Selenium supplementation has been shown to have therapeutic value in chronic liver disease. We aimed to investigate the association between serum selenium, severity of liver fibrosis, and mortality in patients with Nonalcoholic Fatty Liver Disease (NAFLD).
Patients or material and methodsA total of 33,944 patients were identified from the Third National Health and Nutrition Examination Survey. NAFLD was diagnosed by hepatic ultrasound after the exclusion of other forms of liver diseases. The severity of liver fibrosis was determined by NAFLD Fibrosis Score >0.676. Multivariate logistic regression analysis was used to investigate the relationship between serum selenium level and liver fibrosis. Association between serum selenium and all-cause mortality in NAFLD patients was also evaluated.
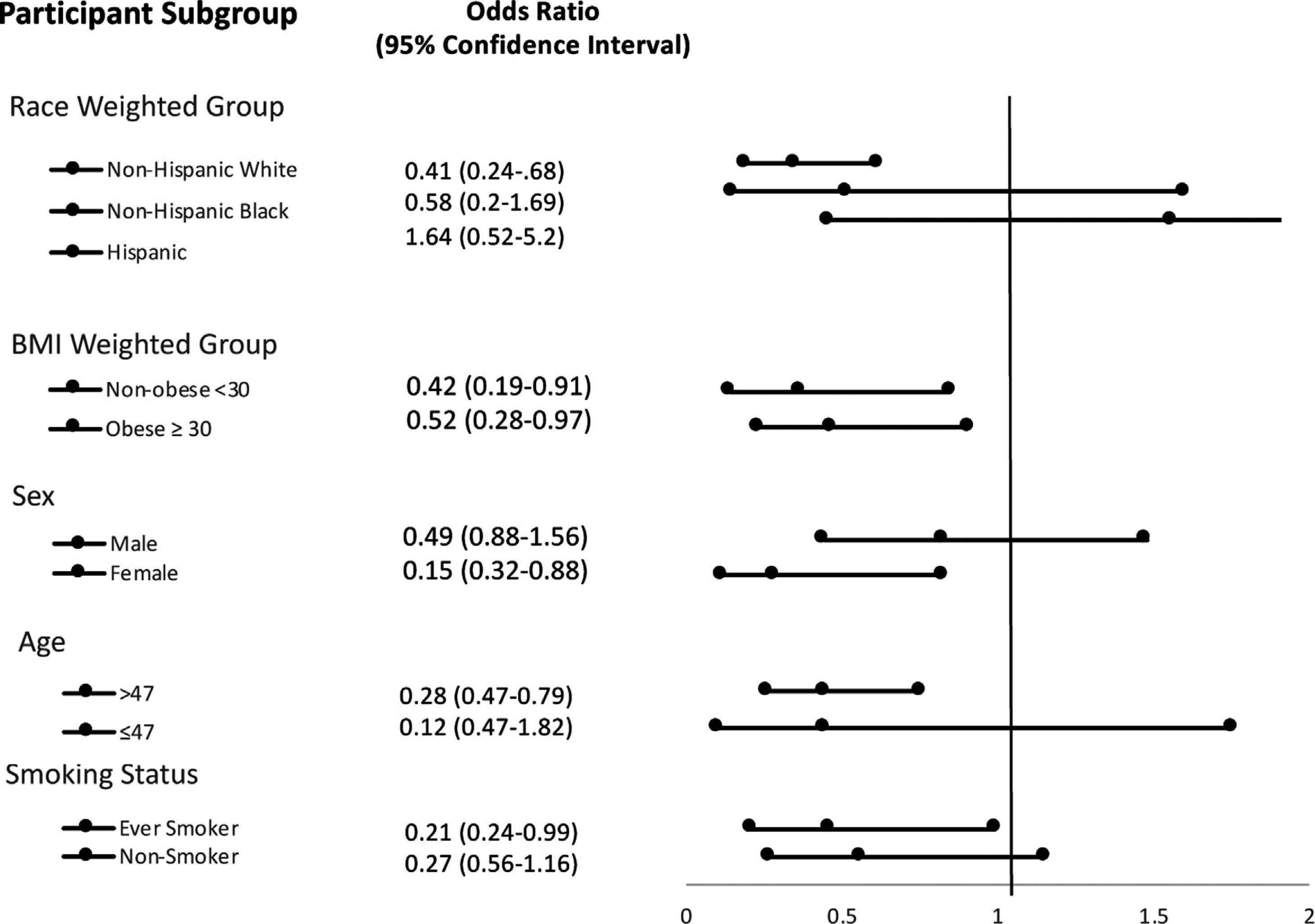
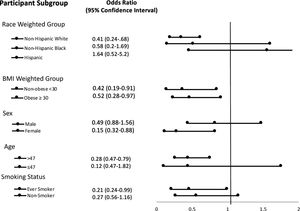
ResultsMultivariate logistic regression analysis demonstrated odds ratio of advanced liver fibrosis (NFS > 0.676) was significantly reduced with increasing serum selenium levels; OR 0.55, [95% CI 0.32−0.94] in the highest selenium quartile. On stratification analysis, the following populations had a significantly reduced risk of advanced liver fibrosis: non-Hispanic white = OR 0.41 [0.24,0.68]; female = OR 0.32 [0.15−0.66] and age >47 = OR 0.47 [0.28−0.79]. The relationship was significant regardless of BMI as noted by BMI ≤ 30 Kg/m2= OR 0.42 [0.19−0.91] and BMI > 30 Kg/m2=OR 0.52 [0.28−0.97]. Hazard ratio for all-cause mortality was HR 0.72 [0.56−0.95].
ConclusionsThe risk of advanced liver fibrosis is inversely associated with serum selenium levels, particularly in older patients, Caucasians, and females. All-cause mortality decreased with increased selenium levels. Selenium may play a role in the prevention of liver fibrosis in NAFLD.
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide and increases the risk of end-stage liver disease and hepatocellular carcinoma (HCC) [1]. There is growing evidence that NAFLD is the hepatic manifestation of a multisystem disease, also affecting extra-hepatic organs leading to increased risk of type 2 diabetes mellitus, cardiovascular disease, and chronic kidney disease [2]. While risk factors such as obesity, diabetes, and a sedentary lifestyle may increase the risk of NAFLD, studies have shown that environmental exposures may further contribute to the pathogenesis of NAFLD [3–5].
