Liver resection is the standard treatment for resectable liver tumors and metastases. However, mortality and morbidity remain significant concerns, particularly for patients with chronically elevated central venous pressure (CVP), which increases perioperative complication risks. The optimal parenchymal transection technique for these patients remains unclear, necessitating further research.
Materials and MethodsThis study established an innovative porcine model for high-CVP liver resection. Animals were divided into two groups: a control group (CVP ≤ 5 mmHg, low-CVP) and an intervention group (CVP ≥ 10 mmHg, high-CVP). A left lateral liver resection was performed using three parenchymal transection techniques: clamp-crush (CC), harmonic scalpel (HS), and stapler (ST). The primary endpoint was intraoperative blood loss, while secondary endpoints included transection time and bile leakage.
ResultsNo differences were found for blood loss or transection time among the low-CVP subgroups. In the high-CVP group, the HS and ST techniques were associated with significantly reduced blood loss and faster transection times than the CC technique. While transection times for the HS and ST were similar between the low- and high-CVP groups, they were significantly longer with the CC technique in the high-CVP group. The incidence of bile leakage was comparable across all three techniques.
ConclusionsThis pilot study demonstrates superior outcomes for HS and ST techniques in high-CVP liver resections. Insights from this large animal model provide a basis for investigating optimal transection techniques for chronically elevated CVP, bridging preclinical research and clinical practice.
Liver resection (LR) is the primary treatment option for resectable hepatic tumors and metastases. Despite recent advances in surgical techniques and perioperative care, LR remains a high-risk procedure. Mortality rates in specialized high-volume centers have been reported to be below 5 %; however, morbidity remains high, with rates of up to 42 % [1]. Intraoperative blood loss poses a significant risk for adverse outcomes following LR and has been linked to increased morbidity and mortality, prolonged surgery, postoperative hepatic failure, and tumor recurrence [2–4].
Intraoperative blood loss during hepatic surgery is typically encountered during parenchymal transection, making it the most critical step in LR. The main source of bleeding during parenchymal transection is backflow from the valveless hepatic veins draining into the inferior vena cava (IVC). Thus, control of central venous pressure (CVP) is essential to minimize blood loss during LR [5]. Lowering CVP reduces the impedance to hepatic venous flow, improves venous drainage from the liver, and thus minimizes blood loss. Strategies to achieve low CVP include patient position (reverse Trendelenburg position), fluid restriction, pharmacological approaches (vasodilators, diuretics, anesthetics), and ventilation adjustment [5,6]. However, patients with comorbidities, including liver cirrhosis, cardiac insufficiency, and portal or pulmonary hypertension, frequently exhibit chronically elevated CVP levels. Owing to the limited options for CVP reduction in these patients, they face a higher risk of intraoperative bleeding and subsequently experience increased morbidity and mortality rates after LR [3,7].
A variety of techniques and devices are available for hepatic surgery with the goal of safe, fast, and anatomically and oncologically correct parenchymal transection. Modern devices (including ultrasonic dissectors, high-frequency sealers, and radiofrequency sealers) have largely replaced traditional methods, such as the finger fracture technique, promising reduced blood loss, and improved outcomes [8–10]. While these findings were predominantly obtained in low-CVP cohorts, it remains unknown whether these results are transferable to patients with chronically elevated CVP [11]. Although high-risk patients with chronically elevated CVP would benefit the most from blood-saving procedures, the optimal parenchymal transection technique in these patients remains unknown. Thus, the current state of knowledge is insufficient to address the issue of parenchymal transection technique selection, which is mostly determined by the surgeon's preference rather than evidence-based practices.
This study aimed to assess the impact of different parenchymal transection techniques (clamp-crush (CC), harmonic scalpel (HS), and stapler (ST)) on intraoperative blood loss by developing and validating a novel and innovative porcine animal model of LR at physiologically low and pathologically elevated CVP levels. We hypothesized that intraoperative blood loss during LR at elevated CVP levels is dependent on the parenchymal transection technique and therefore represents a potentially modifiable factor that could enhance critical intraoperative performance indicators.
2Materials and Methods2.1Experimental designThis study compared three different parenchymal transection techniques in a porcine model of atypical (non-anatomic) left lateral lobe (LLL) LR under physiological baseline and pathologically elevated CVP conditions. Animals were divided into two groups: 1) low-CVP (≤ 5 mmHg, n = 10, control) and 2) high-CVP (≥ 10 mmHg, n = 15, intervention). Subsequently, low- and high-CVP animals were randomly assigned to three subgroups based on the parenchymal transection technique: I) CC, II) HS, and III) ST.
2.2AnimalsA total of 25 female German Landrace pigs (aged 3–6 months, weighing 20–40 kg) were used. All animals were housed in the central animal facility of the University of Münster and acclimatized for 7 days. They were provided with free access to water and standard chow. All procedures were conducted in accordance with the German Animal Welfare Law, and the study was approved by the local animal care committee (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, permit reference number 81–02.04.2021.A459).
2.3Anesthesia and hemodynamic monitoringWe used a previously described standardized anesthesia protocol [12]. After a 12-hour fast with ad libitum access to water, animals were anesthetized via intramuscular injections of ketamine (15 mg/kg, 100 mg/mL, WDT, Garbsen, Germany), azaperone (2 mg/kg, 40 mg/mL, Elanco, Bad Homburg, Germany), and atropine (10 μg/kg, 0.5 mg/mL, B. Braun, Melsungen, Germany). Venous access was established in the ear vein for intravenous administration of propofol (3 mg/kg, 10 mg/mL, Zoetis, Berlin, Germany), followed by endotracheal intubation for mechanical ventilation (Cato®, Dräger, Lübeck, Germany). The tidal volume was set at 7–10 mL/kg to maintain an expiratory carbon dioxide partial pressure of 40 ± 5 mmHg and inspiratory oxygen fraction of 30 ± 5 % with an initial positive end-expiratory pressure (PEEP) of 0–5 cm H2O. Anesthesia was maintained with propofol (3 mg/kg/h, Zoetis), and continuous analgesia was provided via intravenous fentanyl (0.005 mg/kg/h, 0.05 mg/mL, Dechra, Aulendorf, Germany).
