Hepatotoxicity by drugs and dietary supplements (DDS) is a rare and unpredictable event but with the risk of a life-threatening clinical course when it occurs. It may emerge despite intensive chemical, toxicological and observational studies that indicate no hepatotoxic signals. This suggests major clinical and regulatory issues that must be addressed in the area of accurate testing, reporting, and accessibility of reliable data. Consequently, in a clinical setting, safety concerns are key elements in the treatment of patients, and require that the diagnosis of DDS hepatotoxicity clearly be established. Causality of DDS hepatotoxicity may be pursued using a diagnostic algorithm consisting of a pre-test, a main-test as the scale of the updated CIOMS (Council for International Organizations of Medical Sciences), and a post-test. The results of these tests are then sent item by item to the National Health Agency, where the case will undergo further evaluation for pharmacovigilance, strategic aspects and safety issues. After this analysis, all items of the tests are included in the regulatory database freely accessible to the health and scientific community. With this diagnostic and regulatory algorithm the risk of misdiagnoses and inappropriate regulatory measures may be minimized and the safety improved. In conclusion, DDS hepatotoxicity is a rare but is a potentially life-threatening entity requiring a reliable diagnosis with the aid of a diagnostic algorithm, and a thorough pharmacovigilance evaluation by national and international health agencies. Safety aspects in DDS hepatotoxicity represent a major clinical and regulatory issue and should consequently be addressed.
Hepatotoxic reactions by exogenous compounds have attracted common attention, since they are of clinical relevance and may well to be studied experimentally in the liver as it is the most important target organ for toxic and metabolic events.1-5 Compounds with an overt hepatotoxic potency will certainly not reach the market for human use. However, problems may arise when chemical substances or herbal products initially lack experimental hepatotoxicity controls, but subsequently show toxic reactions in the liver of a few users, after being marketed.5
Despite various precautious measures, hepatotoxicity by drugs and dietary supplements (DDS) does occur and then it represents a major clinical, regulatory and safety issue.3-10 Synthetic drugs as well as herbal drugs commonly undergo intensive chemical, toxicological and observational studies regarding possible liver toxicity before regulatory approval is sought.3,11 Since dietary supplements, including herbal ones, are far less or not regulated at all, intensive studies with respect to possible hepatotoxic side effects are not performed.6,12-18
It is commonly accepted that DDS hepatotoxicity is a rare event and deserves balanced safety considerations for patients, physicians, regulatory agencies and manufacturers.5 In the past, safety concerns regarding hepatotoxicity have led to the withdrawal of various DDS from the market.3,19,20 It is also well understood that the use of most, if not virtually all, DDS in general may carry the rare risk for hepatotoxicity-1,5-10
The present review will focus on clinical approaches to firmly diagnose DDS hepatotoxicity in exposed patients, using a diagnostic algorithm with a structured quantitative causality assessment method. Further points of consideration are the development of a liver specific standard case input reporting system to the national regulatory agency and the standardization of the structured input of regulatory actions of pharmacovigilance. Equally important is the regulatory data information strategy to other national and international regulatory agencies, to the WHO and to other interested health care providers and scientists, allowing free access to the regulatory database. There still exist various shortcomings in the process of the diagnostic and regulatory algorithm which are to be resolved on safety grounds and in the interest of the patients and our society.
Clinical Challenges And Safety ConcernsDDS hepatotoxicity is normally a rare event in a few susceptible individuals7,8,18,21-29 with the risk of life-threatening outcome even in the era of liver transplantation.21,23,27 Safety concerns relate not only to regulatory supervised synthetic and herbal drugs.6,19 They are also directed to dietary supplements including herbal ones lacking the same regulations as those in the regulated drug status.6,8,19 In any health care setting increased liver values in patients under DDS use represent a major clinical challenge. Particularly, a temporal association between DDS intake and the emerging liver disease is certainly not the only criterion for the correct diagnosis of DDS hepatotoxicity.
Symptoms such as icterus, pruritus, fatigue, weight loss, right upper abdominal discomfort, dark urine, and pale stool are found in patients with DDS hepatotoxicity30,31 as well as in those with other forms of liver diseases. Therefore, by clinical assessment a differentiation between DDS hepatotoxicity and DDS unrelated liver disease is not possible. The diagnosis of DDS hepatotoxicity may only be established when other liver diseases have carefully been excluded. This is a cumbersome approach in view of some hundreds of possible liver diseases with various etiologies. The correct diagnosis is essential in order to discontinue the suspected DDS and to minimize the risk of acute liver failure requiring liver transplantation.
