Background. Liver re-transplantation (re-OLT) remains the only feasible option for patients with graft failure following liver transplantation. Sparse resources and a growing waitlist mandate that available grafts are allocated properly. We studied the differences in patient demographics, characteristics, and survival for those listed for re-OLT in a region with prolonged wait times.
Material and methods. We performed a single-center retrospective study, from 2005 to 2013, of adult candidates listed for liver re-OLT at a tertiary care center within United Network for Organ Sharing (UNOS) region 1.
Results. Of the 48 patients listed for re-OLT, 1(2%) improved while waiting, 14(29%) died while waiting, and 33(69%) underwent re-OLT. Those re-transplanted represented 11% of the center’s adult liver transplant volume during the same time period. Comparing those who died while waiting to those who achieved re-OLT, there was no significant difference in age (median 52 vs. 48 years, p=0.56) or MELD at second listing (median 29 vs. 26, p = 0.90). Waitlisted candidates who failed to achieve re-transplant died on average of 15.5 days (IQR 36 days) days after re-listing. Those re-transplanted achieved 3-year survival of 70% and there was no significant difference in 3-year survival of those re-transplanted within or beyond 90 days of first transplant (70% vs. 69.5%, p = 0.28).
Conclusions. In conclusion, re-OLT is the only viable option for candidates with nonreversible liver graft failure. Inability to achieve re-OLT leads to nearly assured and expeditious death. Despite technical challenges, in experienced hands excellent long term survival following re-OLT can be achieved.
Since the first human liver transplant in 1963, liver transplantation (OLT) has remained the primary therapy for end-stage liver disease. Although there have been considerable advances in immune modulation, surgical technique, and medical management, the demand for liver grafts continues to outpace the supply of organs available for transplantation. Specifically, despite over 6,700 liver transplants in 2014, more than 15,000 patients still remain on the liver transplantation waitlist, and over 3,800 died awaiting OLT.1 Given this, assigning allocation priorities is of paramount importance.
Primary graft failure following OLT poses a dilemma, as life-saving liver re-transplantation (re-OLT) is required in an estimated 10-20% of cases.2–6 Although it is often felt that re-OLT is unavoidable in certain cases, there continue to be significant ethical concerns about whether or not it is justified.2 From a healthcare system standpoint, it has been well demonstrated that re-OLT increases cost and resource utilization.5–7 Similarly, from a patient care perspective, numerous studies have reported decreased survival following re-OLT as compared to primary OLT.6,8–12 Aside from traditional donor and recipient factors, important considerations that have been investigated with regards to survival after re-OLT include optimal timing, with no conclusive evidence showing superiority of early re-transplant (0-30 days) vs. late re-transplant (> 30 days).5,11,13,14 If re-OLT is going to continue to be considered in a sparse resource environment with a growing waitlist, furthering our understanding of these patients is important in order to make informed decisions.
Given this, we sought to compare the differences in patient demographics, characteristics and survival of those patients who underwent liver re-OLT vs. those candidates who were listed for re-OLT but failed to achieve re-transplant. Furthermore, in those who achieved re-OLT, we aimed to see if timing had any impact on overall survival.
Material and MethodsA single-center, retrospective study was performed at a tertiary care center within UNOS Region 1 (United States of America) between January 2005 and January 2013. All adult patients (age > 18 years at the time of transplant) who underwent liver transplantation and required subsequent relisting for re-OLT were included in the study. In order to decrease selection bias, exclusion criteria were determined a priori and included pediatric patients (< 18 years of age) as well as those receiving combined transplants (e.g. simultaneous liver and kidney transplants) who were felt to be inherently different than the analyzed cohort.15 Patients in the study were divided into two groups: those who ultimately underwent re-OLT and those who were removed from the waitlist due to clinical deterioration or death. Causes for liver re-OLT, as previously defined by Markmann, et al.,8 were as follows: primary nonfunction (PNF) - identified as non-technical graft failure requiring re-OLT within 1 week without other identifiable causes, initial poor function (IPF) - identified as non-technical graft failure requiring re-OLT after 1 week without other identifiable causes, acute hepatic artery thrombosis (HAT) requiring emergent re-OLT, chronic cellular rejection, chronic biliary stricture, recurrent disease and other (drug-toxicity induced liver failure). Patients were removed from the waitlist due to death as a result of: sepsis, recurrent disease, chronic cellular rejection, intra-cerebral hemorrhage, acute HAT, chronic biliary stricture and other (post-transplant lymphoproliferative disorder and allograft sickle cell disease). Demographics and re-OLT characteristics were obtained through chart review. Vital status and date of death were determined by performing an audit of the electronic medical records and were verified by the Social Security Death Master File. To decrease information bias, only variables with data that was available through single center retrospective chart review were collected.