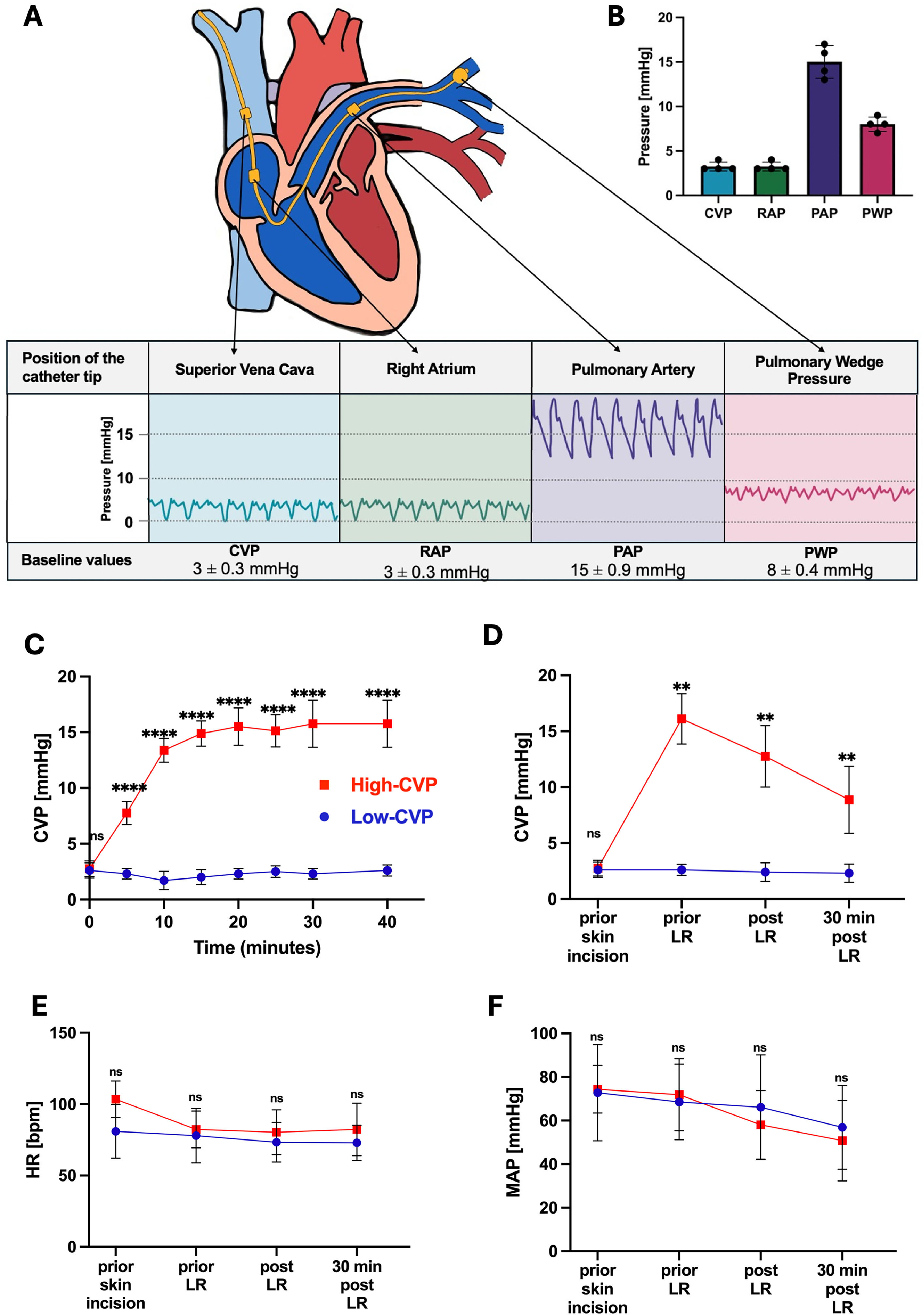
2.4CVP monitoring and modulationIn preliminary experiments, physiological CVP, right atrial pressure (RAP), pulmonary artery pressure (PAP), and pulmonary wedge pressure (PWP) of healthy German Landrace pigs were measured using a 7 French (Fr) Swan-Ganz catheter (Edwards Lifescience, Unterschleissheim, Germany) inserted via an 8.5 Fr venous sheath (Teleflex Medical, Athlone, Ireland) into the right jugular vein (Fig. 1A and B). PWP was recorded at end-expiration following inflation of the pulmonary arterial catheter balloon with 1.5 mL of air. Inflation was confirmed by observing expected changes in the pulmonary artery pressure waveform, which indicated restricted right-to-left blood flow (Fig. 1A).
Baseline and intraoperative hemodynamic parameters. A) Localization of the Swan-Ganz catheter tip and corresponding pressure curves; B) values for central venous pressure (CVP), right arterial pressure (RAP), pulmonary artery pressure (PAP), and pulmonary wedge pressure (PWP); C) CVP increase and stabilization for low-CVP (≤ 5 mmHg, n = 10) and high-CVP (≥ 10 mmHg, n = 15) groups during 30 min run-in period before liver resection (LR); D) CVP; E) heart rate (HR); and F) mean arterial pressure (MAP) comparing low- and high-CVP groups. All data are presented as mean ± SEM, with 4–15 pigs individually analyzed per group. Data were analyzed using 2-way ANOVA, followed by Tukey's post-hoc test. Significance is indicated by the following symbols: ** p < 0.01, ***p < 0.001, **** p < 0.0001, and ns = non-significant. bpm: beats per minute.
For subsequent experiments, invasive hemodynamic monitoring was established through the ultrasound-guided placement of an arterial line (Vygon, Ecouen, France) in the left femoral artery, a quad-lumen central venous catheter for continuous CVP measurements (Teleflex Medical) in the left jugular vein and a 13 Fr high-flow catheter (Achim Schulz-Lauterbach, Iserlohn, Germany) in the right jugular vein. The heart rate (HR), mean arterial pressure (MAP), CVP (Fig. 1), peripheral capillary oxygen saturation, and rectal body temperature were continuously monitored.
CVP was maintained at ≤ 5 mmHg in the low-CVP group and elevated to ≥ 10 mmHg in the high-CVP group using fluid overload (5–7 liters of lactated Ringer's solution) and an increased PEEP of 10 cm H2O. After CVP stabilization in both the low- and high-CVP groups, the values were maintained for a 30-minute run-in period before LR (Fig. 1C).
