Introduction. Hepatic fibrosis is a common pathological process of chronic liver injury. Oxidative stress and inflammation may have prognostic value in disease progression.
Objective. To examine the implication of both aforementioned factors in hepatic fibrosis progression and whether, the antioxidant effect of various biological active constituents such as phenolic, flavonoids and fatty acids of purslane hydro-ethanolic extract can represent a potential target for therapy.
Methods. Purslane exhibited a considerable antioxidant potential in DPPH assay compared to α-tocopherol. Consequently, the current study was designed to examine the prophylactic and curative effects of purslane extract on bile duct ligation (BDL)-induced liver fibrosis in rats in comparison with silymarin as a reference hepatoprotective agent. Purslane (400 mg/kg/day) or silymarin (50 mg/kg/day) were administered orally for 4 weeks, immediately after surgery in order to evaluate the prophylactic effect and for 3 weeks starting 3 weeks after BDL in order to evaluate the curative effect. BDL significantly increased liver enzymes, total bilirubin (TB) and tumor necrosis factor-alpha (TNF-α) in serum along with malondialdehyde (MDA) in liver tissues.
Results. Significant decrease in hepatic antioxidant defense system was noted in BDL-rats. Conversely, administration of purslane reversed all these biochemical parameters which were previously induced by BDL. Considerably, purslane effect was more pronounced in the prophylactic study than that in the curative one.
Conclusion. The present work suggested that purslane had prophylactic and curative value on cholestasis-induced liver fibrosis through inhibition of oxidative stress, decreasing the expression of profibrogenic cytokines, collagenolytic activity and activation of hepatic stellate cells.
Hepatic fibrosis, a major feature of many chronic liver injuries including metabolic, cholestatic, viral and genetic diseases, is a common pathological process regardless of etiology and its progression lead to cirrhosis.1 The biliary obstruction causes hepatocellular necrosis, proliferation of bile ductular epi-thelial cells, activation of stellate cells2 and subsequent release of cytokines and growth factors which stimulate the extracellular matrix deposition leading to hepatic fibrosis.3 Liver dysfunction, oxidative stress, inflammation and others may be contributed.4 There are several approaches aiming to reduce the excessive scar formation via curing the primary disease to prevent injury, reducing inflammation or increase the degradation of scar matrix.5 Disadvantages associated with anti-fibrotics synthetic drugs are toxicity due to chronic administration and reduced therapeutic effect when used in clinical studies.
Anti-fibrotics from natural sources may reduce the risk of toxicity and preserve therapeutic effectiveness in clinical usage. Plants are a rich source of natural bioactive phytochemicals such as antioxidants that can delay or inhibit the oxidation of lipids and other molecules by inhibiting the initiation or propagation of oxidative chain reactions.6
Portulaca oleracea [family: Portulacaceae (purslane)] is a summer annual plant listed in the World Health Organization as one of the most used medicinal plants.7 Purslane is a rich source of omega-3 fatty acids, gallotannins, kaempferol, quercetin, apigenin, glutathione,8 monoterpen-oids, alkaloids, coumarins and flavonoids.9 It is used as antipyretic, anti-scorbutic, antiseptic, antispasmodic, diuretic, antihelmintic, for treatment of urinary disorders and reducing pain swelling and inflammation.10 Recent studies have demonstrated various pharmacological effects of this plant as hypoglycemic,11 antioxidant,12 analgesic and anti-inflammatory,13 neuroprotective14 and wound healing15 effects. Purslane does not have any cytotoxicity or genotoxicity and it is safe for daily use.16
The present study was undertaken to clarify the complication of hepatic fibrosis associated with bile duct ligation (BDL) to address the underlying molecular mechanisms as well as to explore more effective therapeutic agent that will prevent, retard or reverse hepatic fibrois. Consequently, the current speculation aims to perform the preliminary phytochemical screening for total phenolic, total flavonoid and fatty acids contents in purslane hydroethanolic extract, evaluate its in-vitro antioxidant activity, elucidate its biochemical effects on BDL-rats. Moreover, the possible prophylactic and curative effects of purslane on experimental hepatic fibrosis progression induced by biliary obstruction in albino rats were investigated in comparison with silymarin as a reference hepatoprotective agent.