Selenium is an essential element in many biological functions and is an important component of human nutrition. Exposure to selenium can be found in nature, such as rocks and sediment, air, soil, fuel oil, drinking water and nutritional supplementation. It is a major component of many enzymes such as glutathione peroxidase and plays an important role in anti-oxidation, DNA synthesis, reproduction, muscle function, and thyroid metabolism [6]. Selenium concentrations have been studied in many diseases and organ systems including the liver [7]. However, the exact relationship between selenium in patients with NAFLD is unclear.
Prior studies have shown selenium concentrations to be lower in patients with NAFLD and cirrhosis [8–11]. Deficiencies in selenium levels in cirrhosis may contribute to the early development of hepatocellular carcinoma [12,13]. Further, selenium supplementation has been shown to improve liver function by reducing serum AST and ALT levels [14]. Therefore, selenium may play a role in improving severe liver disease [12,15]. Despite these findings, there is a paucity of research in epidemiological studies evaluating the role of selenium in patients with NAFLD.
This analysis looked at the association between serum selenium levels and the progression to advanced liver fibrosis in NAFLD patients in the NHANES-III database. The role of selenium in NAFLD and advanced fibrosis has potential implications for the treatment of liver disease.
2Material and methods2.1Dataset and linkageThe National Health and Nutrition Examination Survey (NHANES)-III (1988–1994) is a survey research program in the United States to assess the health status of the civilian population [16]. NHANES III was conducted in two phases, from 1988 to 1991 and 1991 to 1994. Interviews, physical exams, and laboratory testing were done, as well as ultrasound to assess for hepatic steatosis. Data from NHANES-III was also linked to death certificates from the National Death Index (NDI) as of December 31st, 2011, allowing for mortality analysis [17]. Given the de-identified nature of this study, an institutional review board was not required.