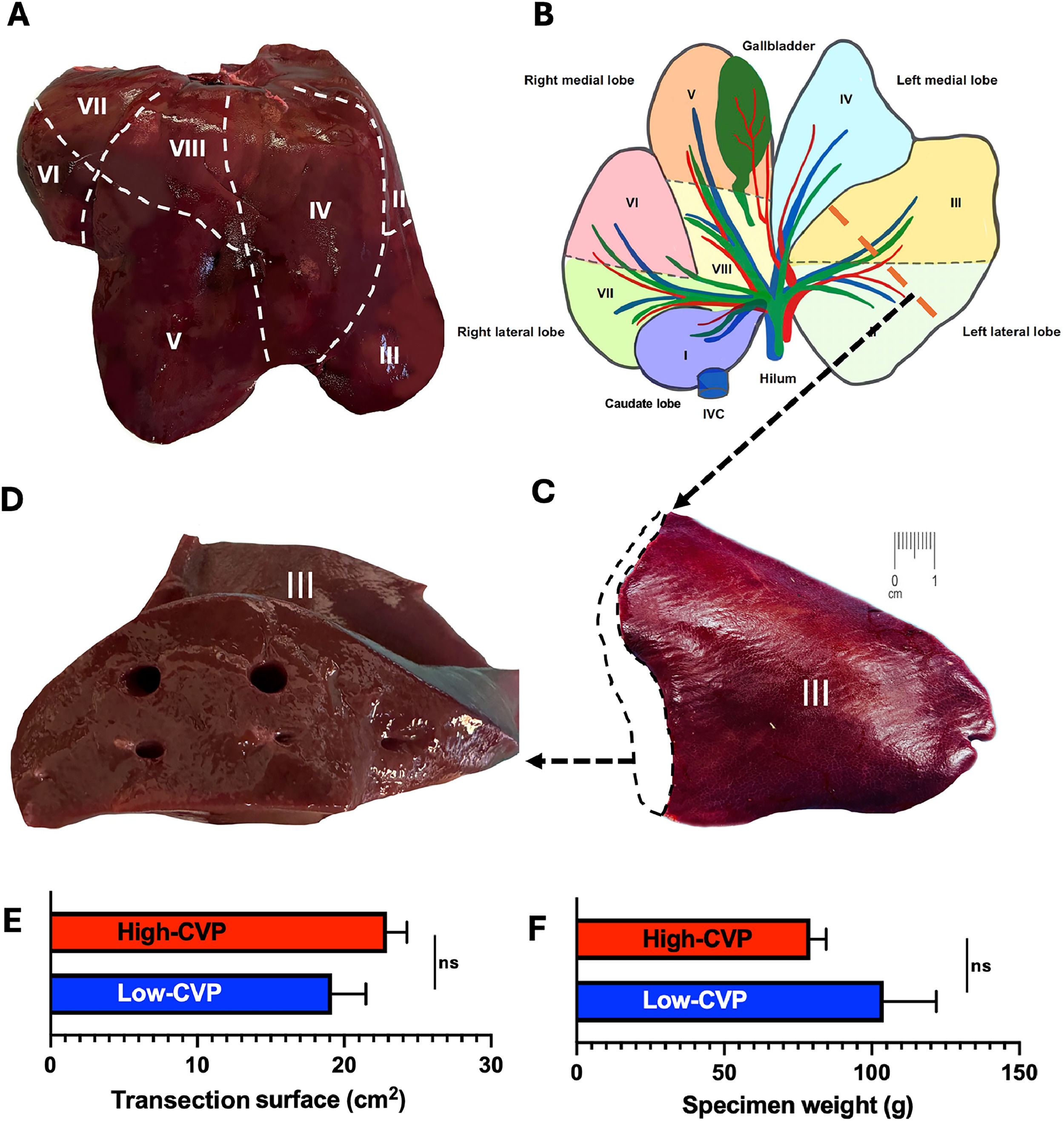
2.5Liver resectionWe established and standardized a porcine model of atypical LR of the LLL. A laparotomy was performed using a midline incision. The liver was mobilized from the surrounding tissue but the hepatoduodenal ligament was not dissected, and no inflow vascular occlusion (Pringle maneuver or left pedical camping) was used to limit anterograde blood flow. In addition, no outflow vascular occlusion was used to limit retrograde blood flow. The LLL (Segments II and III) was exposed and measured from its pedicle (identified by the deep interlobular fissure) to the apex. The line of transection for atypical LLL-LR was defined as bisection of the LLL. The LLL was resected using a coronary incision method, as this area typically contains only two vascular branches, whereas the liver parenchyma is several centimeters thick, which offers optimal anatomical conditions to focus on parenchymal transection techniques (Fig. 2A–D). Notably, specimen weight and transection surface were comparable between the low- and high-CVP cohorts (Fig. 2E and F)
Anatomy of the porcine liver and schematic diagram of left lateral liver resection. A) Porcine liver segments II – VIII (ventral view). B) Schematic drawing of porcine liver segments I – VIII (dorsal view). The bile duct and gallbladder are marked in green, the artery in red, and the portal vein and inferior vena cava (IVC) in blue. The dashed grey lines represent the borders between the liver segments. The yellow dashed line shows the transection line of the left lateral liver resection. C) Resected liver segment III. The dotted line indicates the liver resection area corresponding to a coronal section along the yellow transection line shown in B); D) Liver resection area corresponding to a coronal section along the yellow transection line shown in B). E) Transection surface area (cm2) and F) specimen weight (g) comparing the low-central venous pressure (CVP) and high-CVP groups. All data are presented as mean ± SEM, with 10–15 pigs individually analyzed per group. Data were analyzed using Student's t-test (E) and Mann-Whitney U test (F); ns = non-significant.
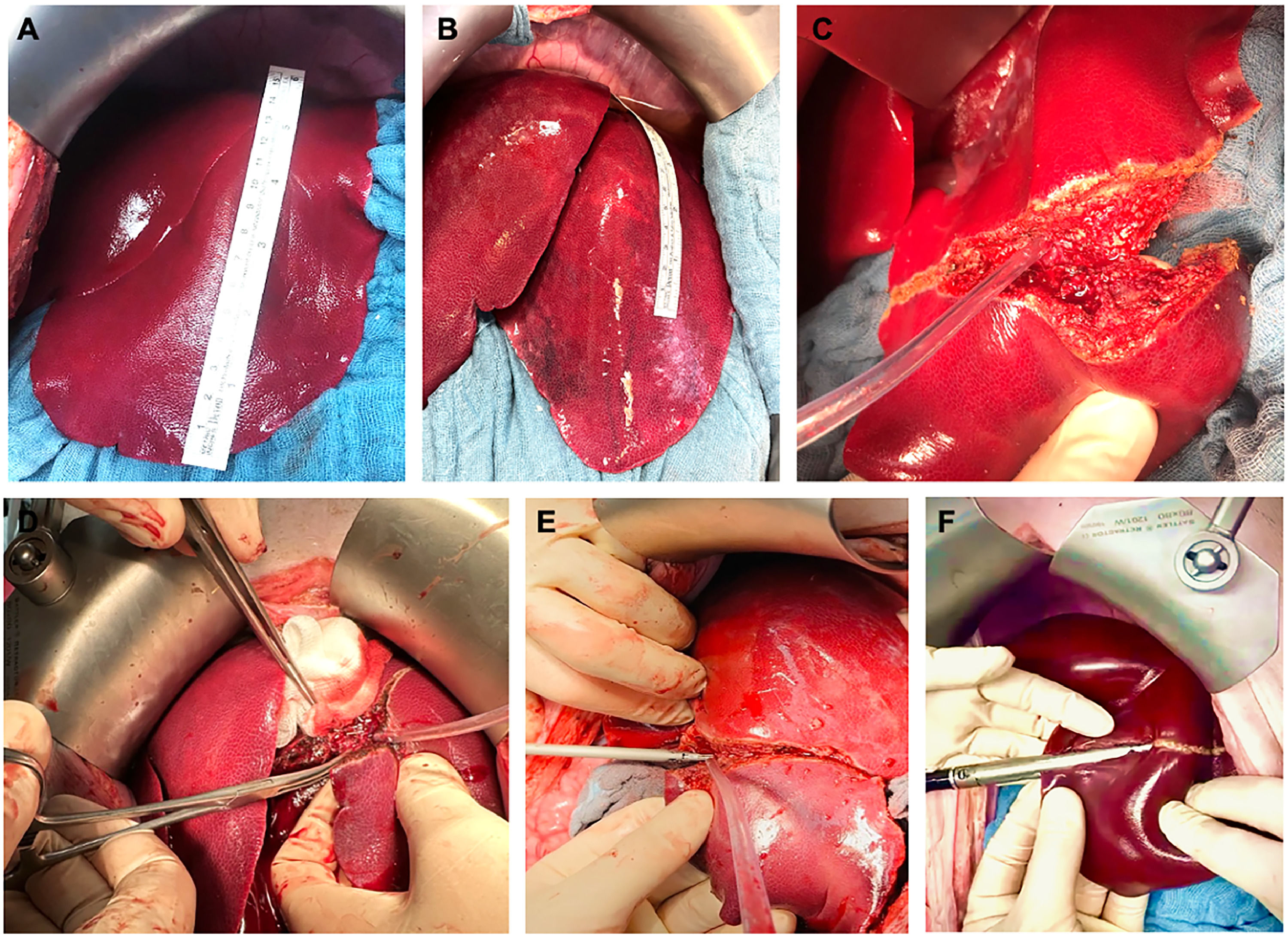
After marking the transection line with electrocautery, parenchymal transection was performed using three different approaches (Fig. 3).
