH almost to its control level restored GSH almost to its control level.
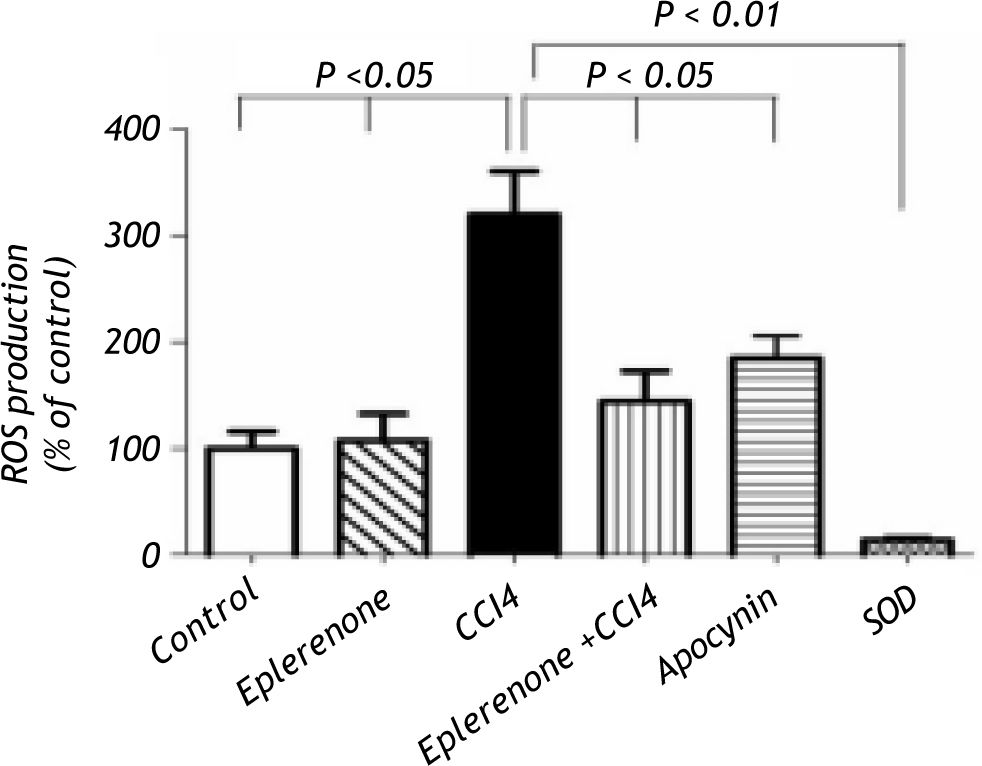
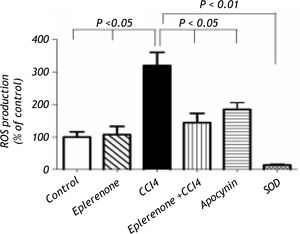
Figure 3, this figure illustrates that eplerenone inhibited ROS production. ROS production was measured by the chemiluminescence enhanced by lucigenin (5 μM) and was marked in liver homogenates of rats treated CCl4. Additionally, apocynin as NADPH oxidase inhibitor, significantly inhibited ROS generation in CCl4-induced liver injury nearly to the control level. To verify the specificity of the chemiluminescence signals enhanced by lucigenin, separate series of experiments using tissue homoge-nates were performed by incubation with SOD (200 U/mL) for the indicated time, and the signal was completely inhibited.
ROS production assessment: control and CCl4-treated groups, in the absence or presence of eplerenone. Values of each bar represent the mean ± SEM. ROS production measured by lucigenin-enhanced chemiluminescence (5 μM) and expressed as arbitrary light unit (percentage of control). ROS production was markedly increased following to CCl4 treatment and eplerenone as well as apocynin inhibited this enhanced ROS generation. In a separate experiment tissue ho-mogenates were incubated with SOD (200 U/mL) for the indicated time, and the signal was completely inhibited. Values of each bar represent the mean ± SEM. *P < 0.05; **P < 0.01 compared to control values.