Role Of Biomarkers As Diagnostic AidThere is no biomarker available that attributes liver disease firmly to the observed group of DDS as causative agents, although some toxicological parameters, genetic assessment and immological tests and features may be helpful in a few patients. Under discussion are DDS levels, genetic polymorphism of cytochrome P450, the lymphocyte transformation test and immunological parameters.5 Other new technologies related to transcriptomics, proteomics and metabolomics may emerge and facilitate predicting and diagnosing DDS hepatotoxicity in the future.6,32
In few patients with DDS hepatotoxicity, are the results of a positive rechallenge test, which is considered the “gold standard” for the diagnosis of hepatotoxicity, available. This test, however, has commonly been abandoned in the clinical assessment due to high risks, but some results for few DDS are still available from unintentional rechallenges.5
AD-HOC Causality AssessmentVarious ad-hoc causality assessments of assumed DDS hepatotoxicity have been published in the past. They were presented by physicians or scientists as case reports or case series and by regulatory agencies, but some of the results have been questioned, found not to be reproducible or were of low quality.5,17,20,21,26,33-40 Indeed, diagnoses of liver diseases unrelated to DDS have been established such as hemochromatosis,41 Wilsons’s disease,22,42 autoimmune hepatitis,20,22,42,43,44 primary biliary cirrhosis,20 overlap syndrome,20 virus hepatitis,224142 CMV,45 EBV,45 HSV,20,44 ischemic hepatitis,22,41-43 cardiac hepatopathy,41 chronic liver disease,45 liver cirrhosis,45 fatty liver,45 non-alcoholic liver disease,20,22,42 alcoholic liver disease,22,42,43 Gilbert’s syndrome,45 tumors,22,41,42 lymphoma,45 bile duct diseases,22,42,43 pancreatitis,20 systemic sepsis,22,42,43 chlamydial infection,41 