MethodsExperimental animalsA total of 120 adult female albino rats weighting (150-200 g) were used. Rats were provided from the farm of National Organization for Drug Control and Research, Giza. They were housed in stainless steel cages at room temperature (24 ± 2 °C) with a 12 h light-dark cycle. They were fed on standard diet and tap water and were acclimatized to the environment for a week prior to experiment. All experiments were carried out in accordance with protocols approved by the local experimental ethics committee.
Establishment of the hepatic fibrosis model and experimental designHepatic fibrosis was induced by BDL.17 The 4th week BDL-rats was used as a model to study the prophylactic effect of purslane (400 mg/kg/day) on hepatic fibrosis18 against reference agent, silymarin extract (50 mg/kg/day),19 from Sigma Chemical Co, St. Louis MO, USA.
Experimental designExperimental rats underwent BDL surgery. Then the experimental work was classified into two parts as follows:
- •
Part one: Prophylactic study. The experiment was lasted for 4 wks. A total of 60 rats were divided randomly into equal 4 groups (15 rats each) as follows:
- ⸰
Group 1: (Sham). Rats were underwent a sham operation and served as negative control group.
- ⸰
Group 2: (BDL). Rats had BDL and served as a positive control group.
- ⸰
Group 3: (BDL+purslane). Rats had BDL and received a daily oral dose of purslane (400 mg /kg) for 4 wks, starting instantly after surgery.
- ⸰
Group 4: (BDL+silymarin). Rats had BDL and received a daily oral dose of silymarin (50 mg /kg) for 4 wks, starting instantly after surgery.
- ⸰
- •
Part two: Curative study. The experiment was done as before yet lasted for 6 wks and BDL-rats received treatment for 3 weeks only, starting 3 weeks after surgery. At the end of the experiment period, all animals were anaesthetized and blood samples were collected, subjected to serum separation and divided into aliquots. Fresh sera were tested for liver enzymes and other aliquots were stored at -20 °C for later biochemical analysis. Rats were then killed by decapitation; the livers were collected, weighed and frozen in liquid nitrogen.
Fresh aerial plant parts were collected from the farm of National Organization for Drug Control and Research, Giza, Egypt in January. Purslane hydroethanolic extract was screened for the presence of total flavonoids, total phenolic and fatty acids contents using the standard methods.20–22
1,1-Diphenyl-2-picrylhydrazyl free radical scavenging activity (DPPH Assay)The antioxidant activity of purslane was determined according to a procedure described by Blois.23
Liver function testsSerum aspartate aminotransferase (AST), alanine aminotransferase (ALT) (24), alkaline phosphatase (ALP) (25), gamma glutamyl transferase (GGT)26 and total bilirubin (TB)27 were determined spectro-photo-metrically using commercial kits (BIO diagnostic co., Egypt).
Determination of oxidative stress biomarkersSuperoxide dismutase (SOD),28 catalase (CAT),29 glutathione peroxidase (GPx),30 reduced glutathione (GSH)31 and malondialdehyde (MDA)32 were estimated in liver tissues as indicators of oxidative stress.
Total protein is needed for other tissue parameters calculation and was determined by the method described by Lowry, et al.33
Tumor necrosis factor alpha (TNF-α) measurementSerum TNF-a was determined using rat TNF-α ELISA kit (RayBiotech, USA).34
Statistical analysisResults were expressed as means ± S.E. Statistical evaluation was done using one- way analysis of variance (ANOVA).35 Values of P < 0.05 were considered significant.
ResultsPhytochemical screeningPhytochemical screening of hydro-ethanolic extract of purslane revealed the presence of various biological active constituents such as total phenolic content (151.4 mg gallic acid equivalent/g), total flavonoids content (6.25mg quercetin equivalent/g) and fatty acids [palmitic acid (C16, 17.9%), stearic acid (C18, 2.98%), oleic acid (C18, 8.69%), linoleic acid [C18 (ω6), 33.7%] and linolenic acid [C18 (ω3), 11.49%].
