After hepatitis A (HAV) mandatory immunization in 2005 in Argentina, the incidence of HAV declined drastically. However, several new autochthonous cases of HAV have been reported since 2017. We aimed to evaluate the clinical and epidemiological characteristics and possible transmission routes of affected patients.
Patients or Materials and methodsWe performed a cross-sectional study of patients residing in Argentina with acute hepatitis A between 30.06.2017 and 31.12.2018.
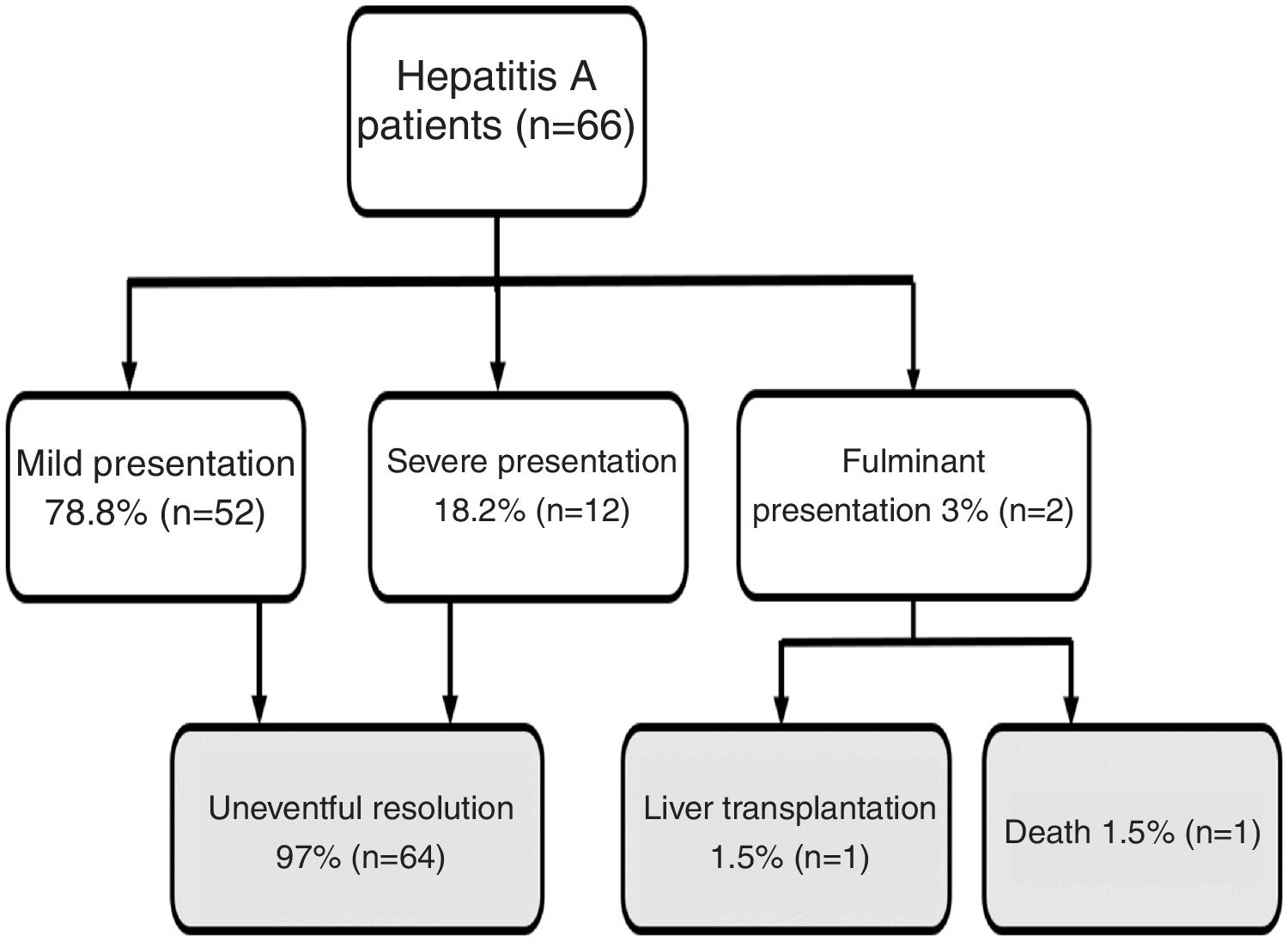
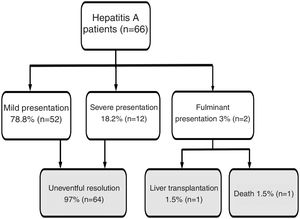
Results66 cases of HAV were registered. Fifty-six patients (86%) were males, with a mean age of 34 ± 12 years old. The most likely routes of transmission were sexual intercourse of men with men, reported by 31 patients. Additionally, 23% and 26% of patients tested positive for HIV and syphilis, respectively. In total, 35% of patients required hospitalization. When assessing outcomes, 79% had a mild presentation and 21% had a severe/fulminant presentation: one patient underwent liver transplantation, and one patient died.