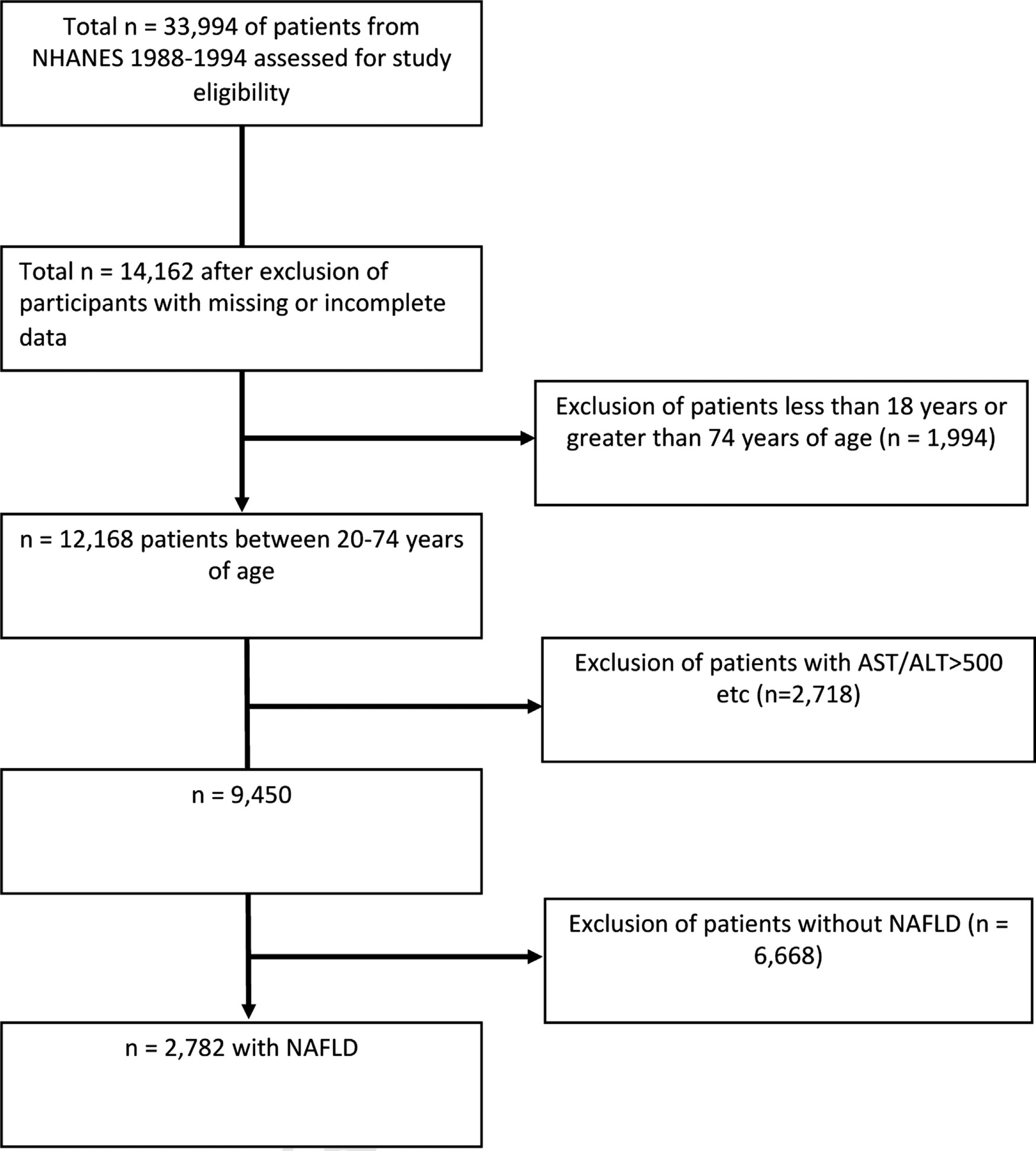
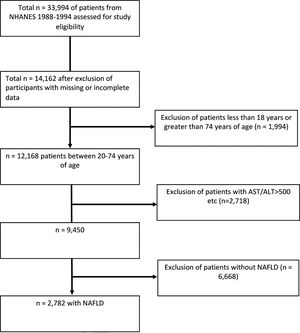
2.2Study populationOf the total 33,994 NHANES III study participants, 12,168 patients remained after exclusion for missing data or were outside the 20–74 age range (Fig. 1). 2718 patients were excluded for the following: positive hepatitis B or C markers, transferrin saturation >50%, >10 alcoholic drinks every week for females and >20 alcoholic drinks every week for males, and AST or ALT > 500 IU/L. 6668 patients without NAFLD were also excluded. A total of 2782 individuals with NAFLD ages 20–74 were included in this study. These individuals had data on hepatic steatosis as diagnosed by ultrasound as well as serum selenium levels.