DPPH assayPurslane extract exhibited a considerable antioxidant potential with half maximal inhibitory concentration (120 μg /mL) comparing to α-tocopherol (20.47 μg /mL).
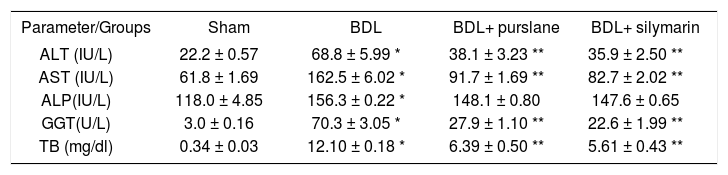
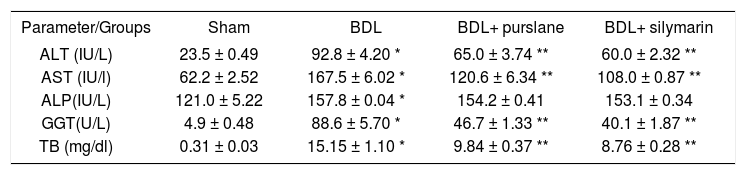
Liver function testsPurslane administration significantly (P < 0.05) reduced serum levels of hepatic enzymes and bilirubin in BDL-rats in comparison with untreated BDL ones (Tables 1 and 2).
Effect of purslane (400 mg/kg, 4wks) in comparison with silymarin (50 mg/kg, 4wks) on the liver functions in BDL-rats.
| Parameter/Groups | Sham | BDL | BDL+ purslane | BDL+ silymarin |
|---|---|---|---|---|
| ALT (IU/L) | 22.2 ± 0.57 | 68.8 ± 5.99 * | 38.1 ± 3.23 ** | 35.9 ± 2.50 ** |
| AST (IU/L) | 61.8 ± 1.69 | 162.5 ± 6.02 * | 91.7 ± 1.69 ** | 82.7 ± 2.02 ** |
| ALP(IU/L) | 118.0 ± 4.85 | 156.3 ± 0.22 * | 148.1 ± 0.80 | 147.6 ± 0.65 |
| GGT(U/L) | 3.0 ± 0.16 | 70.3 ± 3.05 * | 27.9 ± 1.10 ** | 22.6 ± 1.99 ** |
| TB (mg/dl) | 0.34 ± 0.03 | 12.10 ± 0.18 * | 6.39 ± 0.50 ** | 5.61 ± 0.43 ** |
Values are (mean ± SE).
Effect of purslane (400 mg/kg, 3wks) in comparison with silymarin (50 mg/kg, 3wks) on the liver functions in BDL-rats.
| Parameter/Groups | Sham | BDL | BDL+ purslane | BDL+ silymarin |
|---|---|---|---|---|
| ALT (IU/L) | 23.5 ± 0.49 | 92.8 ± 4.20 * | 65.0 ± 3.74 ** | 60.0 ± 2.32 ** |
| AST (IU/l) | 62.2 ± 2.52 | 167.5 ± 6.02 * | 120.6 ± 6.34 ** | 108.0 ± 0.87 ** |
| ALP(IU/L) | 121.0 ± 5.22 | 157.8 ± 0.04 * | 154.2 ± 0.41 | 153.1 ± 0.34 |
| GGT(U/L) | 4.9 ± 0.48 | 88.6 ± 5.70 * | 46.7 ± 1.33 ** | 40.1 ± 1.87 ** |
| TB (mg/dl) | 0.31 ± 0.03 | 15.15 ± 1.10 * | 9.84 ± 0.37 ** | 8.76 ± 0.28 ** |
Values are (mean ± SE).
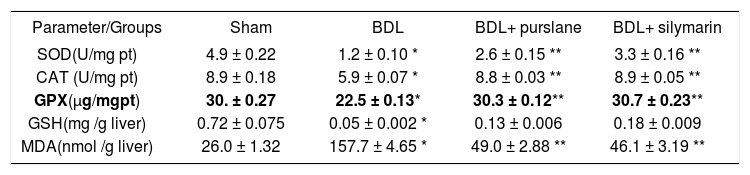
The liver SOD, CAT, GPx and GSH were significantly elevated whereas, MDA levels were significantly reduced in the BDL-rats after purslane/silymarin administration in the two rat-models vs. untreated BDL-rats (Tables 3 and 4).