ConclusionsOur study describes that during the study period, HAV infection affected predominantly young adults, particularly men who have sex with men. An elevated proportion of them was diagnosed with a concomitant sexually transmitted disease, and several patients had a severe presentation of the disease.
Hepatitis A is an acute infection of the liver caused by the hepatitis A virus (HAV). This self-limiting infection was estimated to cause approximately 126 million cases of acute hepatitis in 2012, and it is associated with 35,000 deaths annually [1]. HAV has a worldwide distribution and it is transmitted through fecal-oral contamination. In high endemicity countries, usually low-resource areas with poor sanitation and hygiene conditions, infection occurs in early childhood and is mainly associated with little clinical impact. On the contrary, in low and intermediate endemicity countries, there is a lower circulation of the virus and, thus, a higher proportion of susceptible persons. When an outbreak occurs, it can cause extended person-to-person transmission, with a higher incidence of symptomatic cases [1,2].
The development of an effective vaccine in the 1990 ́s and subsequent massive immunization has modified the incidence of severe or fulminant HAV in vaccinated and unvaccinated similar age groups. This suggests herd immunity, but it also associates with rising susceptibility in the long term due to the lower circulation of the virus [3]. In the year 2005, HAV vaccine was added as a single-dose vaccine to the mandatory child immunization schedule in Argentina, significantly changing its epidemiology. Before introducing universal HAV vaccination, fulminant presentation of HAV was the leading cause of liver transplantation in children [4]. Ten years after this massive immunization strategy, no further cases of fulminant hepatitis due to HAV were reported in the country [5,6].
However, new cases of autochthonous HAV have been reported in Argentina since 2017. Thus, we designed this study to evaluate the demographic and clinical characteristics of these patients and their possible transmission routes to improve current preventive strategies.
Materials and methodsStudy population and designWe performed a cross-sectional study of patients with HAV. To obtain the most representative sample of Argentina, we invited all members of the Argentinian Association for the Study of Liver Diseases that endorsed this study.
Patients diagnosed with acute HAV between 06.30.2017 and 12.31.2018 were included in the study. Hepatitis A diagnosis was done with a positive anti-HAV IgM test (commercially available third-generation immunoassays) in patients with acute hepatitis syndrome, considered with an increase in aminotransferases of at least five times the upper limit of normal value [7]. The study protocol has an Institutional Review Board and Ethics Committee revision and approval. No informed consent was required since only anonymized data previously registered in clinical charts was used. This research was conducted in accordance with the Declaration of Helsinki.
Demographic and clinical dataWe registered patients' age, gender, and current residence at the time of diagnosis. Regarding possible routes of HAV transmission, we considered the following: household contact with a person with hepatitis A, traveling abroad in the three months before diagnosis, and sexual practices.
Biochemical dataWe registered the following data: alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (expressed as the number of times the upper limit of normal value) and total bilirubin values at the onset of the symptomatic period, as well as the highest values observed during follow-up. We also recorded the lowest percentage of prothrombin time observed. Other serologies signaling present or past infection by hepatitis B (anti-HBs, total anti-HB core, IgM anti-HBcore), hepatitis C (anti-HCV), human immunodeficiency virus (HIV test) and syphilis (VDRL) were registered.
Clinical presentation and outcome dataWe registered the type of presenting symptoms at disease onset, such as asthenia, pruritus, abdominal or generalized muscular pain, arthralgias, jaundice, hyporexia, and/or fever. Additionally, we registered the presence of any extrahepatic manifestations, such as Guillain Barre syndrome, pneumonitis, pancreatitis, and glomerulonephritis.
