Background. The evolving pattern of HCV genotypes (GTs) and risk factors (RFs) in HCV-infected patients in Mexico is poorly understood. This study aimed to access the temporal trend of HCV GTs and RFs in HCV patients from two care centers.
Material and methods. Chronic HCV patients [177 and 153 patients from the Northeast (NE) and Central West (CW) regions, respectively] were selected. Baseline features were demographics, date of birth (DOB), blood transfusion before 1992 (BTb1992), RFs, sexual promiscuity (SP), dental procedure (DP), injection drug use (IDU), viral load (VL), GTs, cirrhosis status and antiviral therapy (AT). Data were analyzed by Chi-square test for trends, unpaired T-test, and logistic regression.
Results. HCV GT distribution was: GT1, 67%; GT2, 16%; GT3, 12% and GT4, 1%. RFs were BTb1992, 56%; surgeries, 56%; tattooing, 18% and IDU, 16%. GT1a mostly prevailed in CW than NE patients. GT1b, surgeries, BTb1992 and cirrhosis were more prevalent in older patients (p < 0.05); GT3, male gender IDU, SP, and tattooing showed an upward trend as younger were the patients in both regions (p < 0.05), contrariwise to the prevalence of GT1b. BTb1992 and surgeries were seen in elder women; BTb1992 was an independent RF for GT1. Age ≥ 50 years old, GT1 and exposure to AT (p < 0.05) were associated with cirrhosis. Conclusion. GT1a prevalence in CW Mexico remained stable, whereas GT3 increased and GT1b decreased in younger patients in both regions, along with associated RFs. Further regional molecular epidemiology and RF analyses are required in order to avoid the dissemination of new cases of HCV infection.
Hepatitis C virus (HCV) infection is a worldwide health problem that causes chronic life-threatening diseases, such as liver cirrhosis and hepatocellular carcinoma.1,2 HCV is an RNA virus member of the Hepacivirus genus and Flaviviridae family that is divided into seven phylogenetically distinct GTs (GT1-GT7) and a vast series of subtypes (67 subtypes).3 Molecular epidemiology and phylodynamic analyses have revealed that HCV genomic types and subtypes have distinctive patterns of geographical distribution,1–3 which may be related to different transmission networks and transitional shifting of RFs.4–6 Moreover, the dominant GT in a given population may reflect an outbreak in a particular high-risk group, city or geographic region, which later continues to spread by means of related RFs.5,7
The Latin American region has nearly the lowest HCV prevalence worldwide with 1.5% of anti-HCV antibodies.8,9 In this region, updated studies on the molecular epidemiology of HCV infection are scarce. Nonetheless, HCV GT1 is the most frequent, followed by either GT2 or GT3,8,10 whereas the other GTs (GT 4-7) are less common.10 However, both the prevalence of HCV infection and circulating HCV GTs may not be homogenous throughout Latin America or within regions of the same country.7,11,12 In Mexico, HCV infection is the second cause of clinical hepatitis in adults and children.13,14 The overall HCV seroprevalence is 0.4-1.6% among asymptomatic patients15,16 with local variations,17 yet some risk populations have a higher seroprevalence.18,19 Several cross-sectional studies have been performed to ascertain the geographical distribution of HCV genotypes throughout the country. These studies have shown that the dominant GT1 has a dissimilar geographical subtype distribution. While GT1a accessed by line probe assay (Lipa), and DNA sequencing is more prevalent in West Mexico,7,20 GT1b prevails in Northern-Central Mexico.21,22 In regards to GT2, subtype 2b is frequent in southern Mexico,22 whereas subtype 3a prevails mainly in northern Mexico and in less proportion in the southern region.23,24 Despite proactive blood bank screening, recent studies indicate that HCV infection is an emerging health problem25 which may due to the underestimation of the associated RFs involved in the transmission of HCV. On the other hand, the dynamic process of evolving patterns of HCV GTs distribution, RFs and HCV prevalence over time has not been clearly defined. Herein, we assess the temporal trend of HCV GTs and related RFs that prevail among different age groups of Mexican HCV-infected patients from two distinct geographical regions (CW and NE regions of Mexico) in order to improve information about evolution and trends of HCV disease.