Effect of purslane (400 mg/kg, 4wks) in comparison with silymarin (50 mg/kg, 4wks) on oxidative stress biomarkers in BDL-rats.
| Parameter/Groups | Sham | BDL | BDL+ purslane | BDL+ silymarin |
|---|---|---|---|---|
| SOD(U/mg pt) | 4.9 ± 0.22 | 1.2 ± 0.10 * | 2.6 ± 0.15 ** | 3.3 ± 0.16 ** |
| CAT (U/mg pt) | 8.9 ± 0.18 | 5.9 ± 0.07 * | 8.8 ± 0.03 ** | 8.9 ± 0.05 ** |
| GPX(μg/mgpt) | 30. ± 0.27 | 22.5 ± 0.13* | 30.3 ± 0.12** | 30.7 ± 0.23** |
| GSH(mg /g liver) | 0.72 ± 0.075 | 0.05 ± 0.002 * | 0.13 ± 0.006 | 0.18 ± 0.009 |
| MDA(nmol /g liver) | 26.0 ± 1.32 | 157.7 ± 4.65 * | 49.0 ± 2.88 ** | 46.1 ± 3.19 ** |
Values are (mean ± SE).
Effect of purslane (400 mg/kg, 3wks) in comparison with silymarin (50 mg/kg, 3wks) on oxidative stress biomarkers in BDL-rats.
| Parameter/Groups | Sham | BDL | BDL+ purslane | BDL+ silymarin |
|---|---|---|---|---|
| SOD(U/mg pt) | 4.5 ± 0.33 | 1.18 ± 0.1 * | 2.17 ± 0.19 | 3.0 ± 0.31 ** |
| CAT (U/mg pt) | 8.6 ± 0.13 | 5.7 ± 0.18 * | 8.5 ± 0.09 ** | 8.3 ± 0.08 ** |
| GPX(μg/mgpt) | 30.6 ± 0.25 | 22.2 ± 0.69* | 27.8 ± 0.17** | 28.3 ± 0.42** |
| GSH(mg /g liver) | 0.71 ± 0.072 | 0.03 ± 0.002 * | 0.07 ± 0.008 | 0.16 ± 0.010 |
| MDA(nmol /g liver) | 25.3 ± 2.06 | 262.3 ± 26.82 * | 87.83 ± 4.67 ** | 57.4 ± 3.46 ** |
Values are (mean ± SE).
Serum TNF-α levels were reduced to about 30% after treatment with either purslane or silymarin in the two rat-models comparing to untreated BDL-rats (Figure 1 and 2). All previous effects of both purslane and silymarin were more pronounced in the prophylactic experiment than those in the curative experiment.
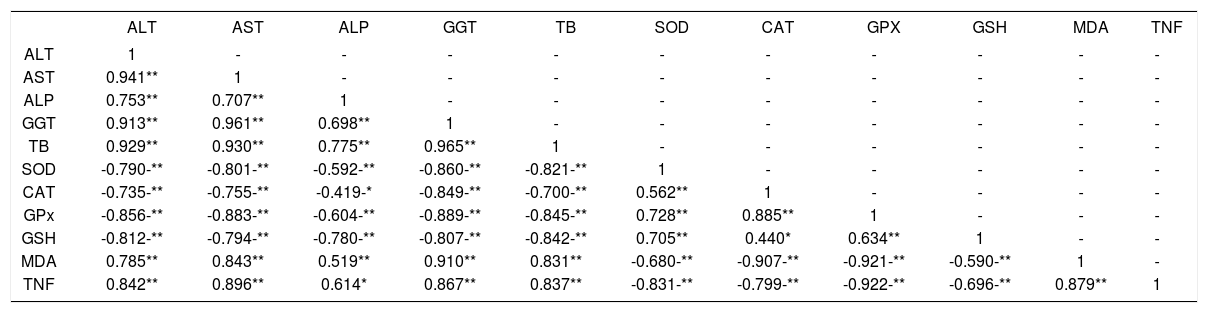
Using the combined results from all experimental animals, it was demonstrated that almost all parameters were significantly correlated with others either positively or negatively as indicated in table 5 and 6.