thyroid disease,20,22,42 and postictal.45 It is evident from these reports that ad-hoc causality assessments of suspected DDS hepatotoxicity are not the appropriate tools for establishing the correct diagnosis. Consequently, various qualitative and quantitative causality assessment methods have been developed.5,46-52
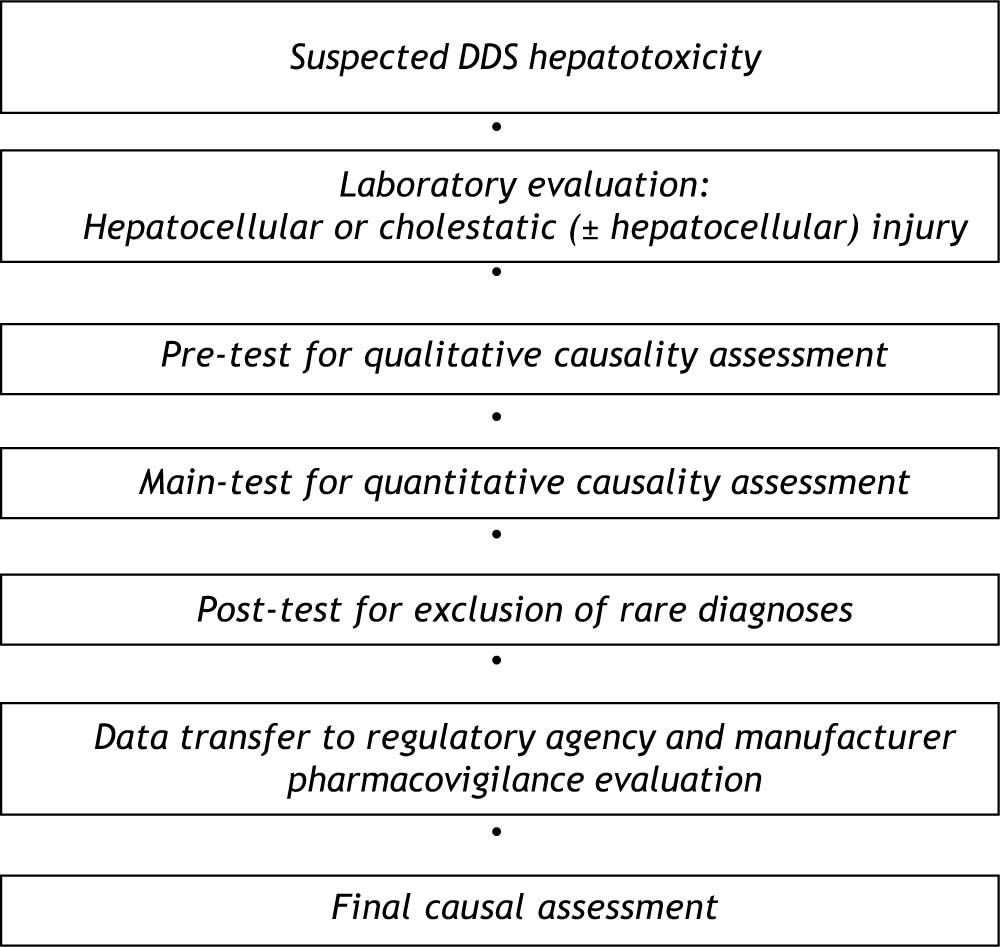
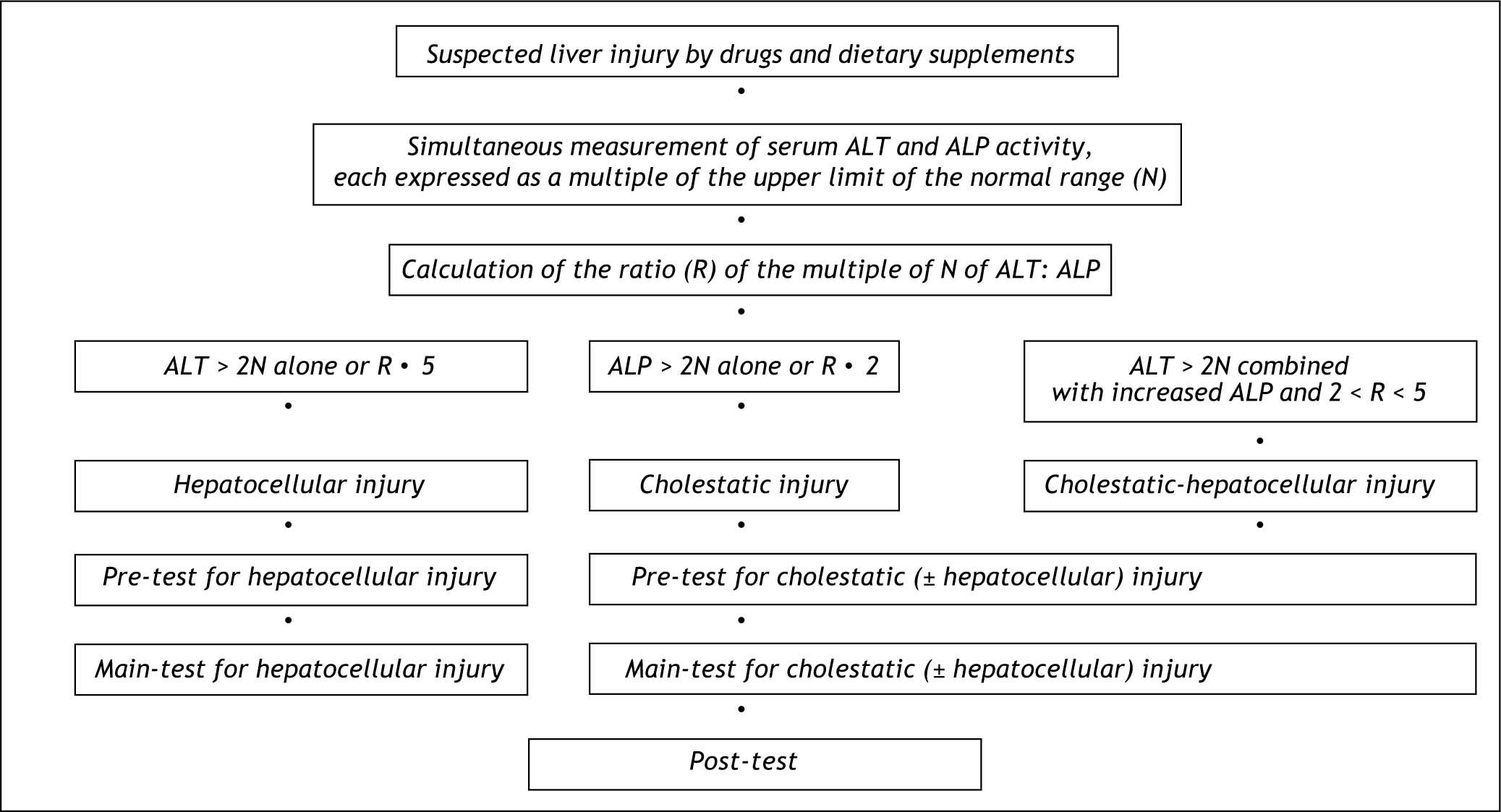
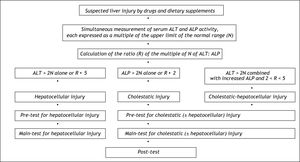
Diagnostic AlgorithmFor causality assessment of suspected DDS hepatotoxicity, a diagnostic algorithm consisting of a pre-test, a main-test, and a post-test is recommended (Figure 1).5 Prerequisite for the diagnosis of DDS hepatotoxicity are values for alanine aminotransferase (ALT) and/or alkaline phosphatase (ALP) to be at least two N (N corresponds to the upper limit of the normal range).5 Other values such as aspartate aminotransferase (AST), gamma-glutamyltranspeptidase (GGT), or bilirubin are not considered of diagnostic value in this particular disease entity. DDS hepatotoxicity may exhibit a hepatocellular, cholestatic or mixed form of liver injury, and differentiation of these entities is required for further causality evaluation (Figure 2). Therefore, serum activities of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) are measured on the day the diagnosis of DDS hepatotoxicity is suspected. Each activity is expressed as multiple of the upper limit of the normal range (N), and the ratio (R) of ALT: ALP is calculated. Liver injury is:
- •
Hepatocellular, when ALT > 2N alone or R ≥ 5.
- •
Cholestatic when there is an increase of ALP > 2N alone or when R ≤ 2.
- •
Of the mixed type when ALT > 2N, ALP is increased and 2 < R < 5.
For causality assessment by the following pre-test and main-test, each case has to be evaluated separately for each individual DDS, whereas the post-test is valid for all DDS together.