Recipient characteristics including age, sex, wait time (for the first and subsequent listings and transplants), MELD score (at first listing, initial transplant, subsequent listing, and re-OLT), and hospital length of stay (LOS) (for the first and subsequent transplants) were compared between those patients that underwent re-OLT and those that died while on the waitlist. Subgroup analyses were then performed on patients receiving re-OLT. As the average waitlist period for those undergoing re-OLT approximated 91 days, a decision was made to define 90 days as a specific cut-off point for further subgroup analysis. Thus, patients who were re-transplanted within 90 days of their initial liver transplant were compared to those re-transplanted after 90 days from their initial liver transplant. This comparison was also made between patients who were re-transplanted within 90 days and those re-transplanted after 90 days from their second listing.
Continuous variables were compared using a Mann-Whitney U test, while categorical variables were compared using 2-group proportion tests. Overall survival following re-OLT was obtained by Kaplan-Meier analysis. Stratified Kaplan-Meier analysis using a right-censored dataset was used to evaluate differences in overall survival between re-OLT groups. Differences in overall survival were assessed using a log-rank test. For all statistical tests, a pre-specified two-sided α of 0.05 was regarded as statistically significant. All statistical analyses were conducted using STATA/MP 11 (StataCorp LP, College Station, TX, USA). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations were used as a guideline for appropriate reporting of the results of this observational study.16 All work was approved by the Massachusetts General Hospital Institutional Review Board (Protocol #2014P000230).
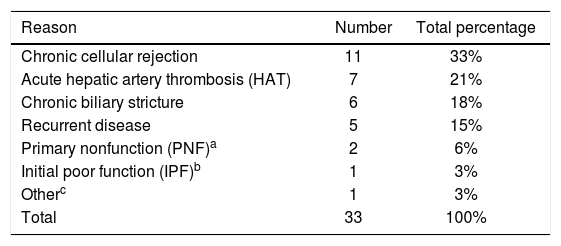
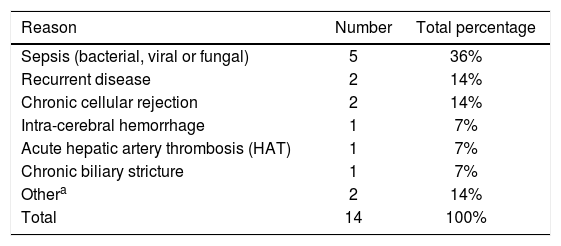
ResultsOver the 8-year study period 346 OLT’s were performed at our institution in 313 adults (age > 18 years at the time of transplant), with 32 patients having undergone a second liver transplant, and 1 patient undergoing a total of 3 transplants. Of patients with graft failure after initial transplantation, 48 were listed for re-OLT with the following results: 1 (2%) improved while waiting, 33 (69%) underwent re-OLT and 14 (29%) died while waiting. Main indications for re-OLT were: chronic cellular rejection (33%), acute HAT requiring emergent re-transplantation (21%), chronic biliary strictures (18%), recurrent disease (15%), PNF (6%), IPF (3%) and other (drug-toxicity induced liver failure - 3%) (Table 1). Causes for death and subsequent removal off the waitlist were: sepsis (bacterial, viral or fungal - 36%), recurrent disease (14%), chronic cellular rejection (14%), intra-cerebral hemorrhage (7%), acute HAT (7%), chronic biliary stricture (7%) and other (post-transplant lymphoproliferative disorder -7%, allograft sickle cell disease - 7%) (Table 2). Those patients that underwent re-OLT represented approximately 11% of the total adult liver transplant volume during this time period and eight of these patients (24%) underwent simultaneous liver/kidney re-transplantation. Median age of those listed for re-OLT was 53.3 (IQR 18.14) years old with a median MELD of 26 (IQR 17) at second listing. Notably, of the five patients undergoing re-OLT for recurrent disease, two (40%) had recurrent hepatitis C virus (HCV) whereas of the two patients who died while awaiting re-OLT, one (50%) had recurrent HCV.