Surgical site during left lateral liver resection using different parenchymal transection techniques. Surgical site after median laparotomy with a ruler on liver segment III. A) Before and B) after central venous pressure (CVP) increase and stabilization ≥ 10 mmHg for 30 min; C) transection surface area during parenchymal transection using D) clamp-crush technique, E) harmonic scalpel, and F) stapler.
CC: The clamp-crush technique involves crushing the hepatic parenchyma using a Kelly clamp to expose and isolate bile ducts and blood vessels. Once the parenchyma is crushed, visible structures are divided and transected by suture ligation, bipolar electrocautery, or clipping (Fig. 3D).
HS: The Ethicon harmonic scalpel® (Johnson & Johnson, Norderstedt, Germany) is an ultrasonic device that simultaneously cuts and coagulates. Parenchymal transection is achieved by ultrasonically activated shears sealing small vessels and biliary structures between the vibrating blades, creating heat, and denaturing proteins to constitute the coagulum (Fig. 3E).
ST: For stapler resection, the parenchyma is fractured with a large surgical clamp, followed by parenchymal transection by serial firings of an Ethicon Echelon Flex™ Endopath® (Johnson & Johnson) with 45 mm and 60 mm white magazines (Fig. 3F).
Clips, sutures, and ligatures were selectively used during parenchymal transection in all groups.
2.6Outcome parametersThe primary outcome was intraoperative blood loss (mL), measured as the amount of blood collected in the suction containers and increase in the weight of the surgical packs. The volumes of rinse fluid and ascites evacuated at the beginning of the operation were subtracted. Blood loss was standardized to the transection surface area (mL/cm²) and specimen weight (mL/g).
Secondary endpoints included surrogate markers for safe and rapid parenchymal transection. (I) Parenchymal transection time (min), defined as the time from the beginning to the end of the parenchymal transection. Parenchymal transection time was standardized to the transection surface area (min/cm²) and specimen weight (min/g). The transection surface area of the liver was measured using image analysis software ImageJ (National Institutes of Health, USA). Photographs of the samples, each containing a scale in centimeters, were provided to the software. The scale was defined in ImageJ by measuring the reference length with the line tool and setting the actual length (in cm) using the "Set Scale" function. The resection area was then manually delineated using a Freehand Selection Tool. The software calculated the area of the selected region, reported in cm2. (II) Bile leakage from the resection plane was assessed using the White test. The cystic duct was dissected and closed using a 2/0 Vicryl ligature (Johnson & Johnson). The main bile duct was dissected below the junction of the cystic duct and closed distally. A 12 Fr silicone bile duct drain was inserted towards the liver, advanced under palpation control just short of the bile duct bifurcation, and secured with a ligature. Thirty minutes post-LR, 20 mL of propofol was injected to provoke bile leakage. The White test was considered positive if visible leakage was observed at the resection plane.
2.7Statistical analysisContinuous variables are presented as mean ± standard error of the mean (SEM), and categorical variables are reported as absolute and relative frequencies. When comparing two groups, the unpaired, two-tailed Student's t-test was used for normally distributed data, and the Mann-Whitney U test was used for data that were not normally distributed. Fisher's exact test was used to analyze categorical variables. Two-way analysis of variance (ANOVA) followed by Tukey's post-hoc test was used to compare multiple groups. Statistical significance was defined as p < 0.05. All experiment-specific n values and statistical tests are indicated in the figure legends. Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
2.8Ethical statementThe present study was approved by the Animal Care Committee of the State Government of North-Rhine Westphalia (LANUV NRW, Recklinghausen, Germany; Approval AZ 81–02.04.2021). A459). All methods adhered to the guidelines set forth by the European Parliament, Council of the European Union (Directive 2010/63/EU).
3Results3.1Central venous pressure and hemodynamic dataGiven the conflicting reports on physiological porcine hemodynamic parameters in the literature [13–17], the initial objective was to establish reliable baseline hemodynamic parameter values for our model. Advanced hemodynamic monitoring was implemented to measure baseline values for CVP, RAP, PAP, and PWP. Measurements were taken with the animals in a supine position, without positional maneuvers, using a PEEP of 0 cm H2O. Using this approach, the following baseline values were established: CVP 3 ± 0.3 mmHg, RAP 3 ± 0.3 mmHg, PAP 15 ± 0.9 mmHg, and PWP 8 ± 0.4 mmHg (Fig. 1A and B). Based on these results, the low-CVP group was defined as having a CVP ≤ 5 mmHg. The animals were then randomly assigned to either the low- or high-CVP cohort.
The baseline CVP values were comparable between the low- and high-CVP groups, with no abnormal values observed. CVP values in both groups stabilized during a 30-minute run-in period before LR. Although the mean CVP significantly increased in the high-CVP group after CVP adaptation, there were no differences in HR or mean MAP between the cohorts (Fig. 1C-F). However, elevated CVP levels led to hyperemic swelling of the liver (Fig. 3A and B).