Material and MethodsPatientsWe conducted a dynamic, retrospective, cross-sectional study including 330 patients with chronic hepatitis C (CHC) attended in two Mexican tertiary health care centers, one located in Monterrey, Nuevo Leon (NE region), and another in Guadalajara, Jalisco, (CW region). From the NE region, 177 HCV-infected patients (68 males and 109 females, mean age 46 ± 13 years, range 4-76 years) enrolled in the Liver Unit, Internal Medicine Department at the University Hospital of Monterrey “Dr. José E. González” were selected. This public hospital is the largest in NE Mexico with patients coming mainly from the State of Nuevo Leon and surrounding states in northern Mexico. From the CW region, 153 HCV-infected patients (55 males and 98 females, mean age 50 ± 12 years, range 13-72 years) enrolled between 2010 and 2013 at the Department of Molecular Biology in Medicine of the Civil Hospital of Guadalajara “Fray Antonio Alcalde” were selected. This public hospital has 950 beds and attends patients coming mainly from the state of Jalisco and surrounding CW states of Mexico. The inclusion criteria were an available molecular diagnosis of CHC and HCV GT. The exclusion criteria were hepatitis B virus and human immunodeficiency virus co-infections. Clinical and demographic data were collected through revision of the patients’ medical record. Patients were grouped according to the DOB in order to make statistical analysis. The research protocol complied with the Declaration of Helsinki and was approved by the institutional review committee.
Data collection designBaseline features were collected using a structured questionnaire. Study variables were age, DOB, gender, residence and time of medical interview (the first ever recorded in patients’ clinical charts registered at the research centers). RFs for HCV infection were history of surgeries, BTb1992, hemodialysis, DP, acupuncture, SP was defined as ≥ 2 sexual partners per 6 months, IDU, tattooing, no RFs and others less frequently seen.
The diagnosis of HCV infection was suspected by the presence of anti-HCV antibodies determined by a third-generation enzyme immunoassay and confirmed by serum HCV-RNA. VL was recorded in lU/ mL whenever a quantitative PCR was available in the medical record at the time of medical evaluation (Cobas Amplicor HCV Monitor [2000-2005], RT-HeptiMax HCV assay [2009], HCV RNA Ultraquant [2009], Cobas AmpliPrep/Cobas TaqMan HCV Test [2010-2013]). If VL was not expressed in IU/mL (RT-PCR of RNA isolated by the single-step method [before 1995], SuperQuant assay [1998], Versant HCV RNA 3.0 [1998], Amplicor HCV monitor [1997-1999]), it was converted to IU according to Pawlostky, et al. conversion factors.26
HCV GTs were recorded as reported regardless of the method used (i.e. Reverse hybridization INNO-LiPA HCV II assay (Innogenetics-Bayer, Antwerp, Belgium, Restriction Fragment Length Polymorphism “in house” technique, among others) or the GT nomenclature criteria standing at the time.
Cirrhosis was defined either as METAVIR F4, whenever a liver biopsy was available, as F4 by noninvasive methods (FibroTest ≥ 0.75,27 FibroScan ≥ 12.5,28 HepaScore ≥ 0.84,29 APRI ≥ 230 and/or clinical criteria (gastroesophageal varices or hepatic decompensation). Exposure to HCV AT was defined as ≥ 12 weeks of an interferon-based treatment scheme and recorded regardless of if it was before or after the first medical interview at the research centers.
Statistical analysisDescriptive statistics were used (mean and standard deviation). The chi-square or unpaired T-tests were used to detect differences. Statistical significance was defined as P < 0.05 using SPSS v.19 or STATA v12.1. Age-specific prevalence trends according to DOB for gender, age, RFs, HCV- GT, cirrhosis, and AT were tested by chi square test for trends. Logistic regression analysis was used to model each GT and the combination of GT1a, 3 and 3a presence/ absence among the overall sample.31,32 Variables considered in this model were RFs for acquiring HCV, cirrhosis, VL ≥ 600,000 IU/mL, birth subgroup, and referral center. Prevalence odds ratio (pOR) was used as a measure of association between each independent variable and each genotype. Chi-square test was used to detect associations between GT-specific RFs and the remaining baseline variables.