Indications for re-transplantation.
| Reason | Number | Total percentage |
|---|---|---|
| Chronic cellular rejection | 11 | 33% |
| Acute hepatic artery thrombosis (HAT) | 7 | 21% |
| Chronic biliary stricture | 6 | 18% |
| Recurrent disease | 5 | 15% |
| Primary nonfunction (PNF)a | 2 | 6% |
| Initial poor function (IPF)b | 1 | 3% |
| Otherc | 1 | 3% |
| Total | 33 | 100% |
Primary nonfunction (PNF) defined as graft failure not due to a technical complication, or without other identifiable causes requiring re-transplantation within 1 week of the original procedure.
Causes of death while on waitlist for re-transplantation.
| Reason | Number | Total percentage |
|---|---|---|
| Sepsis (bacterial, viral or fungal) | 5 | 36% |
| Recurrent disease | 2 | 14% |
| Chronic cellular rejection | 2 | 14% |
| Intra-cerebral hemorrhage | 1 | 7% |
| Acute hepatic artery thrombosis (HAT) | 1 | 7% |
| Chronic biliary stricture | 1 | 7% |
| Othera | 2 | 14% |
| Total | 14 | 100% |
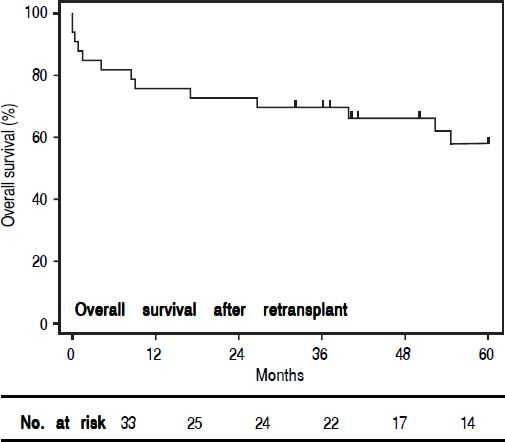
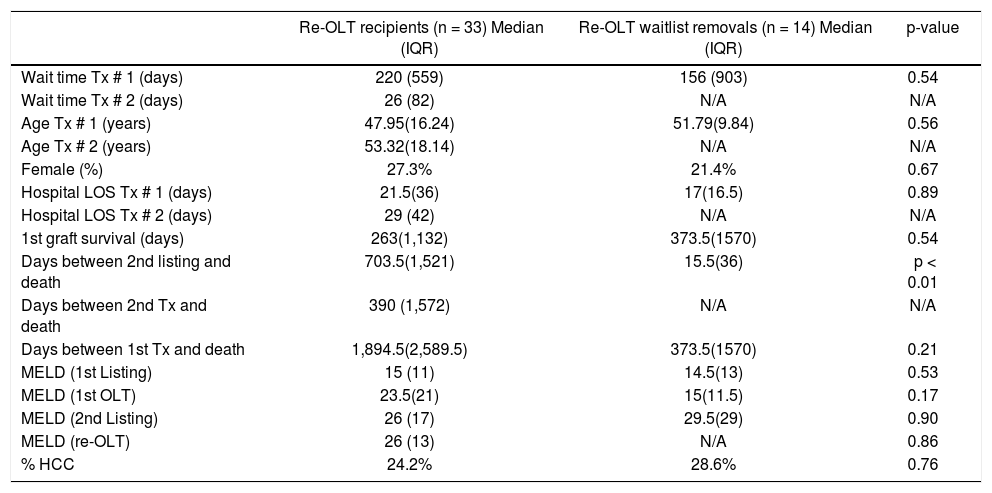
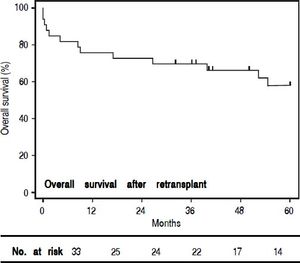
When comparing those patients who underwent re-OLT to those who died on the waitlist (Table 3), there was no significant difference in age, sex or MELD scores at the second listing. Furthermore, in those patients who underwent re-OLT, wait times, MELD scores, and hospital LOS during the primary transplant were not significantly different. Candidates listed for re-OLT who failed to achieve transplant died 15.5 (IQR 36) days following re-listing, in