Gallbladder ultrasound was conducted between 1988 and 1994 as part of NHANES III and was re-assessed for hepatic steatosis. The degree of steatosis was graded as normal, mild, moderate or severe. Quality control and imaging findings were followed per published protocols [18].
2.3OutcomesSelenium levels were categorized into quartiles (<113 ng/mL, 113−122 ng/mL, 123−133 ng/mL, and >133 ng/mL). Laboratory preparation methods were standard procedure from the CDC’s Elemental Analysis Laboratory and is available elsewhere [19].
We determined serum selenium levels by degree of liver fibrosis. The NFS was used to assess fibrosis severity. The NFS score incorporates age, BMI, fasting glucose, AST, ALT, platelet count, and albumin. A score above 0.676 signifies severe fibrosis or cirrhosis of the liver. A score between −1.455 and 0.675 defines intermediate fibrosis, and a score < -1.455 defines mild fibrosis [20]. These cutoffs were used to determine selenium levels in relation to levels of fibrosis. To assess the association between selenium quartiles and all-cause mortality, we used the NHANES-III dataset linked to the National Death Index as of December 31st, 2011, for a maximum follow-up period of 276 months from the date of interview. Median follow-up period was 19.8 years.
Covariates included age, race/ethnicity, sex, alcohol consumption (grams/day), smoking status (‘smoker or former smoker’ as determined by smoking ≥ 100 cigarettes, ‘never smoker’), systolic and diastolic blood pressures, diabetes status (Yes/No) as determined by a high HbA1c (> 6.5%), high fasting blood sugar (> 126 ng/dl), or use of diabetic medication, waist circumference, HDL, LDL, triglycerides, overall metabolic syndrome status (Yes/No), and C-reactive protein.
2.4Statistical analysisTo compare baseline characteristics between the fibrosis and no fibrosis groups, we used Student’s t-test for continuous variables (assumptions for normality were reasonably met) and Rao-Scott Chi-Square test for categorical variables. Comparisons of covariates across selenium quartiles were done using ANOVA for continuous variables and Rao-Scott Chi-square tests for categorical variables followed by post-hoc pairwise comparisons between quartiles using Tukey’s multiple comparison test. Mean NAFLD Fibrosis Score was compared across selenium quartiles using ANOVA, followed by post-hoc pairwise comparisons using Tukey's multiple comparison test.
To determine the association between serum selenium quartiles and fibrosis, a multivariable logistic regression model was used and confounding variables were selected in a series of three additive models. Model A adjusted for age group (
3Results3.1Baseline characteristicsTable 1 lists baseline characteristics by the presence of advanced liver fibrosis. 8.4% (236/2782) of the study population had advanced liver fibrosis, as determined by the NFS score. Those with liver fibrosis were, on average, 16 years older (62.0 vs. 45.8; p < 0.0001) and were less likely to be male (44% vs. 56%; p < 0.01). As expected, those with liver fibrosis had increased blood pressure, waist circumference, BMI, HOMA-IR, and metabolic syndrome (all p < 0.0001).