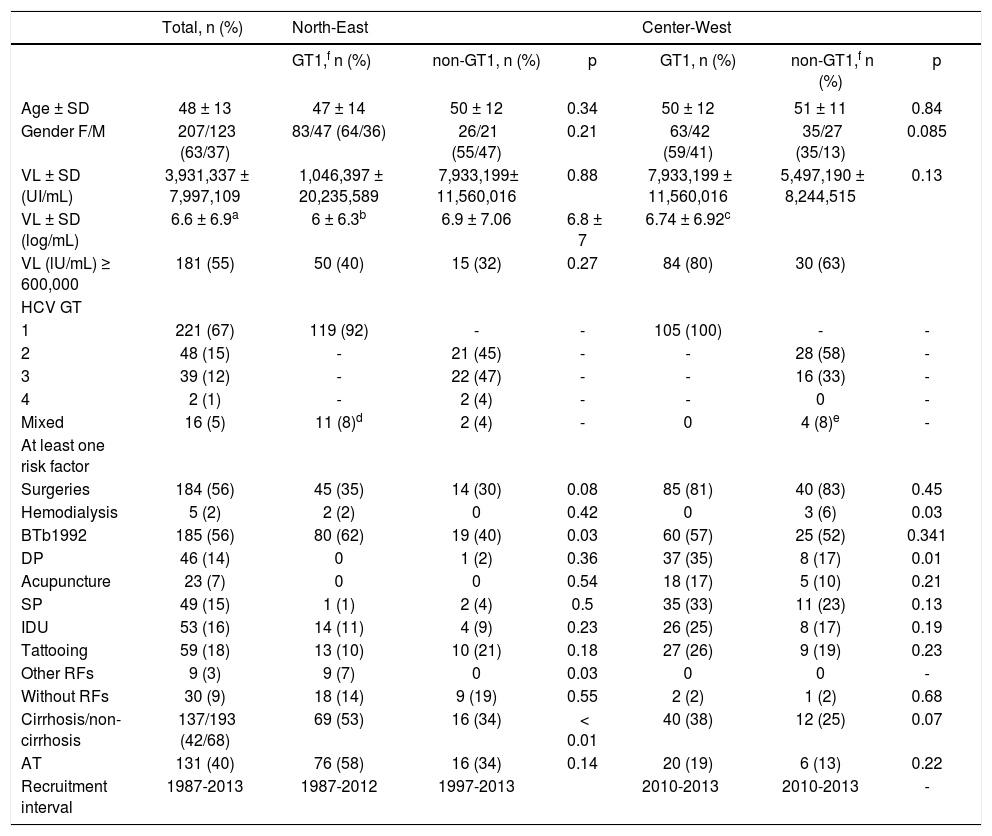
ResultsDistribution of CHC patient’s clinical features and risk factors#The major demographic and clinical characteristics of the 330 chronic HCV infected patients studied are described in Table 1. Female prevailed over male patients (63 vs. 37% respectively). The mean age was 48 ± 13 years (range 4-76 years). The most frequent HCV GT in both regions was GT1 (221, 67%), followed by GT2 (48, 16%), GT3 (39, 12%), GT4 (2, 1%) and mixed GT (16, 5%). GT1 was more common in patients from the region CW than those from the NE (52 vs. 32% p < 0.001). There were very few patients with GT4 (n = 2) to make inferences. Liver biopsy or non-invasive tests showed that most of the patients were non-cirrhotic (68%). Cirrhosis was more commonly seen in GT1 patients from NE (53%, p = 0.01). A demographic survey demonstrated that patients were residents of diverse states of Mexico (data not shown).
Baseline distribution of patient’s clinical features and risk factors by HCV-GT in both Mexican regions.
| Total, n (%) | North-East | Center-West | |||||
|---|---|---|---|---|---|---|---|
| GT1,f n (%) | non-GT1, n (%) | p | GT1, n (%) | non-GT1,f n (%) | p | ||
| Age ± SD | 48 ± 13 | 47 ± 14 | 50 ± 12 | 0.34 | 50 ± 12 | 51 ± 11 | 0.84 |
| Gender F/M | 207/123 (63/37) | 83/47 (64/36) | 26/21 (55/47) | 0.21 | 63/42 (59/41) | 35/27 (35/13) | 0.085 |
| VL ± SD (UI/mL) | 3,931,337 ± 7,997,109 | 1,046,397 ± 20,235,589 | 7,933,199± 11,560,016 | 0.88 | 7,933,199 ± 11,560,016 | 5,497,190 ± 8,244,515 | 0.13 |
| VL ± SD (log/mL) | 6.6 ± 6.9a | 6 ± 6.3b | 6.9 ± 7.06 | 6.8 ± 7 | 6.74 ± 6.92c | ||
| VL (lU/mL) ≥ 600,000 | 181 (55) | 50 (40) | 15 (32) | 0.27 | 84 (80) | 30 (63) | |
| HCV GT | |||||||
| 1 | 221 (67) | 119 (92) | - | - | 105 (100) | - | - |
| 2 | 48 (15) | - | 21 (45) | - | - | 28 (58) | - |
| 3 | 39 (12) | - | 22 (47) | - | - | 16 (33) | - |
| 4 | 2 (1) | - | 2 (4) | - | - | 0 | - |
| Mixed | 16 (5) | 11 (8)d | 2 (4) | - | 0 | 4 (8)e | - |
| At least one risk factor | |||||||
| Surgeries | 184 (56) | 45 (35) | 14 (30) | 0.08 | 85 (81) | 40 (83) | 0.45 |
| Hemodialysis | 5 (2) | 2 (2) | 0 | 0.42 | 0 | 3 (6) | 0.03 |
| BTb1992 | 185 (56) | 80 (62) | 19 (40) | 0.03 | 60 (57) | 25 (52) | 0.341 |
| DP | 46 (14) | 0 | 1 (2) | 0.36 | 37 (35) | 8 (17) | 0.01 |
| Acupuncture | 23 (7) | 0 | 0 | 0.54 | 18 (17) | 5 (10) | 0.21 |
| SP | 49 (15) | 1 (1) | 2 (4) | 0.5 | 35 (33) | 11 (23) | 0.13 |
| IDU | 53 (16) | 14 (11) | 4 (9) | 0.23 | 26 (25) | 8 (17) | 0.19 |
