Methods. The study was designed to evaluate the hepatoprotective activity of aqueous extract of central stem of Musa sapientum (AqMS) against carbon tetrachloride induced hepatotoxicity in rats. Animals were divided into six groups. Group I served as normal control. Group II, III, IV, V & VI were administered CCl4 mixed with olive oil 1:1 (1.5 mL/kg) I.P., twice a week for 5 weeks. Group II was maintained as CCl4 intoxicated control. Group III, IV and V received AqMS at a dose of 25, 50 and 100 mg/kg. Group VI received silymarin 100 mg/kg for 5 weeks orally once daily. Marker enzymes of hepatic functions estimated in serum were AST, ALT and ALP. Antioxidant parameters estimated were MDA and GSH in blood and liver and SOD in blood, after fifth week, animals were sacrificed, livers dissected out and evaluated for histomorphological changes.
Results. There was significant rise in AST, ALT and ALP in CCl4 intoxicated control group II. Treatment with AqMS prevented rise in levels of these enzymes. There was significant rise in MDA and fall in GSH in blood and liver in group II, indicating increased lipid peroxidation and oxidative stress upon CCl4 ad-ministration. Treatment with AqMS prevented rise in MDA & increased GSH in treated group. SOD levels were decreased in group II while groups treated with AqMS showed significant rise (p < 0.05). Maximum hepatoprotective effect was observed with 50 mg/kg dose. Hepatoprotective effect observed with this dose was comparable to standard hepatoprotective drug silymarin. The results of pathological study also support the results of biochemical findings.
Conclusion. the results of the present study indicate that stem of Musa sapientum possess hepatoprotective effect and probably it is due to it’s antioxidant property.
Liver regulates various important metabolic functions. Hepatic damage is associated with distortion of these metabolic functions. Liver diseases such as cirrhosis, fatty liver and chronic hepatitis are important world health issues. Conventional and synthetic drugs used in liver disease are inadequate and sometimes have serious side effects.1
The use of herbal remedies for the treatment of liver diseases has long history starting with Ayurvedic treatment and extending to the Chinese, European and other systems of traditional medicine. A large number of plants and formulations have been claimed to have hepatoprotective effect. Some plants which have shown genuine utility in liver disorders are Silybium marinum,2Picrrorhiza kurroa,3Andographis paniculata, Phyllunthus niruri and Eclipta alba.4
Musa sapientum Linn is an herbaceous plant of Museace family, it has great medicinal value. Fruits and roots have antidiabetic activity.5,6 Rhizome and stem aqueous extract is used to dissolve urinary stones.7 Aqueous extract of flower is useful in dysentery and diarrhea.8 Aqueous extract of stem have antivenom9 and analgesic activity.10 Various biologically important compounds reported from banana plant are tannins, pectins, dopamine, serotonin, noradrenaline, sitostetrol, stigmasitosterol, etc.11–13 However to best of our knowledge this plant has not been explored for its hepatoprotective effect.
We have investigated hepatoprotective effect of aqueous extract of central part of stem of Musa sapientum Linn (AqMS). This central part of stem is true stem of banana plant. What appears like stem of this plant is pseudo stem formed by upright concentric layers of leaf sheaths. The true stem begins as an underground corm which grows upwards pushing its way through the centre of pseudo stem eventually producing inflorescence which later bear fruits. This central part of stem is edible part of plant. We have found aqueous extract of this part of plant to have significant hypoglycemic effect (unpublished data). Hepatic damage was induced in rats by giving them carbon tetrachloride (CCl4) intraperitonealy. Ability of AqMS to ameliorate CCl4 induced heptatotoxicity and its effects on important antioxidant parameters were studied.
Material and MethodsChemicalsTechnical grade carbon tetrachloride (purity 99.4%) was obtained from Merck, India and silymarin was obtained from Sigma Chemicals, USA. Stem of Musa sapientum was collected from local area and was authenticated by National Institute of Science Communication and Information Resources, New Delhi (Voucher no.NISCAIR/RHMD/Consult/2007-08/895/79). All other reagents used were of analytical grade and obtained either from Sisco Research Laboratories or Qualigens Fine Chemicals, Mumbai, India.