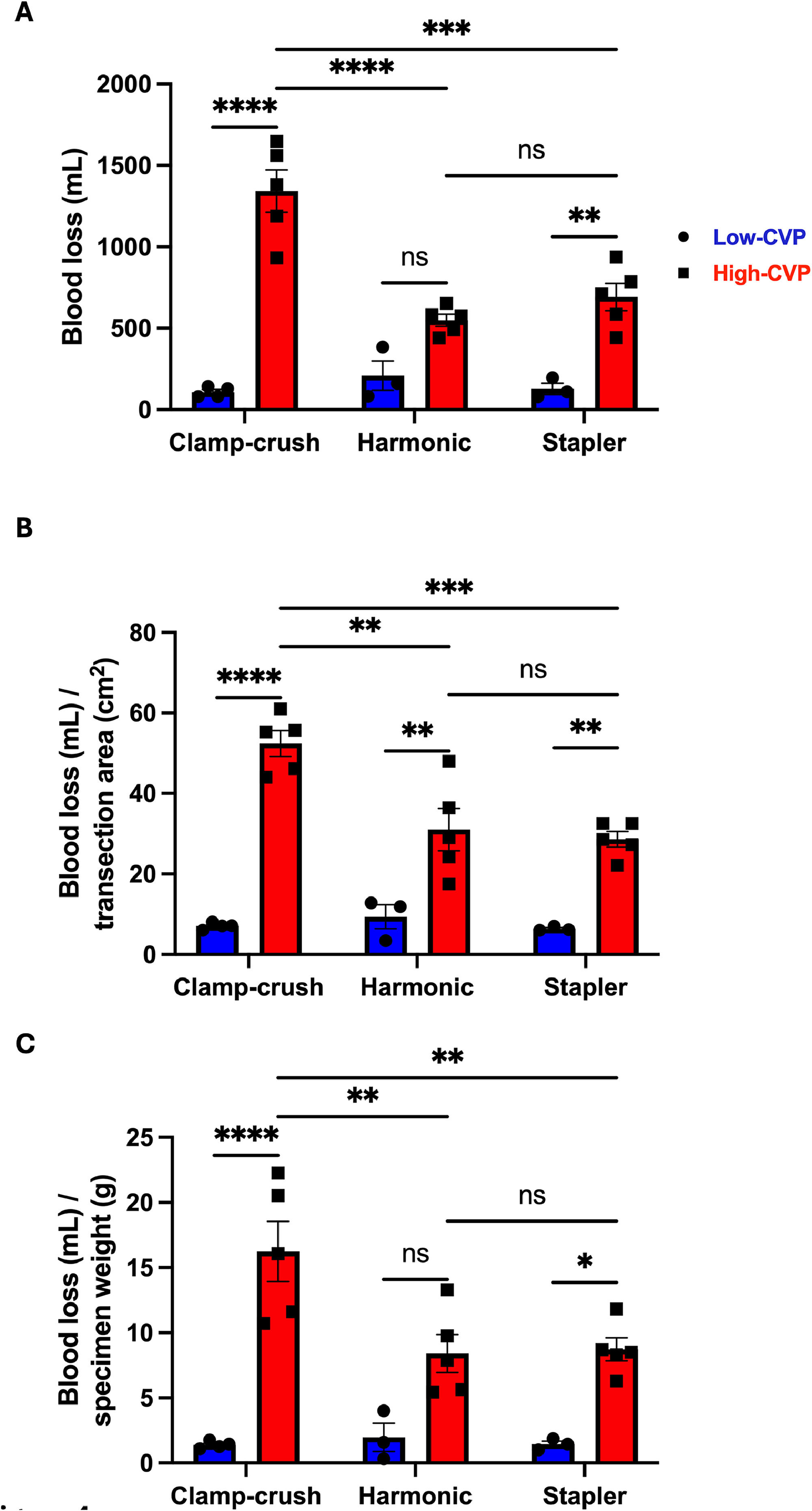
3.2Intraoperative blood lossIn the low-CVP group, blood loss was comparable among all three subgroups (CC: 108 ± 16 mL, HS: 209 ± 90 mL, and ST: 128 ± 35 mL). When comparing the low- and high-CVP cohorts, blood loss in the CC (108 vs. 1342 mL, p < 0.0001) and ST (128 vs. 692 mL, p = 0.0038) subgroups was significantly higher in the high-CVP group. However, the blood loss in the HS subgroup (209 vs. 548 mL, p = 0.1944) was comparable between the low- and high-CVP cohorts. When focusing on the differences in blood loss in the high-CVP cohort stratified by the parenchymal transection technique, it was found that the mean blood loss was significantly higher in the CC subgroup than in both the HS (1342 vs. 548 mL; p < 0.0001) and ST subgroups (1342 vs. 692 mL; p = 0.0001), while blood loss was comparable between the HS and ST techniques (548 vs. 692 mL; p = 0.9664) (Fig. 4A).
Comparison of intraoperative blood loss between different transection techniques under low- and high-central venous pressure (CVP). A) Intraoperative blood loss (mL); B) intraoperative blood loss normalized to the transection surface area (mL/cm2); C) intraoperative blood loss normalized to the specimen weight (mL/g). All data are presented as mean values ± SEM, with 10–15 pigs individually analyzed per group. Data were analyzed using 2-way ANOVA, followed by Tukey's post-hoc test. Significance is indicated by the following symbols: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns = non-significant.
The mean transection surface areas in the low- and high-CVP groups were 19 ± 2 cm² and 23 ± 1 cm² (p = 0.1477), respectively (Fig. 2E). In the low-CVP group, blood loss normalized to the transection surface area was comparable among all three subgroups (CC: 7 ± 0 mL/cm², HS: 9 ± 3 mL/cm², ST: 6 ± 0 mL/cm²). Comparing the low- and high-CVP groups, blood loss per transection surface area was significantly higher in the high-CVP group for the CC (7 vs. 52 mL/cm², p < 0.0001), HS (9 vs. 31 mL/cm², p = 0.004), and ST (6 vs. 29 mL/cm², p = 0.0031) subgroups. When examining differences among parenchymal transection techniques in the high-CVP cohort, the CC subgroup exhibited significantly higher blood loss than both the HS (52 vs. 31 mL/cm²; p = 0.001) and ST subgroups (52 vs. 29 mL/cm²; p = 0.0003). However, there was no significant difference between the HS and ST techniques (31 vs. 28 mL/cm²; p = 0.9926). (Fig. 4B).
3.4Intraoperative blood loss normalized to specimen weightThe mean specimen weights in the low- and high-CVP groups were 104 ± 18 g and 79 ± 5 g (p = 0.1549), respectively (Fig. 2F). In the low-CVP group, blood loss per specimen weight was comparable among all three subgroups (CC: 1 ± 0 mL/g, HS: 2 ± 1 mL/g, and ST: 1 ± 0 mL/g). When comparing the low- and high-CVP cohorts, blood loss per specimen weight in the CC (1 vs. 16 mL/g, p < 0.0001) and ST (1 vs. 9 mL/g, p = 0.0367) subgroups was significantly higher in the high-CVP group. However, there was no significant difference between the low- and high-CVP cohorts in the HS subgroup (2 vs. 8 mL/g, p = 0.0793). When focusing on the differences in the high-CVP cohort stratified by parenchymal transection technique, it was found that the intraoperative blood loss normalized to specimen weight was significantly higher in the CC subgroup than in both the HS (16 vs. 8 mL/g; p = 0.0067) and ST subgroups (16 vs. 9 mL/g; p = 0.0097), whereas no significant difference was observed between the HS and ST techniques (8 vs. 9 mL/g; p > 0.9999) (Fig. 4C).