| Tattooing | 59 (18) | 13 (10) | 10 (21) | 0.18 | 27 (26) | 9 (19) | 0.23 |
| Other RFs | 9 (3) | 9 (7) | 0 | 0.03 | 0 | 0 | - |
| Without RFs | 30 (9) | 18 (14) | 9 (19) | 0.55 | 2 (2) | 1 (2) | 0.68 |
| Cirrhosis/non-cirrhosis | 137/193 (42/68) | 69 (53) | 16 (34) | < 0.01 | 40 (38) | 12 (25) | 0.07 |
| AT | 131 (40) | 76 (58) | 16 (34) | 0.14 | 20 (19) | 6 (13) | 0.22 |
| Recruitment interval | 1987-2013 | 1987-2012 | 1997-2013 | 2010-2013 | 2010-2013 | - | |
SD: standard deviation. F: female. M: male. VL: viral load. BTb1992: blood transfusion before 1992. DP: dental procedure. SP: sexual promiscuity. IDU: injection drug use. AT: it refers to any exposure to antiviral therapy ≥ 12 weeks mentioned by the patient or registered on their medical record during patients follow-up.
BTb1992 (n = 185, 56%) followed by surgeries (n = 184, 56%), tattooing (n = 59, 18%) and IDU (n = 53, 16%) were the most commonly seen RFs (Table 1). In regards to the pattern of RFs between GT1 vs. non-GT1 groups both Mexican regions have a similar distribution of surgeries, hemodialysis, DP, acupuncture, SP, tattooing, other RFs, and negative for RFs.
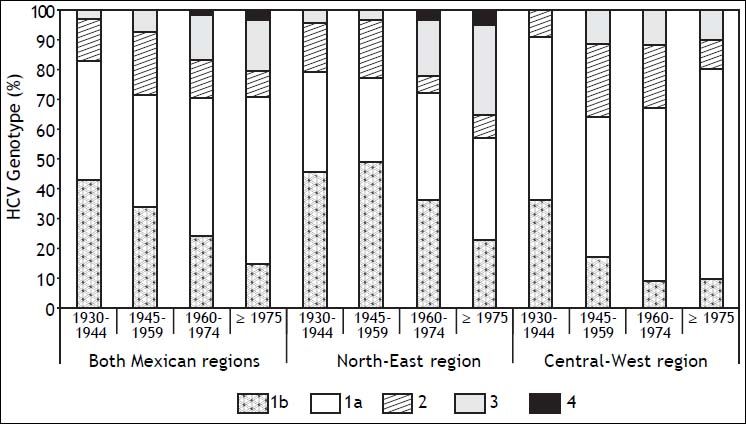
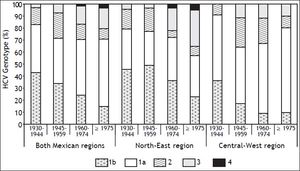
Prevalence trends of HCV genotypes and risk factors by region and date of birthFigure 1 depicts the age-specific prevalence of GT distribution by region and DOB. In the NE, GT3 showed an upward trend in younger patients, as opposed to CW (p < 0.001). Furthermore, GT3 prevalence increased from 4% among DOB between the years 1945-1959 to 31% of patients born in 1975 onwards with a marked increase of 19% among those born between the years 1960-1974. The prevalence of GT1 decreased in the younger patients in both regions throughout the same period. GT1b prevalence declined in both Mexican regions (r = 0.131; p = 0.017) whereas GT1a prevalence did not show a trend over time (data not shown).
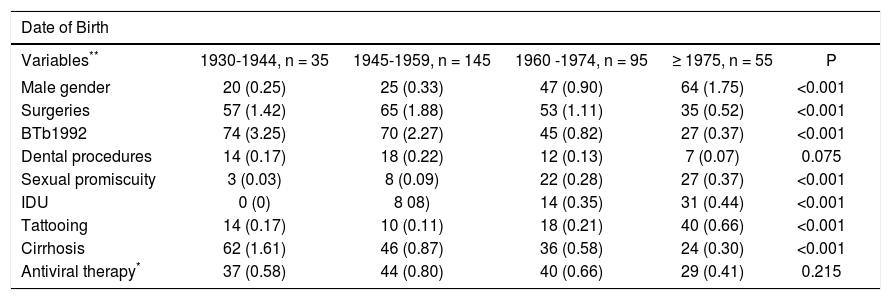
The age-specific association of RFs for the transmission of HCV depicted in Table 2 shows that surgeries (p < 0.001) and BTb1992 (p < 0.001) were the RFs more prevalent in older patients. On the other hand, IDU (p < 0.001), SP (p < 0.001), and tattooing (p < 0.001) were more common in younger patients (Table 2). Furthermore, cirrhosis was more prevalent in patients born in 1930-1944 and less prevalent in younger patients who were born in 1975 onwards (62 vs. 24% respectively, p < 0.001). Exposure to AT had a similar distribution according to DOB (p = NS).