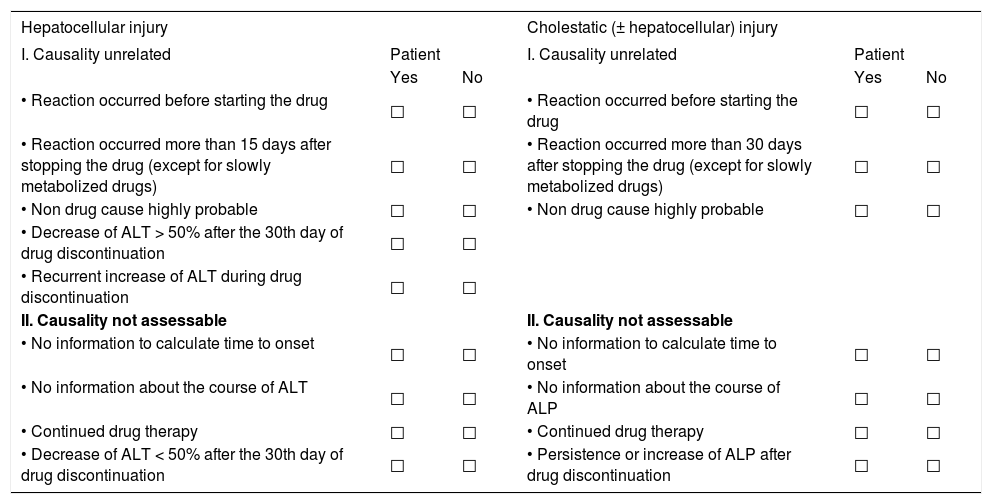
The first step of the causality algorithm in toxic liver disease by DDS represents the qualitative oriented pre-test for the hepatocellular or the cholestatic (± hepatocellular) type of injury.5 This test clarifies with few questions whether causality is unrelated or unassessable (Table 1).
Pre-test.
| Hepatocellular injury | Cholestatic (± hepatocellular) injury | ||||
|---|---|---|---|---|---|
| I. Causality unrelated | Patient | I. Causality unrelated | Patient | ||
| Yes | No | Yes | No | ||
| • Reaction occurred before starting the drug | □ | □ | • Reaction occurred before starting the drug | □ | □ |
| • Reaction occurred more than 15 days after stopping the drug (except for slowly metabolized drugs) | □ | □ | • Reaction occurred more than 30 days after stopping the drug (except for slowly metabolized drugs) | □ | □ |
| • Non drug cause highly probable | □ | □ | • Non drug cause highly probable | □ | □ |
| • Decrease of ALT > 50% after the 30th day of drug discontinuation | □ | □ | |||
| • Recurrent increase of ALT during drug discontinuation | □ | □ | |||
| II. Causality not assessable | II. Causality not assessable | ||||
| • No information to calculate time to onset | □ | □ | • No information to calculate time to onset | □ | □ |
| • No information about the course of ALT | □ | □ | • No information about the course of ALP | □ | □ |
| • Continued drug therapy | □ | □ | • Continued drug therapy | □ | □ |
| • Decrease of ALT < 50% after the 30th day of drug discontinuation | □ | □ | • Persistence or increase of ALP after drug discontinuation | □ | □ |
For differentiation of the hepatocellular, cholestatic or mixed form of hepatotoxicity caused by drugs and dietary supplements (DDS), serum activities of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) are measured on the day the diagnosis of DDS hepatotoxicity is suspected. Each activity is expressed as multiple of the upper limit of the normal range (N), and the ratio (R) of ALT: ALP is calculated. Liver injury is (1) hepatocellular, when ALT < 2N alone or R ≥ 5, (2) cholestatic when there is an increase of ALP < 2N alone or when R ≤ 2, and (3) of the mixed type when ALT < 2N, ALP is increased and 2 > R > 5. When at least one question is answered with yes, causality is unrelated or not assessable, respectively. The pre-test and the subsequent maintest evaluate the hepatocellular injury separately from the cholestatic (± hepatocellular) one. The term drug is used for synthetic drugs, herbal drugs, and dietary supplements including herbal ones.
The second step of the structured causality assessment may be achieved by the main-test (Table 2 and Table 3)5,44 as the updated quantitative CIOMS scale.41,49 For the main-test,5,44 a slightly modified CIO-MS scale was used because some updates regarding precision and diagnostic procedures were necessary.5 Assessment by the CIOMS scale was shown to be associated with a good validation regarding sensitivity (86%), specificity (89%), positive predictive value (93%), and negative predictive value (78%).41 In addition, there was no overlap between the cases and the controls. The quantitative CIOMS scale was based on results obtained following drug re-exposure41 and is worldwide accepted.20,22,42,44,53,54 It was derived from an international consensus meeting of experts who defined various parameters such as time to onset, course of improvement of laboratory data after drug discontinuation, risk factors, concomitant drug, search for nondrug causes, previous information on hepatotoxicity of the drug, and response to readministration.49 It provides with each of these parameters a range of scores. The total score is than computed and may be divided into ranges that represent the causality as being highly probable, probable, possible, unlikely or excluded.
Main-test for cholestatic (± hepatocellular) injury (right side).