Correlation coefficient values between parameters in BDL-rats of prophylactic study.
| ALT | AST | ALP | GGT | TB | SOD | CAT | GPX | GSH | MDA | TNF | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALT AST | 10.848** | 1 | - | - | - | - | - | - | - | - | - |
| ALP | 0.632** | 0.629** | 1 | - | - | - | - | - | - | - | - |
| GGT | 0.895** | 0.960** | 0.701** | 1 | - | - | - | - | - | - | - |
| TB | 0.890** | 0.917** | 0.771** | 0.949** | 1 | - | - | - | - | - | - |
| SOD | -0.799-** | -0.858-** | -0.732-** | -0.894-** | -0.905-** | 1 | - | - | - | - | - |
| CAT | -0.815-** | -0.883-** | -0.496-** | -0.922-** | -0.781-** | 0.767** | 1 | - | - | - | - |
| GPX | -0.829-** | -0.912-** | -0.453-** | -0.891-** | -0.793-** | 0.648** | 0.894** | 1 | - | - | - |
| GSH | -0.640-** | -0.677-** | -0.843-** | -0.633-** | -0.789-** | 0.803** | 0.468** | 0.413* | 1 | - | - |
| MDA | 0.910** | 0.949** | 0.593** | 0.944** | 0.877** | -0.802-** | -0.923-** | -0.951-** | -0.565-** | 1 | - |
| TNF | 0.913** | 0.956** | 0.704** | 0.960** | 0.935** | -0.885-** | -0.914-** | -0.881-** | -0.646-** | 0.936** | 1 |
Correlation coefficient values between parameters in BDL-rats of curative study.
| ALT | AST | ALP | GGT | TB | SOD | CAT | GPX | GSH | MDA | TNF | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALT | 1 | - | - | - | - | - | - | - | - | - | - |
| AST | 0.941** | 1 | - | - | - | - | - | - | - | - | - |
| ALP | 0.753** | 0.707** | 1 | - | - | - | - | - | - | - | - |
| GGT | 0.913** | 0.961** | 0.698** | 1 | - | - | - | - | - | - | - |
| TB | 0.929** | 0.930** | 0.775** | 0.965** | 1 | - | - | - | - | - | - |
| SOD | -0.790-** | -0.801-** | -0.592-** | -0.860-** | -0.821-** | 1 | - | - | - | - | - |
| CAT | -0.735-** | -0.755-** | -0.419-* | -0.849-** | -0.700-** | 0.562** | 1 | - | - | - | - |
| GPx | -0.856-** | -0.883-** | -0.604-** | -0.889-** | -0.845-** | 0.728** | 0.885** | 1 | - | - | - |
| GSH | -0.812-** | -0.794-** | -0.780-** | -0.807-** | -0.842-** | 0.705** | 0.440* | 0.634** | 1 | - | - |
| MDA | 0.785** | 0.843** | 0.519** | 0.910** | 0.831** | -0.680-** | -0.907-** | -0.921-** | -0.590-** | 1 | - |
| TNF | 0.842** | 0.896** | 0.614* | 0.867** | 0.837** | -0.831-** | -0.799-** | -0.922-** | -0.696-** | 0.879** | 1 |
In the present study, BDL-induced hepatic damage and fibrosis was characterized by marked biochemical, pathophysiological and morphological abnormalities produced by interrupted enterohepatic circulation, obstructed hepatic biliary tree, retention of biliary constituents (bilirubin, bile acids and cholesterol), poor lipid digestion and absorption of fat-soluble vitamins and impairment of hepato-cellular transport. The deleterious effect of BDL on liver function is emphasized by significant (p < 0.05) elevation in serum AST, ALT, ALP, GGT and TB concentration in BDL-rats throughout the study periods as compared with sham-operated control group reflecting the toxic effects of the regurgitated bile acids to the liver and the severity of hepatic injury and cholestasis.