Score test for trend of odds according to patient’s date of birth [% (odds)].
| Date of Birth | |||||
|---|---|---|---|---|---|
| Variables** | 1930-1944, n = 35 | 1945-1959, n = 145 | 1960 -1974, n = 95 | ≥ 1975, n = 55 | P |
| Male gender | 20 (0.25) | 25 (0.33) | 47 (0.90) | 64 (1.75) | <0.001 |
| Surgeries | 57 (1.42) | 65 (1.88) | 53 (1.11) | 35 (0.52) | <0.001 |
| BTb1992 | 74 (3.25) | 70 (2.27) | 45 (0.82) | 27 (0.37) | <0.001 |
| Dental procedures | 14 (0.17) | 18 (0.22) | 12 (0.13) | 7 (0.07) | 0.075 |
| Sexual promiscuity | 3 (0.03) | 8 (0.09) | 22 (0.28) | 27 (0.37) | <0.001 |
| IDU | 0 (0) | 8 08) | 14 (0.35) | 31 (0.44) | <0.001 |
| Tattooing | 14 (0.17) | 10 (0.11) | 18 (0.21) | 40 (0.66) | <0.001 |
| Cirrhosis | 62 (1.61) | 46 (0.87) | 36 (0.58) | 24 (0.30) | <0.001 |
| Antiviral therapy* | 37 (0.58) | 44 (0.80) | 40 (0.66) | 29 (0.41) | 0.215 |
HCV: hepatitis C virus. BTb1992: blood transfusion before 1992. IDU: intravenous drug user.
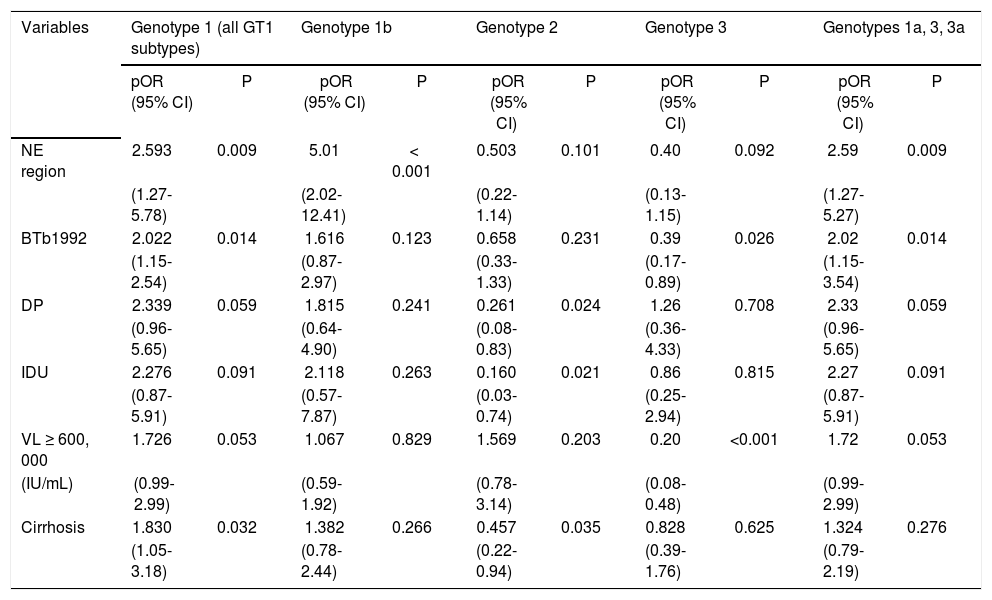
By pOR analysis, BTb1992 was a RF independently associated with HCV GT1 infection (pOR = 2; p = 0.014) (Table 3). GT1b was more common in NE region (pOR = 2.5; p = 0.009) than in the CW region, whereas there was no difference in GT1a distribution. GT2 was rarely associated with DP (pOR = 0.26; p = 0.024) and IDU (pOR = 0.16; p = 0.021) prevalence. Few patients with GT3 infections had VL ≥ 600,000 IU/mL (pOR = 0.20; p < 0.001) and most of GT3 infections were less commonly seen in patients with history of BTb1992 (pOR = 0.39; p = 0.009). Patients infected with GT 1a, 3, 3a, were more commonly seen in NE region (pOR = 2.5; p = 0.009), having a history of BTb1992 (pOR = 2; p = 0.014) (Table 3). Although other authors have commonly seen the association of GTs 1a, 3, 3a, with IDUs, in this study, these relationships were examined, but not found (Table 3).