comparison to those who achieved re-OLT who died 703.5 (IQR 1521) days following re-listing (p < 0.001). The median time to re-OLT was 26 (IQR 82) days. For patients achieving re-OLT, overall 1-, 3-, and 5-year survival was 76%, 70% and 58%, respectively (Figure 1). Median follow-up time for survival analyses was 50 months.
General demographics of patients who achieved re-transplantation vs. those who died on the waitlist.
| Re-OLT recipients (n = 33) Median (IQR) | Re-OLT waitlist removals (n = 14) Median (IQR) | p-value | |
|---|---|---|---|
| Wait time Tx # 1 (days) | 220 (559) | 156 (903) | 0.54 |
| Wait time Tx # 2 (days) | 26 (82) | N/A | N/A |
| Age Tx # 1 (years) | 47.95(16.24) | 51.79(9.84) | 0.56 |
| Age Tx # 2 (years) | 53.32(18.14) | N/A | N/A |
| Female (%) | 27.3% | 21.4% | 0.67 |
| Hospital LOS Tx # 1 (days) | 21.5(36) | 17(16.5) | 0.89 |
| Hospital LOS Tx # 2 (days) | 29 (42) | N/A | N/A |
| 1st graft survival (days) | 263(1,132) | 373.5(1570) | 0.54 |
| Days between 2nd listing and death | 703.5(1,521) | 15.5(36) | p < 0.01 |
| Days between 2nd Tx and death | 390 (1,572) | N/A | N/A |
| Days between 1st Tx and death | 1,894.5(2,589.5) | 373.5(1570) | 0.21 |
| MELD (1st Listing) | 15 (11) | 14.5(13) | 0.53 |
| MELD (1st OLT) | 23.5(21) | 15(11.5) | 0.17 |
| MELD (2nd Listing) | 26 (17) | 29.5(29) | 0.90 |
| MELD (re-OLT) | 26 (13) | N/A | 0.86 |
| % HCC | 24.2% | 28.6% | 0.76 |
IQR: inter-quartile range. Tx: transplant. LOS: length of stay. MELD: Model for End-Stage Liver Disease. HCC: hepatocellular carcinoma.