Regarding clinical disease variants, we registered the occurrence of a biphasic course (defined as the onset of a new episode of jaundice and transaminase elevation after a period of clinical and biochemical remission of hepatitis A, after excluding other causes of acute hepatitis) and/or a cholestatic course of the disease (defined as hepatitis A episodes with a predominant elevation of alkaline phosphatase, jaundice, and pruritus).
Regarding disease severity, the following information was registered: hospitalization due to hepatitis A, severe hepatitis (defined as a patient with hepatitis A diagnosis with the development of coagulopathy: INR less than 1.5 or prothrombin time expressed in activity percentage less than 50%) fulminant hepatitis (defined as severe hepatitis A with the development of encephalopathy) [8]. For this study, when a patient had a clinical course without severe/fulminant hepatitis evolution, it was considered mild hepatitis. The following options were considered when examining the disease outcome: resolved without complications, liver transplantation, or death (Fig. 1).
Flow chart. Hepatitis A patient’s outcome during follow-up.
Note: Mild presentation: clinical course without severe/fulminant hepatitis evolution; Severe presentation: Hepatitis A with coagulopathy (INR less than 1.5 or prothrombin time expressed in activity percentage less than 50%); Fulminant presentation: severe hepatitis A with encephalopathy.
The results are presented as percentages, medians, and interquartile ranges for non-normally distributed data.
ResultsDemographic dataDuring the study period, 66 cases of hepatitis A were registered. In 56 cases (84.8%) the patients were males, with a mean age of 34 ± 12 years old. Regarding their location, most patients (41 patients; 62%) resided in the country's capital city, Ciudad Autónoma de Buenos Aires. The remaining patients were distributed in urban areas across the country: 14 patients in the central region (8 patients in Córdoba and 6 patients in Santa Fe), 3 patients in the north of the country (2 patients in Misiones and 1 patient in La Rioja) and 3 patients in the south region of the country (2 patients in Tierra del Fuego and 1 patient in Neuquén).
The most likely routes of transmission were sexual intercourse of men with men, reported by 31 patients (59% of 52 patients with available data). All described transmission routes are detailed in Table 1.
Characteristics of patients with hepatitis A in Argentina during the study period (n = 66).
| Characteristics | All (n = 66) |
|---|---|
| Possible transmission route | |
| Sexual practices (MSM) - available in 52 patients | 60% (n = 31) |
| Unprotected sexual intercourse - available in 51 patients | 45% (n = 23) |
| Household contact - available in 53 patients | 17% (n = 9) |
| Recent travel abroad - available in 58 patients | 19% (n = 11) |
| More than one reported transmission route | 35% (n = 23) |
| None reported | 15% (n = 10) |
| Accompanying symptoms | |
| Jaundice | 85% (n = 56) |
| Asthenia | 76% (n = 50) |
| Fever | 42% (n = 28) |
| Anorexia | 42% (n = 28) |
| Abdominal pain | 34% (n = 22) |
| Arthralgias and/or myalgias | 32% (n = 21) |
| Two accompanying symptoms at presentation | 95% (n = 63) |
| Three or more accompanying symptoms at presentation | 86% (n = 57) |
| Laboratory findings | |
| Higher total bilirubin (mg/dl; median - IQR 25−75%) | 8 (6−10.4) |
| Higher alkaline phosphatase (times ULN; median-IQR 25−75%) | 1,4 (0.9−2.5) |
| Higher aspartate aminotransferase (times ULN; median-IQR 25−75%) | 24 (4−59) |
| Higher alanine aminotransferase (times ULN; median- IQR 25−75%) | 55 (17−94) |
| Lower values of prothrombin activity (%; median-IQR 25−75%) | 69 (51−81) |
MSM: men who have sex with men, ULN: upper limit of normal.
Concomitant with HAV diagnosis, 23% of patients had a seropositive test for HIV (n = 14 patients, testing available in 61 patients), 26% patients had a positive test for syphilis (n = 13 patients, testing available in 49 patients), and 2% of patients had a positive test for IgM anti-HB core and anti-HCV respectively (1 patient in each group). All concomitantly detected sexually transmitted diseases are detailed in Table 1. When considering associated risk factors, these STD were detected predominantly in MSM. Only 2 patients did not declare having unprotected sex with men as a risk factor: one patient co-infected with syphilis and HIV mentioned unsafe heterosexual practices as a risk factor, and in the other case, no risk factor was declared. The median values and interquartile ratios of bilirubin, transaminases, alkaline phosphatase, and prothrombin time expressed in activity percentage are detailed in Table 2.