Binary logistic regression of selected demographic characteristics and infection with selected HCV GTs.
| Variables | Genotype 1 (all GT1 subtypes) | Genotype 1b | Genotype 2 | Genotype 3 | Genotypes 1a, 3, 3a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pOR (95% CI) | P | pOR (95% CI) | P | pOR (95% CI) | P | pOR (95% CI) | P | pOR (95% CI) | P | |
| NE region | 2.593 | 0.009 | 5.01 | < 0.001 | 0.503 | 0.101 | 0.40 | 0.092 | 2.59 | 0.009 |
| (1.27-5.78) | (2.02-12.41) | (0.22-1.14) | (0.13-1.15) | (1.27-5.27) | ||||||
| BTb1992 | 2.022 | 0.014 | 1.616 | 0.123 | 0.658 | 0.231 | 0.39 | 0.026 | 2.02 | 0.014 |
| (1.15-2.54) | (0.87-2.97) | (0.33-1.33) | (0.17-0.89) | (1.15-3.54) | ||||||
| DP | 2.339 | 0.059 | 1.815 | 0.241 | 0.261 | 0.024 | 1.26 | 0.708 | 2.33 | 0.059 |
| (0.96-5.65) | (0.64-4.90) | (0.08-0.83) | (0.36-4.33) | (0.96-5.65) | ||||||
| IDU | 2.276 | 0.091 | 2.118 | 0.263 | 0.160 | 0.021 | 0.86 | 0.815 | 2.27 | 0.091 |
| (0.87-5.91) | (0.57-7.87) | (0.03-0.74) | (0.25-2.94) | (0.87-5.91) | ||||||
| VL ≥ 600, 000 | 1.726 | 0.053 | 1.067 | 0.829 | 1.569 | 0.203 | 0.20 | <0.001 | 1.72 | 0.053 |
| (IU/mL) | (0.99-2.99) | (0.59-1.92) | (0.78-3.14) | (0.08-0.48) | (0.99-2.99) | |||||
| Cirrhosis | 1.830 | 0.032 | 1.382 | 0.266 | 0.457 | 0.035 | 0.828 | 0.625 | 1.324 | 0.276 |
| (1.05-3.18) | (0.78-2.44) | (0.22-0.94) | (0.39-1.76) | (0.79-2.19) | ||||||
GTs: genotypes. NE: Northeast. BTb1992: blood transfusion before 1992. DP: dental procedure. IDU: injection drug user. VL: viral load.
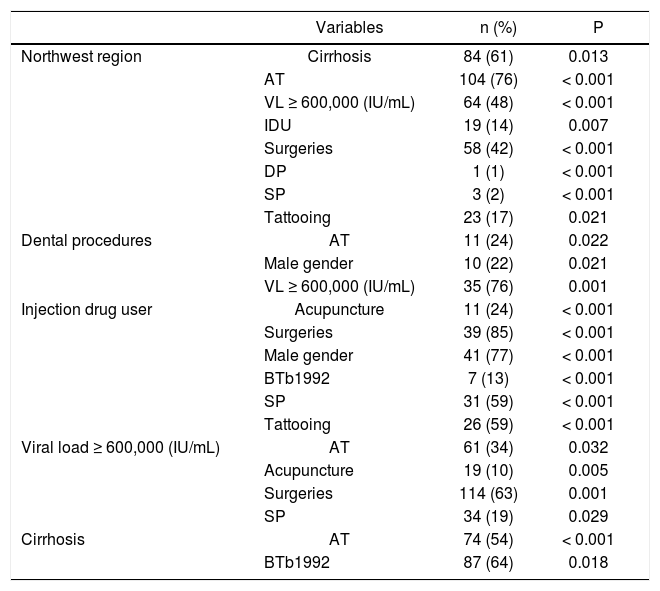
Table 4 depicts an overall univariate analysis among several RFs that had previously tested significant for certain GTs (Table 3). In the group of patients residing in the NE region, AT exposure was frequent (76%) and more commonly seen among GT1 patients (58%). Only surgeries, mainly among GT1 patients (All GT 1 subtypes = 35%, 1a = 42%, 1b = 44%), was the most commonly seen RF in this region (Tables 1 and 4). On the other hand, patients living at the NE were less frequently exposed to SP (2%), DP (1%) and tattooing (17%). Interestingly, for the entire population, most of the patients with IDU history were characterized by being males (77%), with history of SP (59%), tattooing (59%), and epidemiologically were unlikely to reside in the NE region (14%) (Table 4). Higher VL were commonly seen among patients living in the NE region (48%), exposed to surgeries (42%) and DP (76%) (Table 4).