AnimalsMale Wistar rats (aged 10-12 weeks, weight range 150-200 g) were obtained from central animal house facility of the institute. The animals were kept under standard conditions (temp. 22 ± 2 °C, 80% humidity with 12 h light/dark cycle). Food and water were accessible ad libitum. Body weights and food consumption were recorded weekly. Records were maintained to comply with the conditions as desired by Institutional Ethical Committee-Animal Resear-ch, (IEC-AR) University College Medical Sciences, Delhi and the experiments were carried out as per the guidelines given by IEC-AR.
Induction of hepatoxicityHepatotoxicity was induced in rats by giving in-traperitoneal injection of CCl4 in olive oil (1:1, 1.5 mL/kg) twice a week for 5 weeks.14
Preparation of aqueous extract of central part of stem (AqMS)Fresh stems of Musa sapientum Linn were used. The upright concentric layers of leaf sheaths forming the pseudo stem were peeled off to reveal the central pale white stem. This central pale white stem was cut into pieces. One hundred gram stem was crushed with 20 mL water in a mixer followed by filtration through a sterile muslin cloth to get aqueous extract. Whole process was carried out at room temperature. Extract was lyophilized and stored in refrigerator (yield 2%).
Phytochemical studiesPhytochemical analysis of the lyophilized aqueous extract was done for terpenoids, phenolic compounds, saponins and pectins according to the methods described.15 Total phenolic contents were analyzed using Folin Ciocalcateu reagent16 and results are expressed as gallic acid equivalent.
Experimental designAnimals were randomly divided into six groups (six rats per group). Group I served as normal control. Groups II, III, IV, V & VI were treated with CCl4 mixed with olive oil in ratio of 1:1 at a dose of 1.5 mL/kg intraperitonealy twice a week for five weeks. Group II animals were maintained as CCl4 intoxicated control without any drug treatment. Group III, IV & V were administered AqMS at a dose of 25, 50 and 100 mg/kg respectively and group VI received silymarin (100 mg/kg in 5% gum acacia) orally once daily for five week17 in addition to CCl4 twice a week as mentioned above.
Biochemical parametersAspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) were estimated in serum using commercially available kits of Transasia Pvt. Ltd. Mumbai. Malonal-dialdehyde (MDA) was estimated in serum and liver.18 Reduced glutathione (GSH) was estimated in whole blood and liver,19 activity of superoxide dismutase (SOD) was assayed in erythrocytes.20
Assessment of liver damageAfter five weeks overnight fasted rats were anaesthetized with pentobarbital (50 mg/kg i.p.)21 and sacrificed by cervical dislocation. Blood was collected from heart by the heart puncture technique in EDTA vial for estimating SOD and GSH and plain vial for estimating MDA, ALT, AST & ALP. Liver tissue was taken out washed with cold saline and stored at -20 °C for estimating the antioxidant parameters.
Histopathological studiesPart of liver samples was submitted in 10% buffered formaldehyde for histophathological examinations. Sections were cut on glass slides (4-6 pm thick) and stained with hematoxylin and eosin (H & E) for routine histomorphology. The extent of liver damage was assessed by a histopathologist under light Olympus microscope.
Statistical analysisThe results are expressed as means ± S.D. The difference between experimental groups compared by one way ANOVA followed by Tukey’s test at 5% sig-nificance level.
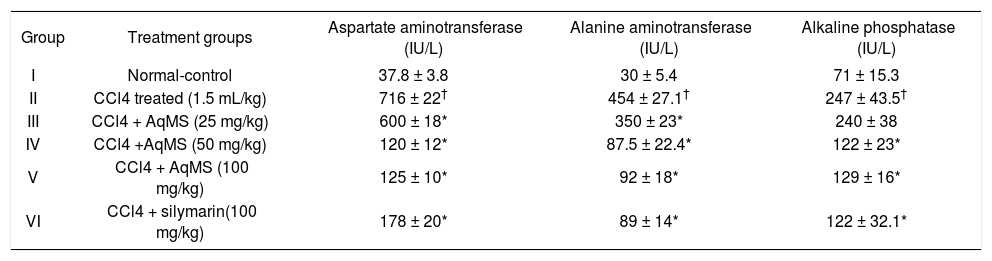
ResultsEffect on serum enzymesTreatment with CCl4 at a dose of 1.5 mL/kg twice a week for five weeks increased levels of AST, ALT and ALP significantly in group II (Table 1). Group III, IV and V which received treatment with AqMS orally once daily in addition to CCl4 twice a week showed significant fall in levels of these parameters. Maximum effect was obtained with a dose of 50 mg/kg which produced 85, 80 and 51% fall in levels of AST, ALT & ALP respectively (p < 0.05). Further increase in dose had no additional benefit. Fall in AST, ALT and ALP with 50 mg/kg dose was comparable to fall in these parameter by standard hepatoprotective drug i.e. silymarin.