Baseline characteristics stratified by presence of liver fibrosis.
| Variables | Fibrosis | No Fibrosis | p-value |
|---|---|---|---|
| 236 (7%) | 2546 (93%) | ||
| Age | 62.0 (0.7) | 45.8 (0.5) | <0.0001 |
| Sex (% male) | 91 (44%) | 1254 (56%) | 0.0022 |
| Systolic BP (mmHg) | 139 (1) | 127 (0.5) | <0.0001 |
| Diastolic Blood Pressure (mmHg) | 78 (0.8) | 79 (0.2) | 0.086 |
| Race/Ethnicity | |||
| Non-Hispanic White | 114 (79%) | 936 (77%) | 0.103 |
| Non-Hispanic Black | 67 (14%) | 690 (11%) | |
| Mexican American | 52 (4%) | 839 (7%) | |
| Other | 3 (3%) | 81 (6%) | |
| Waist Circumference (cm) | 117.8 (0.8) | 106.9 (0.4) | <0.0001 |
| BMI (kg/m2) | 36.6 (0.5) | 32.2 (0.2) | <0.0001 |
| TG in (mg/dL) | 215.4 (15.5) | 214.2 (4.6) | 0.95 |
| Total Cholesterol (mg/dL) | 225.1 (4.9) | 224.1 (1.5) | 0.84 |
| HDL (mg/dL) | 42.7 (1.1) | 42.4 (0.5) | 0.82 |
| A1C | 6.55 (0.19) | 5.61 (0.04) | <0.0001 |
| CRP | 0.76 (0.10) | 0.54 (0.02) | 0.026 |
| AST (IU/L) | 23.4 (1.5) | 23.0 (0.5) | 0.79 |
| ALT (IU/L) | 16.5 (1.5) | 23.5 (0.8) | 0.0001 |
| GGT (IU/L) | 43.0 (6.0) | 41.3 (1.3) | 0.77 |
| Smoked at least 100 cigarettes | 117 (53%) | 1283 (58%) | 0.33 |
| Selenium (ng/mL) | 124.5 (2.3) | 126.6 (1.5) | 0.31 |
| HOMA-IR >2.5 | 180 (77%) | 1554 (54%) | <0.0001 |
| Metabolic Syndrome as % | 211 (93%) | 1633 (65%) | <0.0001 |
Table 2 lists the baseline characteristics by selenium quartile. Those in the highest quartile of selenium (> 133 ng/mL) were older, more likely to be male and non-Hispanic. Those in the highest quartile also had significantly elevated triglycerides and total cholesterol and had significantly lower waist circumference and BMI. Other laboratory markers can be seen in Table 2.
Baseline characteristics of study subjects stratified by Selenium level (Quartiles 1-4).
| Selenium level | |||||
|---|---|---|---|---|---|
| Variables | Quartile 1 (<113) | Quartile 2 (113−122) | Quartile 3 (122−133) | Quartile 4 (> 133) | p-value |