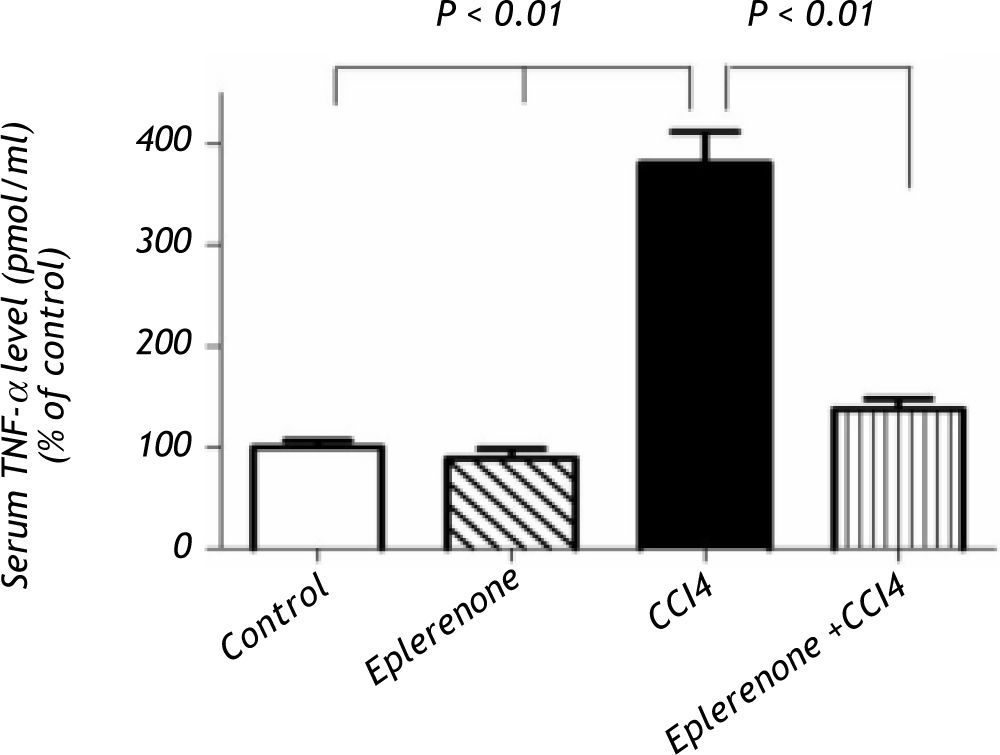
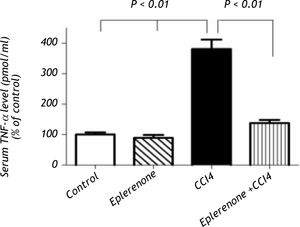
CCl4 produced a significant increase in serum TNF-a level, while pretreatment with eplerenone inhibited this change (Figure 4).
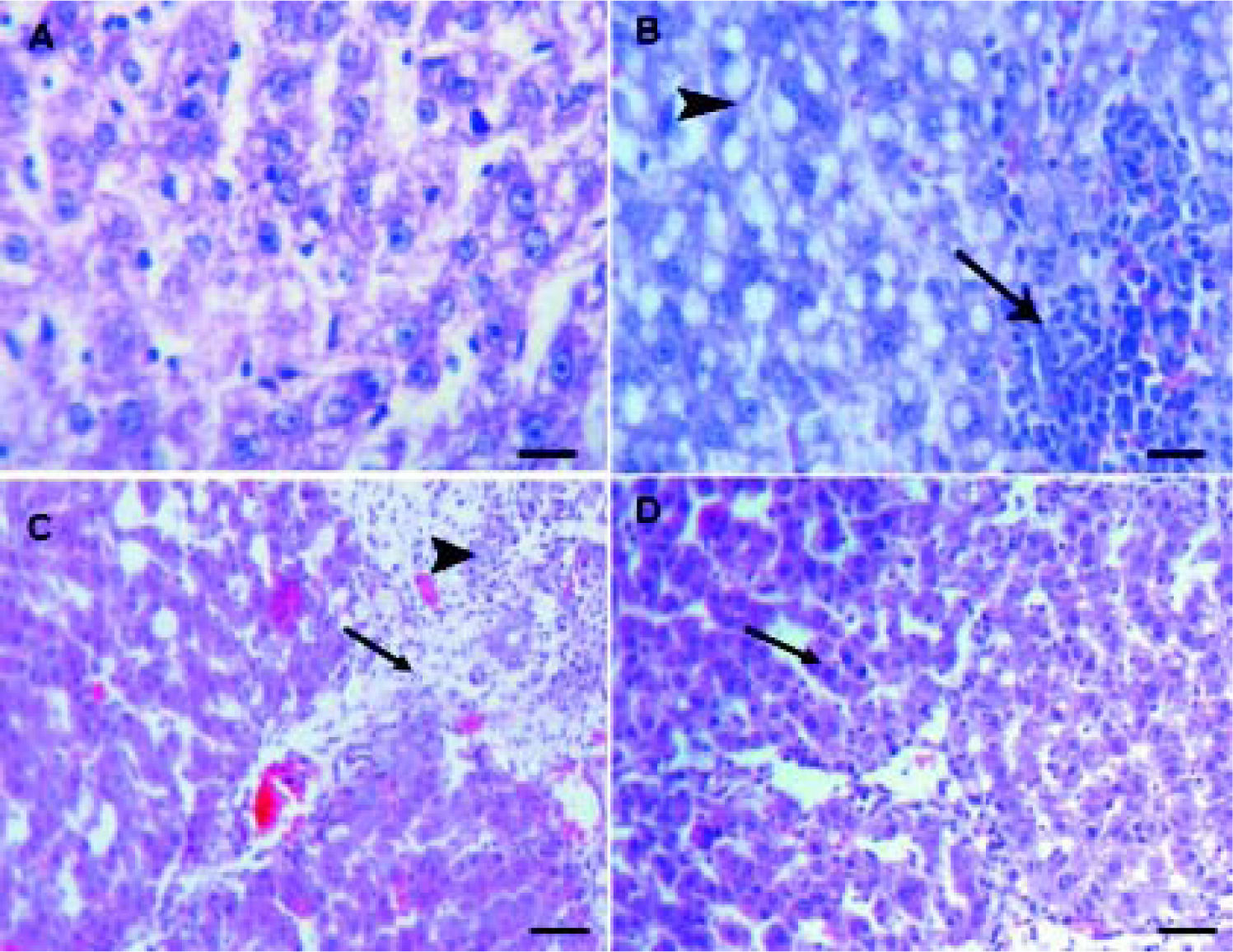
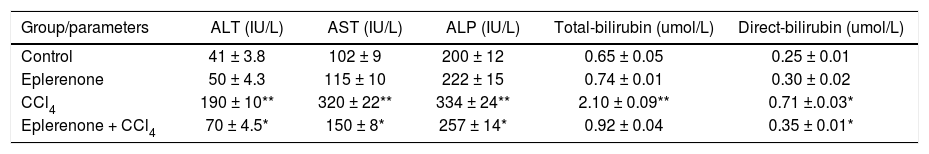
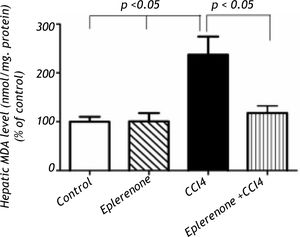
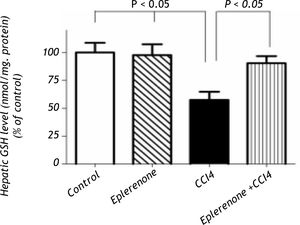
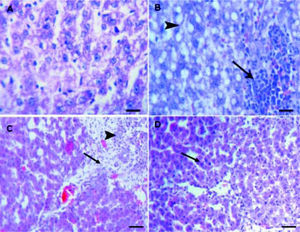
Histopathological examinationHematoxylin and eosin histological slides were evaluated by a blinded investigator and analyzed using a semi-quantitative score for inflammation. In control group, there were no pathological changes in healthy control livers which showed normal lobular architecture with central vein and radiating hepatic cords (Figure 5).
A.Liver of healthy control group showing normal lobular architecture with central vein and radiating hepatic cords. B. CCl4 showing portal fibrosis (arrows), biliary hyper-plasia (arrow-heads) and moderate around cell infiltration in the portal areas. C. Liver of CCl4 showing diffuse fatty change (arrowheads) and mo-nonuclear cell infiltration (arrow). D. The lesions in the liver of CCL4 group pretreated with eplerenone were decreased except for congestion of some portal blood vessels, and few cells with vacuolated cytoplasm, original magnification x 300; scale bar = 50 μn.
The histological examination of the liver showed distorted architecture with distorted central vein and multifocal areas of coagulative necrosis along the hepatic parenchyma in CCl4-treated group. The lesions in the liver of CCl4 group pre-treated with eplerenone were attenuated except for congestion of some portal blood vessels, and few cells with vacuo-lated cytoplasm.
DiscussionCCl4 has been widely used to induce experimental hepatic damage as it induces liver cell necrosis and apoptosis, and can be used to induce hepatic fibrosis or cirrhosis by its repetitive administration.33 We verified the hepatotoxicity of CCl4 by the increased serum aminotransferases and decreased albumin levels compared to that of the control group. Raised serum enzyme levels in CCl4-injected rats can be attributed to the damaged hepato-cellular structural integrity.34 Here, we try to investigate the relevance of using the MR antagonist, eplerenone in hepato-protection of CCl4-induced liver injury and the underlying mechanism of this protection. Of interest, eplerenone administration not only restored the serum aminotransferases to that of control levels but also the serum albumin level as well.