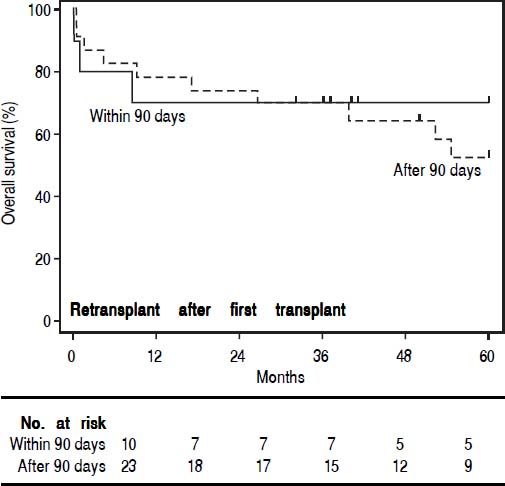
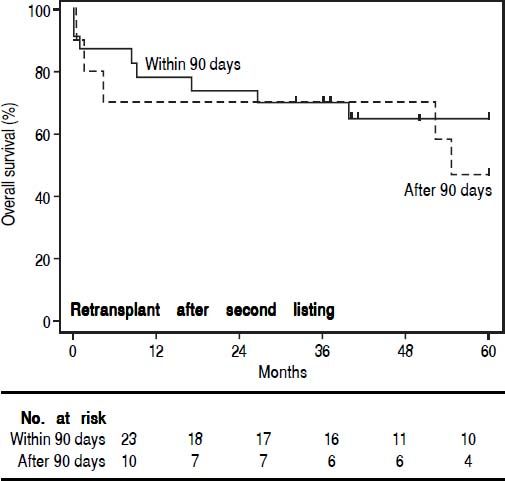
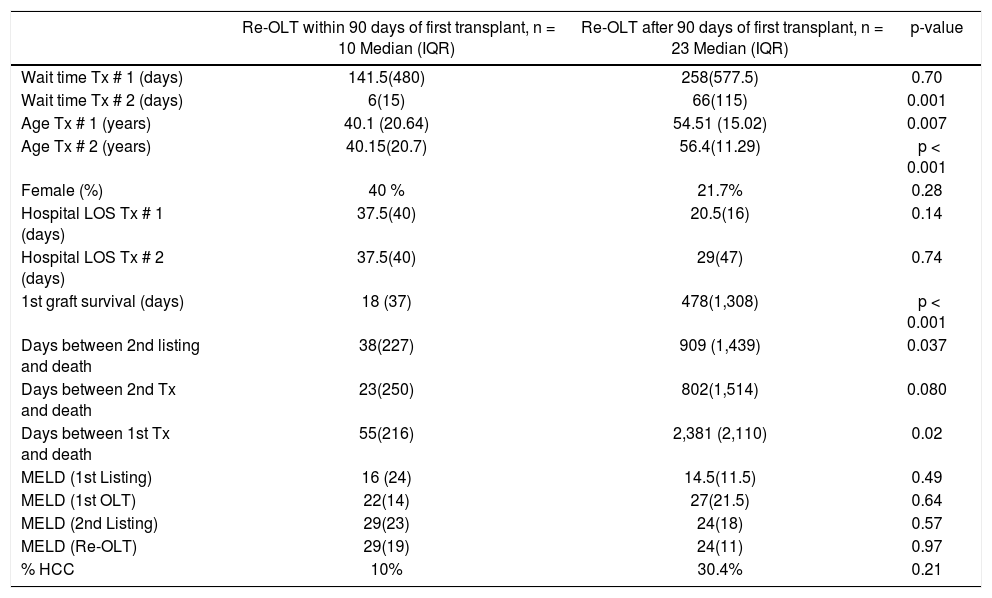
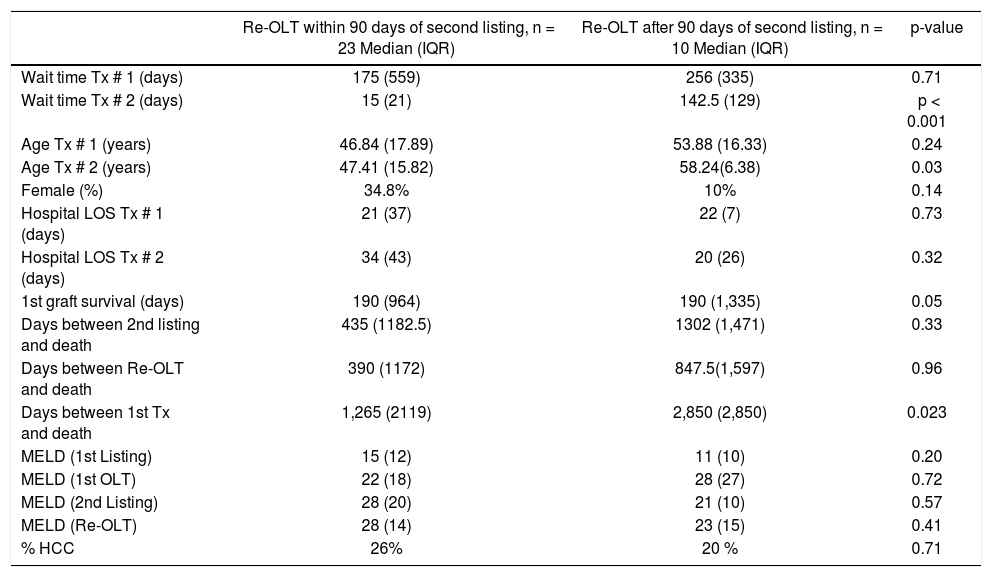
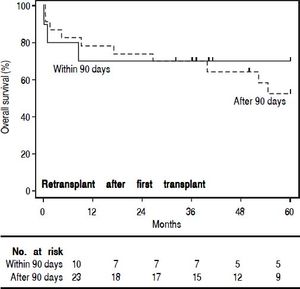
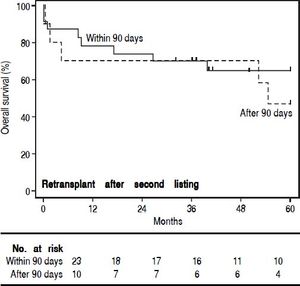
In order to study the impact of re-OLT timing, patients who underwent re-OLT early (within 90 days of their first transplant) were compared to those who underwent re-OLT late (after 90 days following their first transplant) (Table 4). Aside from age at transplant, these groups were similar. Further, survival analysis demonstrated no difference in 3-year survival between the groups (70% vs. 70%, log-rank test p = 0.59) (Figure 2). Lastly, to assess the impact of wait times after re-listing, a comparison was made between patients who underwent re-OLT within 90 days of the second listing and those that underwent re-OLT 90 days after second listing (Table 5). These groups were noted to be similar throughout. Likewise, there was no significant difference in survival estimates at 3-years (70% vs. 70%, log-rank test p = 0.50) (Figure 3).
Comparison of patients who underwent re-transplantation early (within 90 days) vs. late (after 90 days) of the first transplant.