Effect of five weeks treatment with aqueous extract of central stem of Musa sapientum (AqMS) on hepatic enzymes.
| Group | Treatment groups | Aspartate aminotransferase (IU/L) | Alanine aminotransferase (IU/L) | Alkaline phosphatase (IU/L) |
|---|---|---|---|---|
| I | Normal-control | 37.8 ± 3.8 | 30 ± 5.4 | 71 ± 15.3 |
| II | CCl4 treated (1.5 mL/kg) | 716 ± 22† | 454 ± 27.1† | 247 ± 43.5† |
| III | CCl4 + AqMS (25 mg/kg) | 600 ± 18* | 350 ± 23* | 240 ± 38 |
| IV | CCl4 +AqMS (50 mg/kg) | 120 ± 12* | 87.5 ± 22.4* | 122 ± 23* |
| V | CCl4 + AqMS (100 mg/kg) | 125 ± 10* | 92 ± 18* | 129 ± 16* |
| VI | CCl4 + silymarin(100 mg/kg) | 178 ± 20* | 89 ± 14* | 122 ± 32.1* |
The results are the mean ± SD for six rats in each group. Significantly different from normal control:
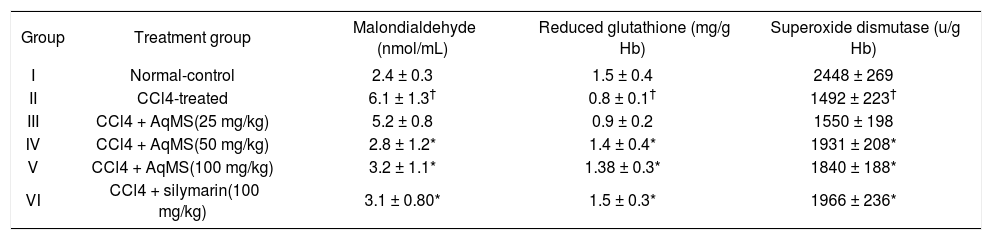
Treatment of rats with CCl4 increased lipid peroxidation as evident by significant rise in the levels of MDA in blood in group II (Table 2). Simultaneous treatment with AqMS decreased MDA in blood by 15% in group III, 47.5% in group IV & 46% in group IV. Amount of reduced glutathione decreased in blood of group II rats by 47%. Group III, IV and V rats which received AqMS treatment as mentioned above had 12.5, 75 and 72% rise in GSH, respectively. Antioxidant enzyme SOD was decreased in erythrocytes of group II rats while treatment prevented fall in activity of SOD in all AqMS treated groups with maximum rise in group IV. Findings with respect to GSH, MDA and SOD in group IV which received AqMS at 50 mg/kg were comparable with group treated with standard hepatoprotective drug silymarin.
Effect of five weeks treatment with aqueous extract of central stem of Musa sapientum (AqMS) on antioxidant parameters in blood.