| Age | 45.4 (0.8) | 47.4 (1.0) | 47.2 (0.8) | 47.4 (0.9) | 0.1 |
| Sex (% male) | 251 (45%) | 277 (54%) | 400 (59%) | 417 (60%) | |
| Systolic BP (mmHg) | 126 (0.9) | 127 (0.9) | 128 (0.7) | 130 (1.0) | 0.027 |
| Diastolic Blood Pressure (mmHg) | 77 (0.5) | 78 (0.6) | 80 (0.6) | 79 (0.5) | 0.0024 |
| Race/Ethnicity: (as %) | |||||
| Non-hispanic white | 196 (69%) | 217 (74%) | 302 (77%) | 335 (83%) | |
| Non-hispanic black | 267 (20%) | 176 (12%) | 170 (9%) | 144 (6%) | |
| Mexican American | 173 (7%) | 192 (7%) | 258 (7%) | 268 (6%) | |
| Other | 16 (1%) | 25 (7%) | 25 (8%) | 8 (5%) | |
| Waist Circumference (cm) | 109.4 (0.8) | 107.8 (0.7) | 107.5 (0.7) | 106.6 (0.8) | 0.011 |
| BMI (kg/m2) | 33.7 (0.4) | 32.5 (0.4) | 32.6 (0.4) | 31.8 (0.3) | 0.001 |
| TG (mg/dL) | 191 (8) | 214 (9) | 225 (12) | 219 (5) | 0.0092 |
| Total Cholesterol (mg/dL) | 215.4 (2.5) | 219.5 (3.2) | 223.0 (2.9) | 233.5 (1.7) | <0.0001 |
| HDL (mg/dL) | 43.1 (0.8) | 41.6 (0.9) | 42.0 (0.6) | 42.8 (0.7) | 0.82 |
| A1C | 5.59 (0.05) | 5.63 (0.05) | 5.61 (0.06) | 5.80 (0.08) | 0.0325 |
| CRP | 0.71 (0.06) | 0.57 (0.03) | 0.54 (0.04) | 0.46 (0.03) | 0.0002 |
| AST (IU/L) | 21.8 (0.9) | 23.5 (0.9) | 24.0 (0.8) | 22.6 (0.4) | 0.36 |
| ALT (IU/L) | 19.3 (0.9) | 23.1 (1.4) | 23.9 (1.2) | 24.4 (1.3) | 0.0015 |
| GGT (IU/L) | 38.3 (2.4) | 41.9 (3.4) | 40.9 (1.9) | 43.4 (2.4) | 0.11 |
| Selenium | 104.2 (0.5) | 117.3 (0.2) | 126.9 (0.2) | 145.5 (1.6) | <0.0001 |
| HOMA-IR (>2.5) | 401 (57%) | 383 (56%) | 475 (54%) | 475 (54%) | 0.78 |
| Metabolic Syndrome as % | 388 (61%) | 401 (66%) | 501 (67%) | 554 (70%) | 0.0372 |
| Smoked at least 100 cigarettes | 320 (58%) | 303 (57%) | 375 (56%) | 402 (60%) | 0.71 |
| Albumin in g/DL | 3.98 (0.03) | 4.07 (0.02) | 4.12 (0.03) | 4.16 (0.03) | <0.0001 |
| Platelet as x10^9/L | 22.4 (0.6) | 23.8 (0.6) | 23.6 (0.5) | 24.8 (0.5) | 0.0034 |
| Fasting Plasma Glucose (mg/dL) | 104.8 (1.7) | 104.6 (1.9) | 105.2 (2.2) | 110.1 (2.8) | 0.11 |