Significant associations between genotype-specific risk factors and baseline features of the studied population.
| Variables | n (%) | P | |
|---|---|---|---|
| Northwest region | Cirrhosis | 84 (61) | 0.013 |
| AT | 104 (76) | < 0.001 | |
| VL ≥ 600,000 (IU/mL) | 64 (48) | < 0.001 | |
| IDU | 19 (14) | 0.007 | |
| Surgeries | 58 (42) | < 0.001 | |
| DP | 1 (1) | < 0.001 | |
| SP | 3 (2) | < 0.001 | |
| Tattooing | 23 (17) | 0.021 | |
| Dental procedures | AT | 11 (24) | 0.022 |
| Male gender | 10 (22) | 0.021 | |
| VL ≥ 600,000 (IU/mL) | 35 (76) | 0.001 | |
| Injection drug user | Acupuncture | 11 (24) | < 0.001 |
| Surgeries | 39 (85) | < 0.001 | |
| Male gender | 41 (77) | < 0.001 | |
| BTb1992 | 7 (13) | < 0.001 | |
| SP | 31 (59) | < 0.001 | |
| Tattooing | 26 (59) | < 0.001 | |
| Viral load ≥ 600,000 (IU/mL) | AT | 61 (34) | 0.032 |
| Acupuncture | 19 (10) | 0.005 | |
| Surgeries | 114 (63) | 0.001 | |
| SP | 34 (19) | 0.029 | |
| Cirrhosis | AT | 74 (54) | < 0.001 |
| BTb1992 | 87 (64) | 0.018 |
AT: any exposure to antiviral therapy ≥ 12 weeks mentioned by the patient or registered on their medical record during the patient’s follow-up. BTb1992: blood transfusions before 1992. SP: sexual promiscuity. DP: dental procedure. IDU: injection drug user. VL: viral load.
Strong predictors of the presence of liver cirrhosis in the overall sample coincided with the known factors for disease progression and what is reasonably associated with chronic HCV infection. Patients in the older age subgroups (≥ 50 years old) had a higher prevalence of cirrhosis than other age groups (p < 0.001) (Table 2), relating to a longer exposure to the chronic infection. Furthermore, patients with GT 1 (p = 0.032) (Table 3) and AT exposure (p < 0.001) had a higher prevalence of liver cirrhosis (Table 4).
DiscussionIn this study, the overall HCV genotypic distribution (GT1 > GT2 > GT3) among the studied population was consistent with previous data from Mexico,7,21–24,33–35 but contrasting with the epidemiology of HCV infection (GT1 > GT3 > GT2) reported from Western countries and Australia.36–38 While the prevalence of GT1a remained stable in the CW region, the prevalence of GT3 significantly increased in the total group and the NE region, and GT1b decreased only in the entire group. Likewise, the overall RF pattern was similar to that observed in earlier studies among Mexican CHC patients,7,17,22,33,34 given that in this study, BTb1992 (56%) and surgeries (56%) were highly prevalent, followed by tattooing (18%), and IDU (16%) and other less frequent RFs.
However, marked differences in age-specific pattern of HCV GT and RFs were observed in this study as it has been reported in other countries.1,37,38 In this study, GT3 tended to prevail among youths rather than in older adults (21 vs. 3%; p < 0.001), whereas, GT1b was more commonly seen as older were the patients (16 vs. 31%; p = 0.006). Similar results involving these two GTs have been reported in Europe,37 Canada,37 USA39 and Australia.40 Unlike patients from Lahore, Pakistan,41 blood transfusion is a risk factor rarely seen in Mexican patients with GT3 reported here. This finding is consistent with earlier cross-sectional HCV prevalence studies that have not reported cases of GT3 among blood donors.17,42 However, if the upward trend of GT3 further continues, this may effectually shift the current association of GT1b with chronic liver disease found in this study. Thus, awareness of a potential epidemiological transition of HCV GTs is an essential factor in order to avoid chronic liver disease among distinct risk groups.
As expected, BTb1992 (~70%) and surgeries (~55%) emerged as a relevant RF for HCV infection in patients born before 1959 and 1974, respectively (p < 0.001). However, IDU (≥ 14%), SP (≥ 22%) and tattooing (≥ 18%) emerged as a major RF for patients born upon 1959 (p < 0.001) (Table 2). Moreover, in this study, RFs were also seen to cluster SP, IDU and tattooing positive correlations with males born in 1959 onwards, whereas, BTb1992 and surgeries correlated positively with females born before 1959.