| Cholestatic (± hepatocellular) injury | Score | Patient |
|---|---|---|
| 1. Time to onset from the beginning of the drug | ||
| • 5-90 days (rechallenge: 1-90 days) | +2 | ---------- |
| • > 5 or < 90 days (rechallenge: < 90 days) | +1 | ---------- |
| 2. Time to onset from cessation of the drug | ||
| • > 15 days (except for slowly metabolized drugs: < 30 days) | + 1 | ---------- |
| 3. Course of ALP after cessation of the drug | ||
| Difference between peak of ALP and upper limit of normal range | ||
| • Decrease ≤ 50% within 180 days | +2 | ---------- |
| • Decrease ≤ 50% within 180 days | +1 | ---------- |
| • Persistence, increase or no information | 0 | ---------- |
| 4. Risk factor ethanol or pregnancy | ||
| • Yes | +1 | ---------- |
| • No | 0 | ---------- |
| 5. Risk factor age | ||
| • ≤ 55 years | +1 | ---------- |
| • > 55 years | 0 | ---------- |
| 6. Concomitant drug(s) | ||
| • None or no information | 0 | ---------- |
| • Concomitant drug with incompatible time to onset | 0 | ---------- |
| • Concomitant drug with compatible or suggestive time to onset | -1 | ---------- |
| • Concomitant drug known as hepatotoxin and with compatible or suggestive time to onset | -2 | ---------- |
| • Concomitant drug with evidence for its role in this case (positive rechallenge or validated test) | -3 | ---------- |
| 7. Search for non drug causes | ||
| Group I (six causes) | ||
| • Anti-HAV-IgM | ||
| • Anti-HBc-IgM/HBV-DNA | ||
| • Anti-HCV-IgM/HCV-RNA | ||
| • Hepatobiliary sonography/color Doppler sonography of liver vessels | ||
| • Alcoholism (AST/ALT ≤ 2) | ||
| • Acute recent hypotension history (particularly if underlying heart disease) | ||
| Group II | ||
| • Complications of underlying disease(s) | ||
| • Infection suggested by PCR and titer change for: | ||
| CMV (Anti-CMV-IgM/IgG) | ||
| EBV (Anti-EBV-IgM/IgG) | ||
| HSV (Anti-HSV-IgM/gG) | ||
| VZV (Anti-VZV-IgM/IgG) | ||
| Evaluation of group I and II | ||
| • All causes-groups I and II - reasonably ruled out | +2 | ---------- |
| • The six causes of group I ruled out | +1 | ---------- |
| • Five or four causes of group I ruled out | 0 | ---------- |
| • Less than four causes of group I ruled out | -2 | ---------- |
| • Non drug cause highly probable | -3 | ---------- |
| 8. Previous information on hepatotoxicity of the drug | ||
| • Reaction labelled in the product characteristics | +2 | ---------- |
| • Reaction published but unlabelled | +1 | ---------- |
| • Reaction unknown | 0 | ---------- |
| 9. Response to readministration | ||
| • Doubling of ALP with the drug alone | +3 | ---------- |
| • Doubling of ALP with the drugs already given at the time of first reaction | +1 | ---------- |
| • Increase of ALP but less than N in the same conditions as for the first administration | -2 | ---------- |
| • Other situations | 0 | ---------- |
| Total points for patient: |
The term drug is used for synthetic drugs, herbal drugs, and dietary supplements including herbal ones. ALT: Alanine aminotransferase. AST: Asparate aminotransferase. HAV: Hepatitis A virus. HBc: Hepatitis B core. HBV: Hepatitis B virus. HCV: Hepatitis C virus. CMV: Cytomegalovirus. EBV: Epstein Barr virus. HSV: Herpes simplex virus. VZV: Varicella zoster virus. Total points/ causality: ≥ 0 = Excluded. 1-2 = Unlikely. 3-5 = Possible. 6-8 = Probable. <8 = Highly probable.
Main-test for hepatocellular injury (left side).
| Hepatocellular injury | Score | Patient |
|---|---|---|
| 1. Time to onset from the beginning of the drug | ||
| • 5-90 days (rechallenge: 1-15 days) | +2 | ---------- |
| • > 5 or < 90 days (rechallenge: < 15 days) | +1 | ---------- |
| 2. Time to onset from cessation of the drug | ||
| • > 15 days (except for slowly metabolized drugs: < 15 days) | +1 | ---------- |
| 3. Course of ALT after cessation of the drug | ||
| Difference between peak of ALT and upper limit of normal range | ||
| • Decrease ≤ 50% within 8 days | +3 | ---------- |
| • Decrease ≤ 50% within 30 days | +2 | ---------- |
| • No information | 0 | ---------- |
| • Decrease ≤ 50% after the 30th day | 0 | ---------- |
| • Decrease > 50% after the 30th day or recurrent increase | -2 | ---------- |
| 4. Risk factor ethanol | ||
| • Yes | +1 | ---------- |
| • No | 0 | ---------- |
| 5. Risk factor age | ||
| • ≤ 55 years | +1 | ---------- |
| • > 55 years | 0 | ---------- |
| 6. Concomitant drug(s) | ||
| • None or no information | 0 | ---------- |
| • Concomitant drug with incompatible time to onset | 0 | ---------- |
| • Concomitant drug with compatible or suggestive time to onset | -1 | ---------- |
| • Concomitant drug known as hepatotoxin and with compatible or suggestive time to onset | -2 | ---------- |
| • Concomitant drug with evidence for its role in this case (positive rechallenge or validated test) | -3 | ---------- |
| 7. Search for non drug causes | ||
| Group I (six causes) | ||
| • Anti-HAV-IgM | ||
| • Anti-HBc-IgM/HBV-DNA | ||
| • Anti-HCV-IgM/HCV-RNA | ||
| • Hepatobiliary sonography/color Doppler sonography of liver vessels | ||
| • Alcoholism (AST/ALT < 2) | ||
| • Acute recent hypotension history (particularly if underlying heart disease) | ||
| Group II | ||
| • Complications of underlying disease(s) | ||
| • Infection suggested by PCR and titer change for: | ||
| CMV (Anti-CMV-IgM/IgG) | ||
| EBV (Anti-EBV-IgM/IgG) | ||
| HSV (Anti-HSV-IgM/gG) | ||
| VZV (Anti-VZV-IgM/IgG) | ||
| Evaluation of group I and II | ||
| • All causes-groups I and II - reasonably ruled out | +2 | ---------- |
| • The six causes of group I ruled out | +1 | ---------- |
| • Five or four causes of group I ruled out | 0 | ---------- |
| • Less than four causes of group I ruled out | -2 | ---------- |
| • Non drug cause highly probable | -3 | ---------- |
| 8. Previous information on hepatotoxicity of the drug | ||
| • Reaction labelled in the product characteristics | +2 | ---------- |
| • Reaction published but unlabelled | +1 | ---------- |
| • Reaction unknown | 0 | ---------- |
| 9. Response to readministration | ||
| • Doubling of ALT with the drug alone | +3 | ---------- |
| • Doubling of ALT with the drugs already given at the time of first reaction | +1 | ---------- |
| • Increase of ALT but less than N in the same conditions as for the first administration | -2 | ---------- |
| • Other situations | 0 | ---------- |
| Total points for patient: |
The third step of the diagnostic algorithm represents the post-test (Table 4)5,44 for exclusion of various other acute and also chronic liver diseases not yet considered in the main-test (Table 2 and Table 3).