Our results revealed the presence of considerable amounts of antioxidants in purslane including phenolic compounds, flavonoids and fatty acids e.g. linoleic, linolenic, oleic, palmitic and stearic acids. The presence of the previous components provides an evidence for the hepatoprotective effect and antioxidant capacity of purslane, as revealed by significant reduction of hepatic enzymes and bilirubin levels of BDL-rats in comparison with untreated BDL-ones throughout the experiment period. The aforementioned data are in harmony with those obtained by other investigators.12,36,37
Purslane administration significantly reduced the levels of these enzymes; the effect was more pronounced in the curative study than that in the prophylactic one. Our consequences agreed with preceding studies38 which postulated that failure of bile salts excretion in cholestasis leads to retention of hydrophobic bile salts within the hepatocytes leading to oxidative damage to mitochondrial proteins and lipids by cytotoxic bile acids, apoptosis and necrosis.
The present work revealed that BDL aggravated the imbalance between free radicals production and the antioxidant defense which results into oxidative stress in the liver and lipid peroxidation as shown by increased MDA content and marked decrease in hepatic GSH content and antioxidant enzymes such as SOD, CAT and GPx, throughout the experimental period, indicating that oxidative stress has a prime role in BDL-induced tissue injury. The current results are in agreement with preceding results.39 The mechanism includes covalent binding of the free radicals with unsaturated fatty acids of bio-membranes resulting in lipid peroxidation and denaturation of proteins and DNA causing series of deteriorative changes in the biological systems, cell inactivation and liver fibrosis.40 The main consequences of BDL in our study is oxidative stress which activates hepatic stellate cells and induces the secretion of growth factors and profibrogenic cytokines thus increase serum TNF-α and IL-6 concentrations via NFκB activation in Kupffer cells leading to enhanced TGF-β and collagen expression.4
The current finding exhibited that purslane attenuated the oxidative stress biomarkers and inhibited the lipid peroxidation. These actions are mainly ascribed to its high content of antioxidant like vitamins A, B, C, E and β-carotene as many lines of evidence have indicated.36,37 The anti-inflammatory effect of purslane and silymarin flavonoids in this experiment may be contributed to explain the decreased level of the pro-fibrogenic cytokine, TNF-α in treated BDL-rats than the untreated group. This finding is in consistence with Lee and others41 who illustrated that omega-3 fatty acid in purslane as anti-inflammatory agent may be a method of reducing long-term complications of liver injury secondary to diseases of cholestasis such as biliary atresia, namely fibrosis and cirrhosis. Furthermore, flavonols as quercetin and catechin were found to inhibit the production of TNF-α and nitric oxide by lipopolysaccharide-activated macrophages. Similarly, apigenin, one of the most potent flavones, inhibits prostaglandin synthesis induced by IL-1and the production of IL-6 and IL-8 activated by TNF-α.42
Collectively, the present data highlights that purslane is considered to be one of the antioxidantsrich plants which had a significant ameliorative effect on liver function, oxidative stress biomarkers and inflammation in comparison with silymarin as a reference agent in BDL-rats. The effects of purslane were more promising in the prophylactic study than in the curative one for management of liver fibrosis.
Abbreviations- •
DPPH: 1,1-diphenyl-2-picrylhydrazyl.
- •
BDL: Bile duct ligation.
- •
TB: Total bilirubin.
- •
MDA: Malondialdehyde.
- •
TNF: Tumor necrosis factor-alpha.
- •
AST: Aspartate aminotransferase.
- •
ALT: Alanine aminotransferase.
- •
ALP: Alkaline phosphatase.
- •
GGT: Gamma glutamyl transferase.
- •
SOD: Superoxide dismutase.
- •
CAT: Catalase.
- •
GPx: Glutathione peroxidase.
- •
GSH: Reduced glutathione.
- •
IL: Interleukin.
- •
HYP: Hydroxyproline.
- •
TIMP-1: Tissue inhibitor metalloproteinase.
- •
RT-PCR: Reverse transcription-polymerase chain reaction.
- •
H & E: Hematoxylin and eosin stain.
I am greatly indebted to Dr. Zeinab Yousef Ali, Assistant Professor of biochemistry, National Organization for Drug Control and Research, Giza for her valuable help and co-operation.