| Group | Treatment group | Malondialdehyde (nmol/mL) | Reduced glutathione (mg/g Hb) | Superoxide dismutase (u/g Hb) |
|---|---|---|---|---|
| I | Normal-control | 2.4 ± 0.3 | 1.5 ± 0.4 | 2448 ± 269 |
| II | CCl4-treated | 6.1 ± 1.3† | 0.8 ± 0.1† | 1492 ± 223† |
| III | CCl4 + AqMS(25 mg/kg) | 5.2 ± 0.8 | 0.9 ± 0.2 | 1550 ± 198 |
| IV | CCl4 + AqMS(50 mg/kg) | 2.8 ± 1.2* | 1.4 ± 0.4* | 1931 ± 208* |
| V | CCl4 + AqMS(100 mg/kg) | 3.2 ± 1.1* | 1.38 ± 0.3* | 1840 ± 188* |
| VI | CCl4 + silymarin(100 mg/kg) | 3.1 ± 0.80* | 1.5 ± 0.3* | 1966 ± 236* |
The results are the mean ± SD for six rats in each group. Significantly different from normal control:
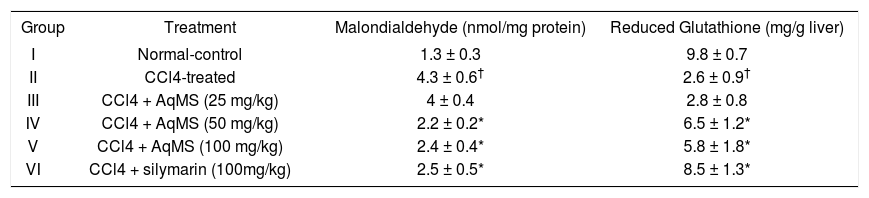
Effect of treatment with AqMS on liver MDA & GSH is shown in table 3. In liver there was 230% rise in MDA in group II. While group III, IV and V showed fall in levels of MDA. Maximum fall was obtained in group IV. GSH was decreased in group II but group III, IV and V which received treatment showed 7, 150, 123% rise in levels of GSH (P < 0.05), respectively. Results obtained in groups IV which received 50 mg/kg dose were comparable with that of hepatoprotective drug silymarin.
Effect of five weeks treatment with aqueous extract of central stem of Musa sapientum (AqMS) on antioxidant parameters in liver.
| Group | Treatment | Malondialdehyde (nmol/mg protein) | Reduced Glutathione (mg/g liver) |
|---|---|---|---|
| I | Normal-control | 1.3 ± 0.3 | 9.8 ± 0.7 |
| II | CCl4-treated | 4.3 ± 0.6† | 2.6 ± 0.9† |
| III | CCl4 + AqMS (25 mg/kg) | 4 ± 0.4 | 2.8 ± 0.8 |
| IV | CCl4 + AqMS (50 mg/kg) | 2.2 ± 0.2* | 6.5 ± 1.2* |
| V | CCl4 + AqMS (100 mg/kg) | 2.4 ± 0.4* | 5.8 ± 1.8* |
| VI | CCl4 + silymarin (100mg/kg) | 2.5 ± 0.5* | 8.5 ± 1.3* |
The results are the Mean ± SD for six rats in each group. Significantly different from normal control:
Phytochemical analysis showed presence of phenolic compounds, terpenoids, saponins and pectins in AqMS. Total phenolic content was found to be 50 mg/gm of lyophilized extract.
Histophathological studiesHistopathology of liver of group I, II, IV & V was done. Group IV in AqMS treated group was selected as it showed maximum hepatoprotective effect. The histology of liver in group I showed a normal cell morphology with hexagonal lobular architecture (Figure 1A). While the group II showed marked hepatocellular damage in the form of severe macro-vesicular steatosis, centrilobular hepatocellular necrosis and periportal inflammation (Figure 1B). These findings seems to be ameliorated by treatment with AqMS in group IV and silymarin in group VI. There was only mild periportal inflammation and reduced amount of steatosis without any significant hepatocyte necrosis (Figure 1C and 1D).
Photomicrograph of rat liver sections H & E X 40; (a) Normal. (b) CCl4 treated (1.5 mL/kg) showing severe macro vascular steatosis, centrilobular hepatocellular necrosis and periportal inflammation. (c) CCl4 + Aqueous extract of Musa sapientum(50mg/kg) (d) CCl4 + silymarin (100 mg/kg); Both group c & d shows only mild periportal inflammation, reduced amount of steatosis without any significant hepatocyte necrosis.
Liver is the principle site for CCl4 induced effect to manifest themselves.22 Plasma membrane, endoplasmic reticulum, mitochondria, and Golgi apparatus are the main subcellular structures affected by CCl4 exposure.23 Damage to plasma membrane of hepatocytes results in release of enzymes in circulation. In the CCl4 treated group ALT, AST, ALP levels increased dramatically compared to normal control group indicating severe hepatocellular damage.