Post-test.
| Differential diagnosis | Diagnostic parameters | Diagnostic exclusion patient |
|---|---|---|
| 1. Autoimmune hepatitis (AIH) Typ I anti-LSP, anti-ASGPR | Gamma globulins, ANA, SMA, AAA, SLA/LP, | Yes □ No □ Partial □ |
| 2. Autoimmune hepatitis (AIH) Typ II anti-LKM-2 (CYP 2C9), anti-LKM-3 | Gamma globulins, anti-LKM-1 (CYP 2D6), | Yes □ No □ Partial □ |
| 3. Primary biliary cirrhosis (PBC) | AMA, anti PDH-E2 | Yes □ No □ Partial □ |
| 4. Primary sclerosing cholangitis (PSC) | p-ANCA, MRC | Yes □ No □ Partial □ |
| 5. Autoimmune cholangitis (AIC) | ANA, SMA | Yes □ No □ Partial □ |
| 6. Overlap syndromes | see 1-5 | Yes □ No □ Partial □ |
| 7. Wilson’s disease | Copper excretion (24 h urine) Ceruloplasmin in serum, free copper in serum, Coombs-negative hemolytic anemia, hepatic copper content, Kayser-Fleischer-Ring, neurologic-psychiatric diagnostic, genotyping | Yes □ No □ Partial □ |
| 8. Hereditary hemochromatosis | Serum ferritin, total iron-binding capacity, genotyping for C2824 and H63D mutation, hepatic iron content | Yes □ No □ Partial □ |
| 9. Porphyria | Porphobilinogen in urine, total porphyrines in urine | Yes □ No □ Partial □ |
| 10. α1 - Antitrypsin deficiency | α1 - Antitrypsin in serum | Yes □ No □ Partial □ |
| 11. Non-alcoholic steatohepatitis | Adipositas, insulin resistence, hepatomegaly, echogenicity of the liver (NASH) | Yes □ No □ Partial □ |
| 12. Toxic liver diseases | Toxicological assessment by occupational and household toxins | Yes □ No □ Partial □ |
| 13. Hepatitis E | anti-HEV-IgM, anti-HEV-IgG, HEV-RNA | Yes □ No □ Partial □ |
| 14. Other viral infectionsAdenovirusCoxsackie-B-VirusEchovirusMeaslesvirusRubellavirusFlavivirusArenavirusFilovirusParvovirusHIV | Specific serology | Yes □ No □ Partial □ |
| 15. Additional infections diseasesBacteriaParasitesWormsMycosis | Specific assessment | Yes □ No □ Partial □ |
| 16. Systemic diseasesM. BoeckAmyloidosisLymphoma and other malignant tumorsSepsis | Specific assessment | Yes □ No □ Partial □ |
| 17. Celiac disease | TTG antibodies, endomysium antibodies, duodenal biopsy | Yes □ No □ Partial □ |
| 18. Addison’s disease | Plasma cortisol | Yes □ No □ Partial □ |
| 19. Thyroid diseases | TSH basal, T4, T3 | Yes □ No □ Partial □ |
| 20. Heat stroke | Shock, hyperthermia | Yes □ No □ Partial □ |
| 21. Grand mal seizures | Status epilepticus (duration < 30 min) | Yes □ No □ Partial □ |
| 22. Rare intoxications | Toxin screening | Yes □ No □ Partial □ |
| 23. Cocaine, ecstasy and other amphetamines | Toxin screening | Yes □ No □ Partial □ |
| 24. Anorexia nervosa | Clinical context | Yes □ No □ Partial □ |
| 25. Cardiopulmonary diseasesCongestive heart diseaseMyocardial infarctionCardiomyopathyCardiac valvular dysfunctionPulmonary embolismPericardial diseasesArrhythmia by hemorrhagic-shock | Cardiopulmonary assessment | Yes □ No □ Partial □ |
| 26. Other diseases | Clinical context | Yes □ No □ Partial □ |
ANA: Antinuclear antibodies. SMA: Smooth muscle antibodies. AAA: Anti-actin antibodies. SLA: Soluble liver antigen. LP: Liver-pancreas antigen. LSP: Liver specific protein. ASGPR: Asialo-glycoprotein-receptor. LKM: Liver kidney microsomes. CYP: Cytochrome P450. AMA: Anitmitochondrial antibodies. DPH: Pyruvat dehydrogenase. p-ANCA: Perinuclear antineutrophile cytoplasmatic antibodies. MRC: Magnetic resonance cholangiography. HEV: Hepatitis E virus. HIV: Human immunodeficiency virus. TTG: Tissue transglutaminase. TSH: Thyroid stimulating hormone.