A higher level of serum selenium was significantly associated with a lower likelihood of liver fibrosis. After the full adjustment model C, patients had a 45% reduced risk of advanced liver fibrosis (aOR 0.55, 95% CI 0.32−0.94; p < 0.05) in the highest selenium quartile (>133 ng/mL).
Stratification analysis of subgroups are listed in Fig. 2. In serum selenium quartile 4, non-Hispanic whites, females, age >47 and those with a smoking history had a significantly decreased association with liver fibrosis.
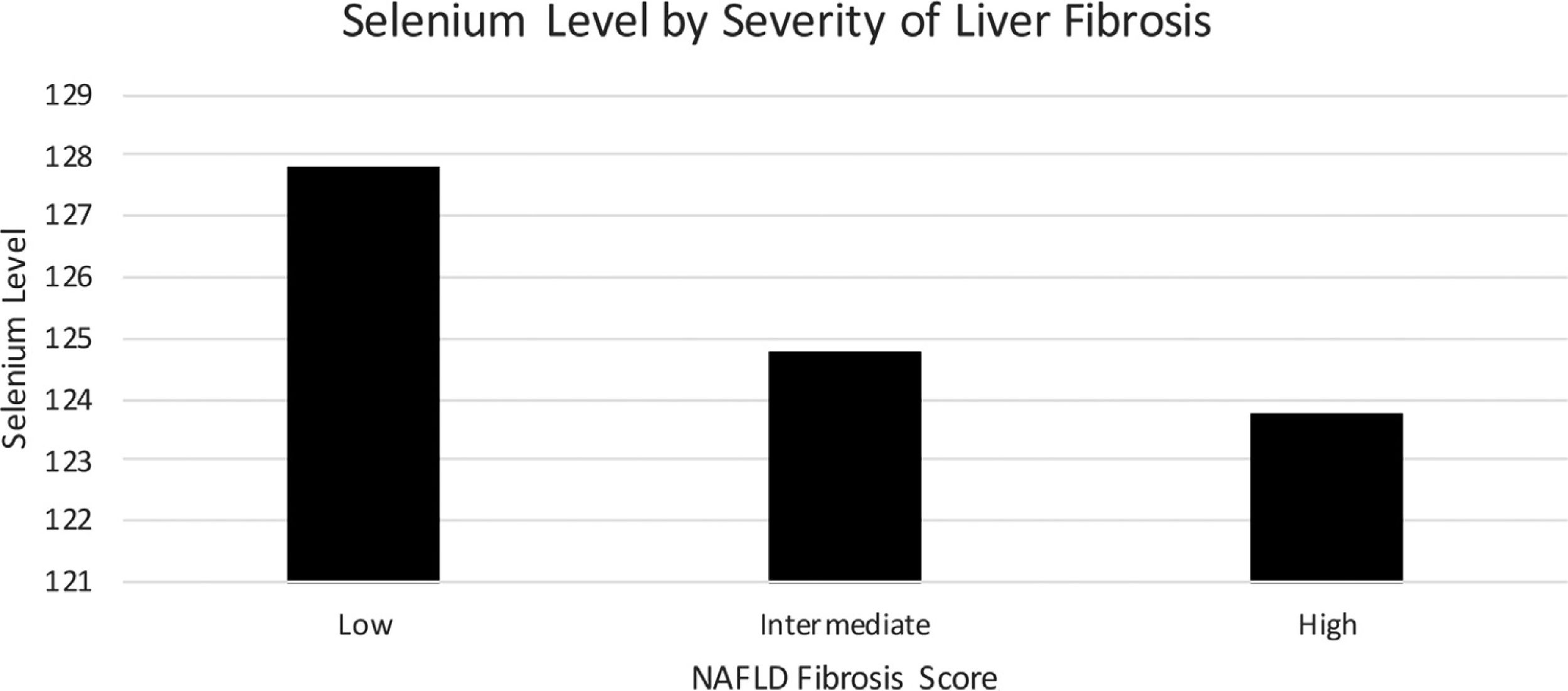
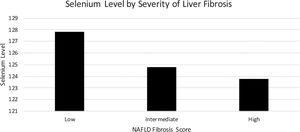
Mean serum selenium levels by degree of fibrosis as determined by NFS are listed in Fig. 3 using multivariate logistical regression model C. Mean serum selenium levels for low, intermediate, and high levels of fibrosis were 127.8 (1.7) ng/mL, 125.8 (1.6) ng/mL, 123.8 (2.3) ng/mL, p < 0.05, respectively (Table 3).
Multivariate Logistical Regression models of Serum Selenium Quartile and Liver Fibrosis.
| Analysis | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p value |
|---|---|---|---|---|---|
| Univariate OR | 1.00 | 0.50 (0.33, 0.76) | 0.72 (0.52, 1.00) | 0.47 (0.27, 0.81) | 0.004 |
| Multivariate Adjusted-A | 1.00 | 0.53 (0.35, 0.80) | 0.78 (0.56, 1.08) | 0.51 (0.30, 0.87) | 0.02 |
| Multivariate Adjusted-B | 1.00 | 0.55 (0.32, 0.92) | 0.74 (0.51, 1.07) | 0.55 (0.32, 0.91) | 0.0539 |
| Multivariate Adjusted- C | 1.00 | 0.55 (0.32, 0.92) | 0.73 (0.50, 1.07) | 0.55 (0.32, 0.94) | 0.0488 |
We determined the hazards of all-cause mortality by selenium quartile. After adjustment for age, sex, and race confounders, those in the highest quartile of selenium had a 28% lower hazard of mortality compared to those in the lowest quartile (HR [95% CI]: 0.72 [0.56, 0.95]). Those in the fully adjusted multivariate model had a 22% lower hazard of mortality compared to the lowest quartile although this relationship was not significant (HR [95% CI]: 0.78 [0.60, 1.02]).