The ability of a hepatoprotective drug to reduce injurious effect or preserve the normal hepatic physiological function is the index of its hepatoprotective effect. Treatment with AqMS lowered levels of these enzymes. Similar effects have been obtained with other plants with hepatoprotective activity.21,24,25
CCl4 metabolism begins with formation of trichloro methyl free radical CCl3• through the action of Cytochrome P450 oxygenase system. The major cytochrome P450 isoenzyme involved is CYP2E1, however at higher concentration CYP3A contributes significantly in this activation. CCl3• radical is converted trichlromethyl peroxy radical CCl3COO• in the presence of oxygen.
CCl3COO• is more active then CCl3• and both react with various biologically important substances like proteins, nucleic acids and lipids, altering their functions. CCl3• and CCl3COO• both can abstract a hydrogen from polyunsaturated fatty acids and initiate the process of lipid peroxidation which can affect in two ways:
- 1.
By compromising membrane function, and
- 2.
By covalent binding of reactive intermediate leading to the liver cell necrosis.26,27
CCl4 also affects hepatocellular levels of Ca2+ and continued exposure leads to increased cytosolic Ca2+. Uncontrolled levels of Ca2+ in cells destroy cytoskeletal structures and activate number of catabolic enzymes whose actions result in cell death via apoptosis or necrosis.28,29 One of the causes for cell damage due to CCl4 is loss of Ca2+ homeostasis.
CCl4 treatment increased lipid peroxidation as evident by rise in MDA, a marker of lipid peroxidation in group II. Others have also reported similar rise in MDA on CCl4 intoxication.30,31 Treatment with AqMS and sylimarin decreased levels of MDA. Sylimarin is known to decrease MDA due to its free radical scavenging activity.32 A positive correlation has been reported in phenolic contents and antioxidant activity.33 Saponins have also been reported to confer hepatoprotective and antioxidant properties.34
Phytochemical studies show the presence of saponins and phenolic compounds in aqueous extract of Musa sapientum. Phenolic content of AqMS was found to be 50 mg/gm. Therefore decrease in MDA may be due to antioxidant activity of the saponin and phenolic compounds present in AqMS. Reduced glutathione (GSH) is a non enzymatic antioxidant, widely distributed in liver cells. It’s main functions is mainly concerned with the removal of free radicals species such as H2O2, superoxide radicals and maintenance of membrane protein thiols.35 AqMS treatment prevented depletion of GSH in liver and blood.
CCl4 has been shown to induce the formation of free radicals which may decrease the activity of SOD as has been observed in CCl4 treated animals, however increase in SOD activity in AqMS treated group indicates that this treatment prevents formation of free radicals.
The results of histophathological study also support the results of biochemical parameters and are the in-situ evidence of hepatoprotective effect of AqMS. There was marked hepatocellular damage in CCl4 alone treated group. Simultaneous treatment of AqMS with CCl4 exhibited significantly less damage to the hepatic cells compared to rats treated with CCl4 alone. The reduction in cellular damage seen in AqMS treated group was morphologically similar to silymarin treated group.
ConclusionIt may be concluded from the present study that AqMS possess hepatoprotective activity against model hepatotoxicant CCl4 and maximum beneficial effect was observed at a dose of 50 mg/kg. The hepatoprotective action is probably related to its potent antioxidant activity. Further investigations are required to characterize the active hepatoprotective principle.
Abbreviations- •
AqMS: Aqueous extract of Musa sapientum.
- •
i.p: Interaperitoneal.
- •
AST: Aspartate aminotransferase.
- •
ALT: Alanine aminotransferase.
- •
ALP: Alkaline phosphatase.
- •
MDA: Malonaldialdehyde.
- •
GSH: Reduced glutathione.
- •
SOD: Superoxide dismuitase.
- •
H&E: Hematoxylin & eosin.
- •
S.D.: Standard deviation.
- •
%: Percent.
- •
ANOVA: Analysis of variance.
- •
IU: International unit.
- •
nmol: Nano mole.
- •
mg: Milligram.
- •
g: Gram.
- •
Hb: Hemoglobin.
- •
mL: Milliliter.
- •
ICMR: Indian council of medical research.
The authors acknowledge the Indian Council of Medical Research, New Delhi for providing financial assistance.