It is noteworthy that oxidative stress and lipid pe-roxidation mediated by oxygen free radicals has been implicated as a common link between chronic liver damage and hepatic fibrosis.35 Reactive oxygen metabolites are shown to mediate microvascular disturbances by various chemical substances.36
During the initial phase of CCl4 toxicity following its administration, a large amount of CCl4 is converted to trichloromethyl radical or other radicals, which in turn accelerate several metabolic pathways.37 These radicals appear to affect the adjacent lipids in the tissues and induce lipid peroxidation.
Hepatocytes are well recognized as being continuously exposed to ROS in various liver diseases including cholestasis. Antioxidant molecules such as GSH and anti-oxidative enzymes such as SOD, and catalase, ordinarily provide hepatocytes with resistance to oxidative stresses.38 In the present study, administration of CCl4 resulted in marked elevation of oxidative stress markers as evident by increased lipid peroxidation product MDA, ROS production and reduction in antioxidant molecule contents such as GSH. These findings suggest that oxidative stress reactions might be an important contributing factor for the CCl4-induced liver dysfunction. It is well established that oxidative stress and lipid peroxidation are involved in the pathogenesis of liver injury.39
On the other hand, aldosterone can increase oxi-dative stress by both increasing ROS production and reducing ROS scavenging capacity of the cells.40
A reduction in antioxidant capacity can also participate in the increase in oxidative stress induced by aldosterone since we have observed that eplerenone is able to increase glutathione levels in hypertensive rats, the most important systemic antioxidant agent.41 Interestingly, the current study revealed that eplerenone was able to normalize the elevated biochemical oxidative stress markers, ROS production and restore GSH normal level that plays an important role in the antioxidant defence mechanism in liver of CCl4-treated rats, suggesting antioxidant properties of eplerenone. Hence, the hepatoprotective effect of eplerenone appears to be due to the suppression of oxidative stress and lipid peroxidation.
Recently, it has been demonstrated that spironola-ctone decreased aldosterone-induced oxidative stress via activation NADPH oxidase enzyme as a major source of ROS thereby reducing cardio-vascular-renal damage.42 According to this concept, an eligible explanation to the inhibitory effect of eplerenone on ROS production may be, at least, owing to inhibition of NADPH oxidase enzyme. This assumption is evident by the ability of the NADPH oxidase inhibitor, apocynin to inhibit ROS generation-induced by CCl4 in liver homogenates.
An increasing number of evidence indicates that activation of the MR has been implicated in mediating the inflammation observed in vessels, heart and renal cortex of rodent models of diabetes and hyper-tension.43-45 MR blockages reduced expression of pro-inflammatory and pro-thrombotic factors in adipose tissue and increased expression of adiponectin in heart and adipose tissue of obese, diabetic mice.46 Moreover, administration of spironolactone attenuated TNF-α level in chronic constriction injury may be due to its anti-inflammatory properties.47 Further, it has been reported that the central administration of spironolactone prevented the rise in TNF-α in the heart failure model.48 Because TNF-α appears early in the cytokine cascade,49 it has been suggested that other proinflammatory cytokines might also be reduced. Data from the present study support that concept we have shown that pretreat-ment with eplerenone to CCl4-induced liver injury group abrogated TNF-α level. This effect may be accounted for the anti-inflammatory properties of eple-renone. The present study demonstrates a potential role of MR blockage in attenuation of the inflammatory process. This role is supported by the fact that the administration of MR antagonist reduced atherosclerotic lesion in different models of atherosclerosis.50-52 This reduction in atherosclerotic process was accompanied by a reduction of inflammatory markers.51,52
Noteworthy, the histopathological findings complemented the results of CCl4 on the liver as evident by the hepatic necrosis, diffuse fatty changes, portal fibrosis and mononuclear cell infiltration. Such lesions were induced via the formation of free radicals, especially the ROS. Importantly, the damaging effects of CCl4 were subsided and attenuated on pre-treatment with eplerenone. Thus, anti-oxidative stress and anti-inflammatory properties of eplerenone appears to play a key role in the attenuation of inflammation, and then preserve the structural integrity of the hepatocellular membrane resulting in amelioration of liver enzyme functions such as ami-notransferases and ALP.
ConclusionIn conclusion, the present study demonstrated a potential protective effect of MR antagonist, eplerenone in liver injury-induced by CCl4. The corresponding mechanisms of this protection may be mediated via anti-inflammatory and anti-oxidative stress properties of MR blockage by eplerenone. Thus, eplerenone might have promising beneficial anti-oxidative and anti-inflammatory effects thereby protecting against liver damage. Nevertheless, larger prospective clinical studies to determine the therapeutic effects of eplere-none in liver injury may be warranted.
AcknowledgmentWe are grateful to Prof. Kawkaab A. Abd elaziz, Department of Pathology, Faculty of Veterinary Medicine, Cairo University for her kind help in performing histopathological studies and interpretation of the results.