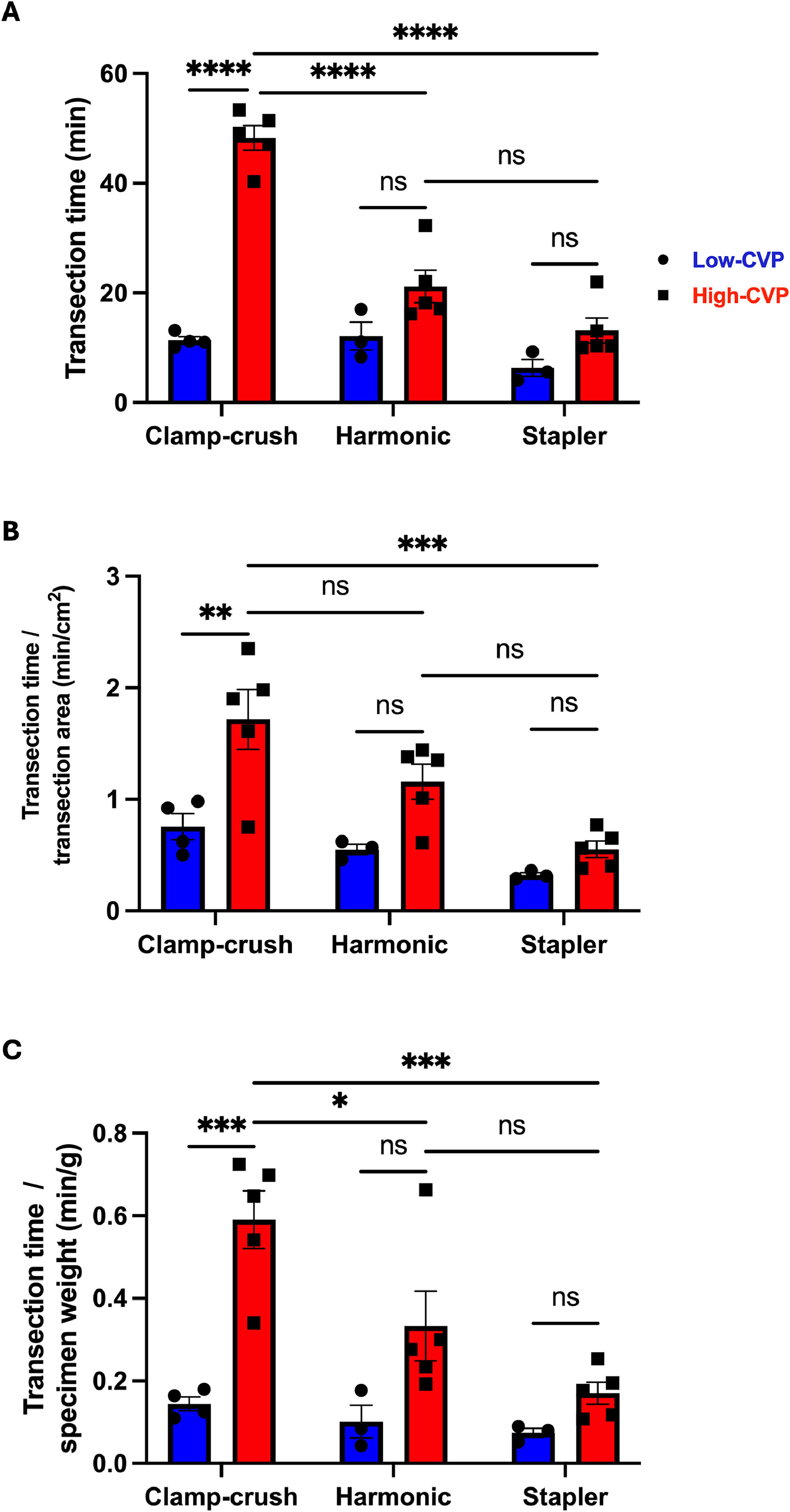
3.5Parenchymal transection timeIn the low-CVP group, parenchymal transection time was comparable among all three subgroups (CC: 11.4 ± 0.7 min, HS: 12.1 ± 2.6 min, and ST: 6.3 ± 1.5 min). When comparing the low- and high-CVP cohorts, parenchymal transection time in the CC subgroup (11.4 vs. 48.3 min, p < 0.0001) was significantly higher in the high-CVP group, while transection time was comparable between the low- and high-CVP cohorts in the HS (12.1 vs. 21.2 min, p = 0.1499) and ST (6.3 vs. 13.2 min, p = 0.4024) subgroups. When examining differences in the high-CVP cohort stratified by parenchymal transection technique, it was found that the mean transection time was significantly higher in the CC subgroup than in both the HS (48.3 vs. 21.2 min; p < 0.0001) and ST subgroups (48.3 vs. 13.2 min; p < 0.0001). However, there was no significant difference between the HS and ST techniques (21.2 vs. 13.2 min; p = 0.1346) (Fig. 5A).
Comparison of transection times between different transection techniques under low- and high- central venous pressure (CVP).
A) Parenchymal transection time (min); B) parenchymal transection time normalized to the transection surface area (min/cm2); C) parenchymal transection time normalized to specimen weight (min/g). All data are presented as mean values ± SEM, with 10–15 pigs individually analyzed per group. Data were analyzed with 2-way ANOVA, followed by Tukey's post-hoc test. Significance is indicated by the following symbols: * p < 0.05, ** p < 0.01, ***p < 0.001, **** p < 0.0001, ns = non-significant.