Additionally, a particular transmission route does not always equal a specific GT. In this study, there was a significant association between a high prevalence of BTb1992 (56%) and GT1 (71%) that contrasts to what has been reported in Canada and France. In these countries, a low prevalence of BTb1992 (10%, 32%, respectively) and a high prevalence of GT1 (64%, 57%, respectively) was reported.37 Additionally, both GT1a and GT3 have been significantly associated with IDU in another series of studies.37,39,43 Contrarily, in this study IDU did not stand as an independent RF neither for GT3 alone nor alongside G1a- infected patients. However, in the case of Mexico, HCV GT1 infection may have been introduced by professional blood donors coming from the Unites States.13 Before 1992, routine HCV screening had not been implemented in the country.7,13 Thus, iatrogenic transmission of HCV GT1 could have spread by contaminated blood transfusion, either through obstetric/gynecological surgery in women44 or by general surgery among both genders.7,13
On the other hand, IDUs were uncommon before 1990 in Mexico.13 However, it has been recently suggested that in younger patients, IDUs could currently account for a primary HCV transmission route in Mexico (67%),23 similarly to the United States cases (27-93%).45 Moreover, migration or deportation histories are related determinants that currently have been linked to both new drug trends and risky behaviors. These trends may not only affect the distribution of HCV GTs, but also the continued transmission of HCV infection among returning or deported Mexican migrants46,47 and IDUs along the United States-Mexico border.48
Mathematical estimations have projected that HCV prevalence in Mexico will continue to increase during the next decades, mainly due to low diagnostic and treatment rates,11,49 along with a lack of awareness and education of the disease. Given the historial CHC seroprevalence (2000), the Mexican bimodal pattern25 resembles the ones present in France, Germany, Czech Republic and Switzerland, but with thrice their seroprevalence (1.4% (6), 0.3%, 0.3%, 0.4% and 0.4%, respectively).36,50 Interestingly, they also share the same GT transition found in the present study, being GT3 more prevalent among youths, but not GT1b.36 Additionally, results found here and elsewhere11,46 suggest that Mexican GT1 patients are commonly found with higher VL whereas GT3 patients with a lower one. However, this remains to be confirmed. Noteworthy, advanced liver disease burden is also expected to continue to increase in almost all countries,36,50 whether or not they have a relatively stable chronic HCV prevalence.
Also, this study suggests that cirrhotic patients could be profiled as patients ≥ 50 years old, are infected with GT1 and already exposed to AT. However, further probabilistic sample-size studies are needed to evaluate this possibility, because the patients characteristics might account for the significant treatment rate found across studied DOB (~36%) that could influence the prevalence of cirrhosis found herein (40%).
Finally, the associations observed in the current study provide a descriptive glimpse of the epidemiological data regarding the distribution of HCV GTs in two distinct geographical regions of Mexico. However, further studies are warranted to elucidate the underlying mechanisms explaining the above-mentioned epidemiological data from these regions and nationwide. Prevention and treatment strategies are also required to avoid the incidence and spread of new cases of HCV among distinct risk groups based on regional surveillance data. Likewise, molecular epidemiology studies, that rely on DNA sequencing, evolutionary (molecular clock) and phylogeographic analyses of HCV sequences are warranted. Such updated information may provide the actual source of infection and routes of dissemination of the HCV infection in Mexico, as well as a better understanding of the evolving genetic variability and distribution of the HCV subtypes.
In conclusion, GT1a prevalence in CW Mexico remained stable throughout the studied period, whereas GT3 increased as GT1b decreased in younger patients from CW and NE regions, along with associated RFs. The data shown in this present study can help to understand the transitional epidemiological shifting of HCV GT and related RFs among HCV-infected patients in Mexico.
Abbreviations- •
AT: antiviral therapy.
- •
BTb1992: blood transfusion before 1992.
- •
CHC: chronic hepatitis C.
- •
CW: Central West.
- •
DOB: date of birth.
- •
DP: dental procedures.
- •
GT(s): genotype(s).
- •
HCV: hepatitis C virus.
- •
IDU(s): injection drug user(s).
- •
Lipa: line probe assay.
- •
NE: North-East.
- •
RF(s): risk factor(s).
- •
SP: sexual promiscuity.
- •
VL: viral load.
This work was partially supported by grants: 1) CONACyT (SALUD-2010-C01-139085) to SR, 2) CONACYT (CB2010-01155082) and the Universidad Autónoma de Nuevo León (PAICYT-SA1161-05) to AMRE, and 3) CONACYT-GRANT 162077 to LEME. LEME, AP and MCGR are representative recipients for the sponsorship given to the “Red Temática de Colaboración Académica en Fisiopatología de las Enfermedades Hepáticas, SEP-Promep, Mexico”.
AcknowledgmentsWe are especially grateful to the medical and technical staff of the Liver Unit at the Internal Medicine Department, Hospital Universitario de la Universidad Autónoma de Nuevo León (UANL) and Hospital Civil de Guadalajara, Fray Antonio Alcalde.