| Re-OLT within 90 days of first transplant, n = 10 Median (IQR) | Re-OLT after 90 days of first transplant, n = 23 Median (IQR) | p-value | |
|---|---|---|---|
| Wait time Tx # 1 (days) | 141.5(480) | 258(577.5) | 0.70 |
| Wait time Tx # 2 (days) | 6(15) | 66(115) | 0.001 |
| Age Tx # 1 (years) | 40.1 (20.64) | 54.51 (15.02) | 0.007 |
| Age Tx # 2 (years) | 40.15(20.7) | 56.4(11.29) | p < 0.001 |
| Female (%) | 40 % | 21.7% | 0.28 |
| Hospital LOS Tx # 1 (days) | 37.5(40) | 20.5(16) | 0.14 |
| Hospital LOS Tx # 2 (days) | 37.5(40) | 29(47) | 0.74 |
| 1st graft survival (days) | 18 (37) | 478(1,308) | p < 0.001 |
| Days between 2nd listing and death | 38(227) | 909 (1,439) | 0.037 |
| Days between 2nd Tx and death | 23(250) | 802(1,514) | 0.080 |
| Days between 1st Tx and death | 55(216) | 2,381 (2,110) | 0.02 |
| MELD (1st Listing) | 16 (24) | 14.5(11.5) | 0.49 |
| MELD (1st OLT) | 22(14) | 27(21.5) | 0.64 |
| MELD (2nd Listing) | 29(23) | 24(18) | 0.57 |
| MELD (Re-OLT) | 29(19) | 24(11) | 0.97 |
| % HCC | 10% | 30.4% | 0.21 |
IQR: inter-quartile range. Tx: transplant. LOS: length of stay. MELD: Model for End-Stage Liver Disease. HCC: hepatocellular carcinoma.