In the low-CVP group, transection time per area was comparable among all three subgroups (CC: 0.8 ± 0.1 min/cm2, HS: 0.6 ± 0 min/cm2, ST: 0.3 ± 0 min/cm2). When comparing the low and high-CVP cohorts, the mean transection time per area was significantly longer in the CC subgroup (0.8 vs. 1.7 min/cm2, p = 0.0073), while they were comparable in the HS (0.6 vs. 1.2 min/cm2, p = 0.3162) and ST (0.3 vs. 0.6 min/cm2, p = 0.9989) subgroups. When focusing on the differences in the high-CVP cohort stratified by parenchymal transection technique it was found that mean transection time per area was significantly extended in the CC subgroup compared to ST subgroup (1.7 vs. 0.6 min/cm2, p = 0.0005) with no significant differences observed between CC and HS (1.7 vs. 1.2 min/cm2, p = 0.2392) or between HS and ST resections (1.2 vs. 0.6 min/cm2, p = 0.1583) (Fig. 5B)
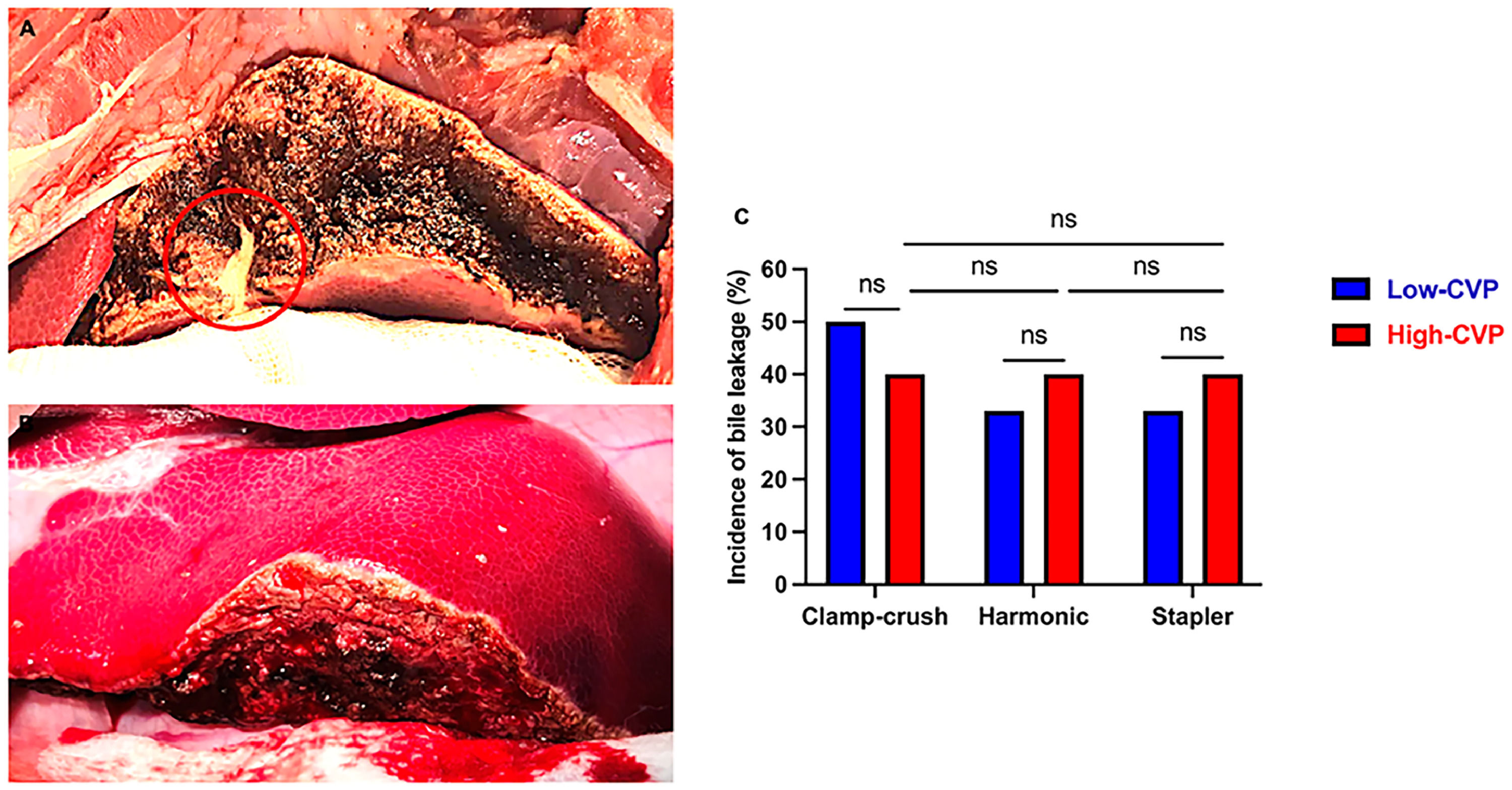
3.7Bile leakageIn the low-CVP group, a positive white test result (Fig. 6) was observed in 50 % of CC resections and 33 % of both HS and ST resections. In the high-CVP group, a positive white test was observed in 40 % of CC, HS, and ST resections, respectively. No significant differences were found between the low- and high-CVP groups (p > 0.9999) or among the various transection techniques in the high-CVP cohort (p > 0.9999) (Fig. 6C).
Bile leakage from resection surface. Frontal view of the resection plane in liver segment III A) with (red circle) and B) without bile leakage following the white test, C) incidence of bile leakage across transection techniques under low and high central venous pressure. All data are presented as percentages, with 10–15 pigs individually analyzed per group. Data were analyzed with Fisher´s exact test. ns = non-significant.
Hepatectomy is a highly effective surgical approach for treating various liver diseases, especially malignancies, and approximately 20,000 LRs are performed annually in Germany [18]. Globally, it is estimated that 700,000 LRs are conducted annually. However, despite recent advances in surgical techniques and perioperative care, LR remains a technically challenging procedure that poses a significant risk to patients. Numerous studies have shown that postoperative morbidity and mortality are closely linked to intraoperative blood loss, primarily occurring during parenchymal transection [2,4]. Accordingly, liver surgeons have always sought technical advancements to minimize excessive hemorrhage.
Over two decades ago, the implementation of a low CVP strategy emerged as an effective method to reduce intraoperative blood loss in liver surgery [19]. This strategy is based on the principle that maintaining a lower CVP correlates with a decreased venous pressure within the liver, thereby minimizing blood loss. Consequently, a widely adopted and highly recommended approach is to conduct LRs at a low CVP, as CVP levels exceeding 5 mmHg are strongly linked to increased intraoperative blood loss [6,20]. The Enhanced Recovery After Surgery Society advocates balanced fluid management to achieve euvolemia in liver surgeries, endorsing the low-CVP strategy as pivotal for mitigating blood loss [20].
However, LR is now more frequently performed in patients with relevant comorbidities such as liver cirrhosis, cardiac insufficiency, and portal or pulmonary hypertension, where CVP is chronically elevated and often impossible to reduce through volume restriction or pharmacological interventions. These patients are at a high risk of intraoperative bleeding and thus have increased morbidity and mortality rates following LR [3]. Therefore, because CVP management is limited in these patients, the surgical resection technique for parenchymal transection is a major contributor to postoperative outcomes. Although high-risk patients with elevated CVP would benefit the most from blood-saving procedures, the optimal parenchymal transection technique in these cases remains unknown. The present study provides the first evidence of reduced intraoperative blood loss and improved transection time when using HS or ST compared to the CC technique for LR in a translational large-animal model of pathologically elevated CVP.
Since the introduction of the CC technique in the 1970s by Lin [21], numerous innovative transection techniques have been developed to reduce blood loss and improve postoperative outcomes in LR [10,22,23]. The introduction of vascular staplers and advanced sealing devices has significantly improved the feasibility of complex LRs. These tools ensure secure sealing of intraparenchymal structures, thereby reducing blood loss and potentially decreasing the need for prolonged hilar vascular inflow occlusion, which is generally not recommended in cirrhotic patients owing to potential complications linked to hepatic ischemia reperfusion injury [11,24,25]. A recent systematic review and network meta-analysis of 22 randomized controlled trials involving 2360 patients examined 10 different parenchymal transection techniques, including bipolar cautery, LigaSure, CC, HS, Cavitron Ultrasonic Surgical Aspirator, ST, Hydrojet, BiClamp forceps, and radiofrequency-assisted devices, used either individually or in combination [10]. Bipolar cautery has emerged as the most effective approach for minimizing blood loss and improving operation time. Conversely, HS has been shown to reduce overall complications, major complications, and transfusion rates, whereas stapler resection is associated with shorter operation times. However, the majority of the included RCTs were conducted under strict low-CVP (ranging between 0 - 5 mmHg) protocols [10]. Thus, the available data regarding the impact of different parenchymal transection techniques on intraoperative blood loss, transection time, and postoperative outcomes in patients with chronically elevated non-reducible CVP are currently limited. As LR becomes more common in high-risk patients [11], there is an urgent and unmet need to develop intraoperative strategies that focus on minimizing intraoperative blood loss and reducing the operation time to improve postoperative outcomes.