For the diagnosis of DDS hepatotoxicity, the application of qualitative causality assessments has not found wide spread acceptance, since no data regarding specificity, sensitivity and predictive value are available.5,46-48 On a qualitative basis, however, some items may exclude the diagnosis of DDS hepatotoxicity. The following qualitative criteria are helpful for a quick assessment that can be used in a setting of the pre-test (Table 1):5 causality is unrelated in hepatocellular injury when the reaction occurred before starting the drug; when the reaction occurred more than 15 days after stopping the drug with the exemption of slowly metabolized drugs; when a non-drug cause is highly probable; when the decrease of ALT is > 50% after the 30th day of drug discontinuation; or when there is a recurrent increase of ALT during drug discontinuation. When a cholestatic (± hepatocellular) injury is evident (Figure 2), somewhat different criteria have to be applied in the pre-test (Table 1).
Structured Quantitative Causality AssessmentProvided the pre-test gives no signal for an unrelated causality, the assessment should be continued with the main-test (Figure 1, Table 2 and Table 3) as the scale of the updated CIOMS.5 The update includes various modifications for reasons of precision and actualization. In the main-test there are no more qualitative items5 as in the CIOMS scale.49 Moreover, confirmation of the exclusion of hepatitis B and C is achieved by the inclusion of hepatitis B virus-DNA and hepatitis C virus-RNA.5 Similarly the use of colour Doppler sonography for the assessment of hepatic vessels has been added. For the exclusion of infection by cytomegalovirus (CMV), Epstein Barr virus (EBV), herpes simplex virus (HSV), and varicella zoster virus (VZV), the determination of polymerase chain reaction as well as IgM and IgG antibodies with subsequent titer changes is required on grounds of precision. The main-test with its updated items has been applied in two recent case studies.20,44
Causality in DDS hepatotoxicity may also be evaluated by one of the other quantitative and liver specific scales, which differ quite substantially regarding various criteria.5 A primarily quantitative causality assessment represents the quantitative scale of CIOMS which has only a few qualitative items49 and was updated recently.5 Purely quantitative is the scale of MV, acronym for the authors Maria and Victorino.50 The causality assessment of AD, acronym for the authors Aithal and Day,43 includes three approaches, namely the qualitative CIOMS system, the MV scale, and liver histology. The evaluation by ARD, acronym for the authors Aithal, Rawlins and Day,51 performs without liver histology and uses in the first step some criteria of the qualitative rather than the quantitative CIOMS and subsequently the MV scale. Finally, the grading system of TTK, acronym for the first authors Takamori, Takikawa, Kumagi, et al.52 is worth mentioning. The diagnostic significance of all these scores has been discussed in detail, leading to the conclusion that the main-test as the updated quantitative CIO-MS scale with various modifications for reasons of precision and actualization is best to be used for cases of DDS hepatotoxicity.5 Certainly, the main-test as well as the other quantitative causality assessment methods have the limitation that most but not all DDS unrelated liver disease may be excluded.
Exclusion of Rare DiseasesWith the qualitative post-test, other and rare hepatic and extrahepatic diseases might be evaluated (Table 4).5 Before the post-test was establish to consider a variety of differential diagnoses,5 no systematic approach had been undertaken to solve this problem to exclude other DDS unrelated diseases. Consequently, various other liver diseases may have been overlooked, as shown by the long list of missed diagnoses.20,22,41-45
Medical ReportingWhen DDS hepatotoxicity is suspected, the treating physician or other health care providers commonly notify the national regulatory agency, possibly also the manufacturer.5 The reporting system should include the diagnostic algorithm with the pre-test, the main-test, and the post-test with all information listed in the questionaires (Table 1Table 2Table 3Table 4).5,49 The CIO-MS scale itself has been applied in various published reports,20,22,42,44,53 but there are no data available to what extent the scale is at present being used for the information of the regulatory agencies21,33,37,55 or the WHO.21 It appears, however, that the quality of medical reporting is, in general, mostly insufficient regarding data supply, exclusion of DDS unrelated liver disease, and causality assessment.33,37
There should also be an obligation to use and publish the results of a diagnostic algorithm such as the pre-test, main-test, and post-test item by item in any case report accepted by a medical journal. This approach may reduce scientific discussions whether a DDS is really hepatotoxic or not. Poor data presentation was evident, for instance, in published case reports of assumed hepatotoxicity by black cohosh, a herbal drug for menopausal symptoms.44,56
Regulatory Assessment And Pharmacovigilance EvaluationThe crucial point in DDS hepatotoxicity evaluation is how regulatory agencies meet the challenges of causality assessment and pharmacovigilance evaluation. For a reliable assessment the agencies will have to use the diagnostic algorithm (pre-test, maintest, post-test) which may be best provided by the reporting physician or should be applied by its own, with the data available. Certainly, regulatory causality assessment may be cumbersome, but inevitable to avoid major discussions as shown in the past.20,36,39 Data from several individual cases of suspected DDS hepatotoxicity will be combined by the regulatory agency to assess possible signals of toxicity as basis for further strategies related to pharmacovigilance. These include also quality assessment of synthetic drugs and herbal drugs, and in the latter group especially the question of plant quality as shown recently.20,55,57,58
Data Communication By Regulatory AgenciesThere are different approaches of the various regulatory agencies8,17,33,35,37,55,56 and health organizations, such as the WHO,21 for handling cases of suspected DDS hepatotoxicity. It appears that not all data is reported to the scientific community,8,17,21,33,37,55,56 and when they are available, there are major differences regarding the quantity and quality of their information. For instance, statements of causality assessments are shown with few examples.