Comparison of patients who underwent re-transplantation early (within 90 days) vs. late (after 90 days) of the second listing.
| Re-OLT within 90 days of second listing, n = 23 Median (IQR) | Re-OLT after 90 days of second listing, n = 10 Median (IQR) | p-value | |
|---|---|---|---|
| Wait time Tx # 1 (days) | 175 (559) | 256 (335) | 0.71 |
| Wait time Tx # 2 (days) | 15 (21) | 142.5 (129) | p < 0.001 |
| Age Tx # 1 (years) | 46.84 (17.89) | 53.88 (16.33) | 0.24 |
| Age Tx # 2 (years) | 47.41 (15.82) | 58.24(6.38) | 0.03 |
| Female (%) | 34.8% | 10% | 0.14 |
| Hospital LOS Tx # 1 (days) | 21 (37) | 22 (7) | 0.73 |
| Hospital LOS Tx # 2 (days) | 34 (43) | 20 (26) | 0.32 |
| 1st graft survival (days) | 190 (964) | 190 (1,335) | 0.05 |
| Days between 2nd listing and death | 435 (1182.5) | 1302 (1,471) | 0.33 |
| Days between Re-OLT and death | 390 (1172) | 847.5(1,597) | 0.96 |
| Days between 1st Tx and death | 1,265 (2119) | 2,850 (2,850) | 0.023 |
| MELD (1st Listing) | 15 (12) | 11 (10) | 0.20 |
| MELD (1st OLT) | 22 (18) | 28 (27) | 0.72 |
| MELD (2nd Listing) | 28 (20) | 21 (10) | 0.57 |
| MELD (Re-OLT) | 28 (14) | 23 (15) | 0.41 |
| % HCC | 26% | 20 % | 0.71 |
IQR: inter-quartile range. Tx: transplant. LOS: length of stay. MELD: Model for End-Stage Liver Disease. HCC: hepatocellular carcinoma.
In this investigation we found that patients who are relisted for transplant and do not receive re-OLT are nearly certain to undergo an expeditious death. Conversely, those who achieve re-OLT have good overall survival. With regards to timing of re-OLT, there was no significant difference in outcomes of those receiving early intervention (within 90 days of first transplant) and those waiting for shorter periods of time (less than 90 days from second listing). Hepatic re-transplantation remains controversial amongst the transplantation community as the decision to re-transplant utilizes valuable resource,11 increases overall hospital costs,7 poses significant technical challenges to the transplant surgery team,17 and comes at the cost of delaying transplantation for other candidates on the waitlist.
In the current study, candidates who were removed from the waiting list for re-OLT due to death were found to suffer mortality approximately 15 times faster than those who were not removed and ultimately achieved re-OLT. This finding was unexplained by acuity of illness after initial transplantation as no significant difference in MELD scores at second listing was found between those who died while on the waitlist and those who went on to achieve re-OLT. Rather, it is likely that the rapid death in those not achieving re-OLT was secondary to the etiology of their end-organ failure. Specifically, we found that the most common cause for death and subsequent removal off the waitlist to be due to sepsis, followed by recurrent disease and chronic rejection. These etiologies are similar to those found by Watt, et al. who noted sepsis to be the cause of death for 50% of their re-OLT candidates.18 Taken together, these data suggest that in those with evidence of sepsis, progression of end-organ failure is likely fatal and re-OLT may be futile. With regard to recurrent disease, the advent of second generation direct acting antivirals (DAA) will likely both decrease the previously noted 20-30%, 5-year post-transplant cirrhosis rate in patients who are transplanted with HCV as well the need for re-OLT in this population.19 Indeed, in our cohort two of the patients receiving re-OLT and one of the patients who died with awaiting re-OLT had recurrent HCV and may have benefitted from DAA therapy.