4DiscussionPrior studies have shown the wide impact of selenium on human health, ranging from antioxidant and anti-inflammatory effects to tumor prevention [21]. Selenium deficiency may have numerous deleterious health effects including advanced liver fibrosis and hepatocellular carcinoma (HCC) [8–13]. We found that patients with advanced liver fibrosis had lower levels of serum selenium. Mild, moderate, and severe liver fibrosis (as defined by the NAFLD Fibrosis Score) had respective median serum selenium levels of 127.8, 124.8, and 123.8, (p < 0.05) in a nationwide cohort. Our results are consistent with similar studies that have reported plasma selenium concentrations to be decreased in patients with cirrhosis [8–12,22].
Bettinger et al. performed a pilot study that showed reduced levels of selenium in liver cirrhosis and HCC when compared to healthy control patients [12]. A meta-analysis by Zhang et al. showed an inverse correlation of selenium level and risk of HCC in human patients [23]. Selenium is known to strengthen immunity and counteract free radicals that lead to progression and development of cancer [24]. Selenium protects membrane lipids from oxidative damage and reactive oxygen species (ROS) and may decrease development of HCC [25]. Given these findings, adequate selenium levels can be beneficial for slowing progression of advanced liver fibrosis and hepatocellular carcinoma.
4.1Socioeconomic factorsIn the present study, we found that patients >47 years, females, and non-Hispanic Caucasians had decreased advanced liver fibrosis with increased serum selenium levels. In our primary data analysis study, we found that Caucasians were more likely to have higher selenium levels while blacks and Mexicans had lower levels of serum selenium. Selenium is found in nutrient-dense foods such as meats, seafood, grains, and nuts that may be reflected in socioeconomic status [26,27]. This is important to note when considering vulnerable populations at risk of lower dietary selenium. Prior studies have shown similar findings [28]. Regional variations, geography, and access to food and water are thought to partly explain some of the racial and ethnic disparities in selenium levels. Gender differences in selenium levels have been associated with insulin resistance, adiposity, and lipid metabolism [29]. Smoking in a known risk factor for NAFLD and cirrhosis, and prospective trials will be needed to elucidate the effects of smoking. Therefore, these are important considerations when exploring the therapeutic value of supplementation in liver disease and mortality.
4.2MortalityFew studies have been done in evaluating selenium in patients with NALFD and its effects on disease progression and mortality. This study demonstrated that all-cause mortality was significantly decreased in a large U.S. population cohort. NAFLD patients with increased selenium levels had a 28% lower hazard of mortality compared to those in the lowest quartile. The role of selenium supplementation and its impact on all-cause mortality has been controversial. Recently, studies have shown promising results for selenium supplementation in decreasing total in-hospital mortality in critically ill patients [30]. Further randomized controlled trials are needed to better understand these associations.
4.3Clinical implicationsFew studies have been done on evaluating the role of selenium supplementation and its impact on clinical outcomes in patients with liver disease. One prior study showed that supplementation with selenium in animal models can improve liver disease and decrease transaminases [31]. Additional studies have shown selenium supplementation to have therapeutic value in liver disease [31–34]. Selenium is an important antioxidant and is known to counteract oxidative stress that may contribute to worsening liver fibrosis. It remains to be explored whether selenium supplementation might be beneficial in liver fibrosis patients and decrease progression to hepatocellular carcinoma.
4.4LimitationsOur study was limited by a few factors. This study is a cross-sectional analysis and prospective randomized controlled trials are needed. We also did not use liver biopsy for diagnosis, which is the gold standard. Nevertheless, we believe that this study provides important insight into the association between selenium deficiency and liver disease.
5ConclusionsThis is the first large population-based study in the United States looking at the association between selenium and hepatic fibrosis in patients with NAFLD. Our findings suggest an inverse relationship between advanced fibrosis as well as decreased mortality in patients with elevated levels of selenium. Selenium plays an important role in liver disease and additional studies may help us understand the impact on cirrhosis and hepatocellular carcinoma.
Author contributionsAll authors worked in all 4 aspects of authorship as per ICMJE guidelines.
FundingNone.
Conflict of interestThe authors have no conflicts of interest to declare.
None.