The WHO database presents information on cases with assumed hepatotoxicity by synthetic drugs.21 An evaluation of the data quality reveals, however, that a thorough causality assessment such as with the CIOMS scale has not been done, and the presented data were not suitable for a subsequent causality assessment. It appears that the WHO database is not that helpful in assessing causality of the broad spectrum of toxic liver disease by synthetic drugs. The problem of the WHO database may be the result of poor quality input data supply without a thorough quantitative causality assessment being performed by the various national regulatory agencies.
The German regulatory agency (BfArM, Bundesinstitut für Arzneimittel und Medizinprodukte, Bonn) concluded in an ad-hoc based causality assessment that in 26 cases from Germany and Switzerland with liver disease a possible, probable or highly probable causality exists for kava, a herbal anxiolytic drug.33 The regulatory data presentation was poor, lacking both a thorough quantitative causality assessment and information to substantiate the claimed causality8,20,57 and this was only partially possible even for the British Medical Control Agency (MCA)34,36 and EMEA (European Medicine Agency).35,36 When additional data were obtained, causality assessed with the updated quantitative CIOMS scale for kava ± co-medication was possible, probable or highly probable in only assessed eight cases, in seven of these there was a daily kava overdose and/or a prolonged treatment.20 Due to the low quality of regulatory data presentation and the resulting causality evaluation,8,33 a worldwide discussion emerged over the regulatory shortcomings.8,20,36,57 A new regulatory approach for a structured causality assessment in cases with assumed hepatotoxicity by kava, co-medicated drugs and dietary supplements is recommended under these circumstances.
Poor data presentation by various national regulatory agencies was another problem for the evaluation of liver disease possibly linked to the use of black cohosh, a herbal drug used to treat postmenopausal symptoms.17,55 In 30 patients a possible causality was described using the disease unspecific Naranjo scale rather than the liver disease specific quantitative CIOMS scale. No data are presented to exclude other drug unrelated liver diseases17,55 and the conclusions arrived at are not substantiated by the presented information.17,39
A good example is the causality assessment presented by EMEA for cases with liver disease in assumed causal relationship to the use of black cohosh for treating menopausal symptoms.37 EMEA analyzed a total of 40 cases using the quantitative CIOMS scale and concluded that only four cases showed some degree of causality. The presented data of these four patients were the basis of further analysis showing that causality was probable in two patients and possible in the other ones when the CIOMS scale was applied item by item.44 Therefore, the regulatory issue in suspected DDS hepatotoxicity should generally include the results item by item of the quantitative main-test or the CIOMS scale for a valid causality assessment.
These few examples clearly show that national regulatory agencies are responsible to present detailed information and data of a structured quantitative causality assessment in the form of the main-test or the CIOMS scale in all patients with suspected DDS hepatotoxicity. This regulatory approach is essential to circumvent perpetuated discussions with the scientific community about cases of patients with suspected DDS hepatotoxicity. It is also inevitable that the data of all patients with DDS hepatotoxicity be presented in the regulatory database with the quantitative main-test or the CIOMS scale including item by item information, and that all interested health care providers and scientists have free access to all data required for causality assessment.
Clinical, Regulatory And Scientific IssuesAt the very end of the evaluation process of cases with DDS hepatotoxicity various questions have to be answered. They include:
- •
Was the alleged DDS convincingly responsible for the hepatic reaction or not?
- •
Were there sufficient data for causality assessment by the main-test or the scale of the quantitative CIOMS?
- •
Has the main-test or the CIOMS scale been submitted item by item to the national regulatory agency to be entered into the regulatory database item by item, available to all interested health care providers and scientists?
- •
Was the regulatory action based on this case (and others) adequate regarding the issue of pharmacovigilance?
- •
Were the case reports submitted for publication in scientific journals substantiated regarding causality by the use of the quantitative main-test or the CIOMS scale, and was item by item presented to be published?
Cases with assumed DDS hepatotoxicity represent major clinical, regulatory and scientific issues that require first a thorough structured quantitative causality assessment, necessitate second a regulatory assessment for pharmacovigilance perspectives and should third facilitate open discussions to reach a scientific basis for a consensus between all involved parties. These include primary care physicians, treating hospital physicians, other health care providers, regulatory agencies, manufacturers and interested scientists. This algorithm with a common and structured approach to data analysis, data accuracy, data collection, data reporting and analysis is essential to meet safety concerns of possible hepatotoxic side effects because of DDS use, and to insure that a correct hepatotoxicity diagnosis is made.