In our study, no differences were found among the three investigated transection techniques when analyzing the intraoperative blood loss and transection time in the low-CVP control groups. This is in line with the current literature and demonstrates that the CC, HS, and ST techniques can be safely applied when conducting low-risk LR [26]. However, a systematic review and network meta-analysis of parenchymal transection techniques during hepatectomy in humans indicated that the ST technique had significantly shorter transection times than the CC technique [10]. Although the CC technique has largely been replaced by modern transection techniques in clinical liver surgery, it remains the most commonly used control group in RCTs comparing different parenchymal transection techniques [10]. Therefore, this study included a CC control group and demonstrated acceptable results in the low-CVP cohort. However, when LR was conducted under high-CVP conditions, the use of HS or ST resulted in significantly improved results. Compared to CC, both HS and ST demonstrated a significant reduction in intraoperative blood loss and transection time, even after adjusting for specimen weight and transection area. These results provide the first evidence that modern devices used for parenchymal transactions during LR improve clinically relevant endpoints in a translational large animal model of elevated CVP. These results are of particular interest to various stakeholders because of the associated costs that often prevent the use of modern devices. When comparing HS and ST under high-CVP conditions, no significant differences were found in blood loss or transection time.
To our knowledge, there is currently no validated model to investigate the impact of different transection techniques under conditions of elevated pathological CVP during LR. Therefore, we developed and validated an innovative large animal model using pigs that offers ideal anatomical and physiological similarities to humans for liver research [13]. Their liver structure closely mirrors that of humans in size, lobar anatomy, vascular architecture, and bile duct system, allowing for the effective translation of findings to human medicine. Additionally, pig liver functions, including metabolic processes, enzyme activities, and responses to injuries and surgeries, are very similar to those in humans. This similarity ensures that the results from pig LR studies are highly relevant and applicable to human clinical settings [27–29].
4.1LimitationsThis study investigated different parenchymal transection techniques for open LR. However, there is a growing trend towards minimally invasive (laparoscopic and especially robotic) LR, with significant differences [30]. As stated above, bleeding during parenchymal transection depends on the difference between the intra-abdominal pressure (IAP) and CVP. Conceptually, when a pneumoperitoneum is used, the IAP is between 10 and 14 mmHg; thus, it acts directly against CVP and decreases blood loss [31]. Additionally, chronically elevated CVP is typically observed in patients with liver cirrhosis, and recent data suggest that these patients benefit the most when operated using minimally invasive approaches [32,33]. Therefore, further research is necessary to extend our findings to the field of parenchymal transection during minimally invasive LR.
Additionally, the relatively small group sizes in this study, guided by ethical considerations, may have limited the statistical power to detect subtle differences in secondary outcomes such as bile leakage. While the design adequately addresses the primary endpoint, we recognize that the evaluation of secondary endpoints may be less robust due to these constraints. Future studies with larger cohorts could provide more comprehensive insights into these secondary outcomes.
In addition, our model is based on acute CVP elevation, which does not replicate the chronic pathophysiological conditions observed in diseases such as cirrhosis, cardiac insufficiency, or pulmonary hypertension. In these chronic conditions, elevated CVP leads to significant alterations in the internal milieu, including impaired hepatic reserve function or altered coagulation profiles. Since our model did not recapitulate chronic conditions such as fibrotic liver changes or congestive hepatopathy, the clinical relevance of our results may be limited. Thus, additional studies using models that replicate these chronic diseases more accurately are necessary to enhance the applicability of these findings to real-world scenarios.
In our study, elevated CVP was maintained only until the completion of LR, with CVP rapidly returning to baseline levels during the 30-minute postoperative observation period (Fig. 1D). To better assess the impact of high CVP on bile leakage or hemorrhage, maintaining an elevated CVP until the secondary endpoint of the experiment would have provided a more comprehensive understanding of its role in postoperative bleeding and bile leakage.
This study focused on intraoperative parameters, such as blood loss and bile leakage, measured 30 min postoperatively. However, long-term outcomes, including recovery, complications, and survival rates, were not assessed. These long-term outcomes are crucial for evaluating the optimal transection technique, particularly in high-risk patients with chronically elevated CVP, as postoperative complications such as bleeding and bile leakage remain major concerns. Therefore, future studies should extend the follow-up period to assess these outcomes and provide a more comprehensive understanding of how different transection techniques affect the postoperative recovery and complications.
Finally, while this study examined different transection techniques under high CVP conditions, it is important to note that techniques commonly used in clinical practice, such as the Pringle maneuver, were not included. The Pringle maneuver, which involves intermittent occlusion of the portal triad to reduce blood loss, is frequently used in patients with an elevated CVP. The use of the Pringle maneuver was omitted for two main reasons: first, the porcine spleno-mesenterico-portal system is highly sensitive to portal occlusion; second, our aim was to evaluate the selected resection methods in a rather demanding scenario and solely focus on blood loss by intentionally excluding inflow control techniques. However, omitting the Pringle maneuver weakens the translational aspect of our model and is a significant limitation. Consequently, future studies should incorporate the Pringle maneuver to further enhance the clinical relevance and realism of the model.
5ConclusionsThe results show the superiority of precise parenchymal dissection techniques, specifically ultrasound dissector and stapler. These findings support our hypothesis that intraoperative blood loss during LR at an elevated CVP may depend on the parenchymal transection technique. Therefore, it is a modifiable factor that directly improves clinically relevant intraoperative outcomes. This innovative and translational large animal model may serve as a suitable basis for further investigation of the optimal LR technique in chronically elevated CVP, functioning as a "bench-to-bedside" model.
CRediT authorship contribution statementFelicia Kneifel: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization. Annika Mohr: Methodology, Formal analysis, Investigation, Resources, Data curation, Writing – review & editing. Alexander D. Bungert: Investigation, Resources, Data curation. Tristan Wagner: Investigation, Resources, Data curation. Mazen Juratli: Writing – review & editing. Haluk Morgul: Validation, Writing – review & editing. Finnja Marie Krug: Investigation, Resources, Data curation. Tim-Gerald Kampmeier: Investigation, Data curation, Writing – review & editing. Christian Ertmer: Investigation, Data curation, Writing – review & editing. Andreas Andreou: Writing – review & editing. Philipp Houben: Validation, Writing – review & editing. Shadi Katou: Validation, Writing – review & editing. Andreas Pascher: Validation, Writing – review & editing, Supervision. Benjamin Strücker: Methodology, Writing – review & editing, Supervision, Project administration. Felix Becker: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization, Supervision, Project administration.