In contrast, re-OLT may portend significant benefit in specific patient populations. When comparing our current results to those from a study performed within the same region in 1993,20 we note that while the re-OLT rate remained the same (11%), the 1-year survival improved (from 48% to 76%) both within the region, as well as na-tionally.6–8,20,21 This improvement can likely be attributed to technical progress, advancements in postoperative critical care, and better immunosuppressive regimens. Specific patient factors that have been noted to be associated with survival in re-OLT patients include recipient age, bilirubin levels, creatinine, UNOS status, and the need for mechanical ventilation.4,14
Although this study was not designed to identify specific donor and recipient factors associated with survival after re-OLT, analysis was performed to help determine the impact of re-OLT timing on outcomes, as this often poses a clinical challenge. While certain complications such as primary graft non-function require immediate intervention, management of other more insidious manifestations of hepatic failure after initial transplantation is more variable. Late or so called “elective” re-transplantations were argued for by Azoulay, et al.,6 who demonstrated similar survival between elective re-transplant recipients and primary OLT recipients, while Powelson, et al.20 recommended that if re-OLT is required, it should be performed either in the immediate transplant period (0-72 h) or delayed until after 30 days so that it is performed after the patient has stabilized from their initial operation. However, when Kim, et al.11 compared outcomes between those re-transplanted within 30 days to those re-transplanted after 30 days of primary transplantation, late (> 30 days) re-OLT was associated with a 6.7-fold increase in the risk of death, while the survival in those re-transplanted early (< 30 days) was similar to primary OLT. Supporting these findings, Facciuto, et al.,13 also demonstrated that late re-transplantation (> 6 months following primary OLT) was associated with increased mortality. Given the inconclusive evidence in the literature, in the current study we compared outcomes at both 90 days from transplant and 90 days from second listing and found no significant difference in overall survival. These findings suggest that there is no clear cutoff with regards to timing after initial liver transplant, or duration of time on the waitlist, that should be rationed as a reason to pursue or forego re-OLT. Rather, the decision of when to re-transplant a candidate should be made individually based on each recipients clinical condition.
Limitations of our study are largely inherent to its retrospective design along with limited sample size given the relatively uncommon occurrence of the procedure being studied (re-OLT). Additionally, as the verifiable institutional database included primarily recipient level data, the lack of donor characteristics, such as donor age, gender, co-morbidities and mechanism of death, made it difficult to perform a more in-depth analysis incorporating variables that may otherwise impact outcomes in our cohort. Further challenges of our study are largely inherent to its retrospective design and type II limitations due to small sample size given the relatively uncommon occurrence of the procedure being studied (re-OLT). Finally, given that recipients in our study were those residing in a region known to have long wait list times (UNOS region 1), generalizability of our observations to short waitlist, low MELD regions is limited.
ConclusionDespite recent improvements in surgical technique, donor selection criteria, and postoperative management of the liver transplant patient, re-OLT remains the only viable option for candidates experiencing non-reversible graft failure. Indeed, the inability to achieve re-OLT leads to nearly assured and expeditious death. Although hepatic re-OLT may pose technical challenges not seen with primary transplantation, in experienced hands good long term survival following re-OLT can be achieved regardless of the timing of primary graft failure. Prior to proceeding with re-OLT, however, the underlying etiology of end-organ failure should be determined as re-OLT in patients with sepsis is often futile.
Abbreviations- •
DAA: direct acting antiviral.
- •
HAT: hepatic artery thrombosis.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis c virus.
- •
IPF: initial poor function.
- •
IQR: inter-quartile range.
- •
LOS: length.
- •
MELD: Model for End-Stage Liver Disease.
- •
OLT: orthotopic liver transplantation.
- •
PNF: primary nonfunction.
- •
Re-OLT: re-transplantation.
- •
Tx: transplant.
- •
UNOS: United Network for Organ Sharing.
None of the authors have financial or other potential conflicts of interest with any companies/organizations whose products or services are discussed in this manuscript.
AcknowledgementsThis work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.