Sexually transmitted diseases concomitantly diagnosed with acute Hepatitis A.
| Positive tests for STD at the time of acute HAV diagnosis | Positive cases/ total number tested |
|---|---|
| HIV positive | n = 8 /61 tested |
| HIV positive + VDRL positive | n = 6/49 tested |
| VDRL positive | n = 7/49 tested |
| Anti-HB core IgM positive | n = 1/59 tested |
| Anti-HCV positive | n = 1/63 tested |
Jaundice was the main clinical sign at onset in 56 patients (85%). Other less frequently described symptoms are detailed in Table 1. The majority of patients (95%) reported at least 2 accompanying symptoms. Regarding the course of the disease, 12 patients (18%) had a cholestatic presentation and 6 patients (9%) a biphasic course. Twenty-three patients (35%) required to be admitted for the management of accompanying symptoms. Patients' clinical evolution (mild, severe, and fulminant presentation) and outcome (uneventful resolution, liver transplantation, death) are depicted in Fig. 1. Notably, 14 patients (21%) had a severe/fulminant presentation, and 2 patients (3%) underwent liver transplantation or died.
DiscussionOur study's main finding is the high number of identified cases of hepatitis A in the years 2017–2018 in Argentina compared to prior years since the introduction of mandatory immunization in infants [9]. These observed cases had different epidemiological features compared to the past: affected patients were predominantly young male adults, the main reported transmission route was likely to be through sexual contact (men who have sex with men), with an elevated rate of co-infection with other sexually transmitted diseases. Finally, the clinical impact was notorious: one-third of affected patients required hospital admission to manage accompanying symptoms, whereas 18% had a severe presentation and 3% a fulminant course. These were the first cases with an ominous course after the introduction of the mandatory childhood single-dose immunization for hepatitis A at a 12-month age in 2005 in Argentina.
The risk of severity due to hepatitis A is related to the age of the infected host: young children often have an oligosymptomatic presentation, whereas this infection in adolescence or adulthood usually has a more severe presentation [1,10]. In our study, patients had a mean age of 34 years old, and the majority presented with jaundice and asthenia as predominant symptoms. We observed an elevated rate of clinical variants: 27% of patients had either a cholestatic or biphasic course of the disease.
The fact that young adults were affected by hepatitis A correlates with the epidemiologic transition observed in our country. The incidence of hepatitis A has decreased significantly in the last decade in Argentina [11]. In the year 2005, 50% of the population had anti-hepatitis A positivity at the age of 5–15 years old, whereas in 2015, the presumed age to reach this seroprevalence escalated to 15–35 years old [1]. This correlates with a recent publication that found a seroprevalence of 46% and 76% in 26–35 years old patients of middle-high income and low income respectively in a central region in Argentina [12]. Thus, a significant proportion of the young adult population is currently not immune and should be considered for mandatory immunization.
When analyzing the Argentinian Ministry of Health report regarding acute hepatitis A diagnosis during the last decade, there was a median of 24 cases per year during this period, with three small identified peaks (ranging from 29 to 57 cases) affecting a single city or bordering cities in the years 2009, 2014 and 2015. In comparison, during the years 2017–2018, there were 126 cases with a broader distribution throughout the country's central region, affecting males in 66% of cases with a median age of 30 years old [9]. These new autochthonous cases of HAV in Argentina can be defined as an outbreak since they fit the Center for Disease Control and Prevention (CDC) definition as a sudden increase in the number of cases of a disease above what is normally expected in a limited geographic area [13]. To be noted, we describe the clinical and epidemiological characteristics of the HAV cases treated by hepatologists who chose to participate, representing one-half of the total cases of HAV detected in the country during this time.
We found a person-to-person spread as the main apparent transmission route of the HAV infection. Sexual transmission, particularly in men who have sex with men, seemed to predominate. This change in transmission route has been reported extensively in other intermediate or low-endemicity geographies in the last years [14–16]. In recent publications regarding outbreaks in several European countries, Canada and US [17–19], the affected population resembled ours in the proportion of males, median age, men who have sex with men transmission, and elevated concomitant detection of sexually transmitted diseases. These similar findings stress the role of person-to-person direct transmission in high-risk groups, where unsafe sexual practices seem to be the common finding [20]. Furthermore, based on the epidemiology of recent HAV outbreaks, the CDC recommends vaccinating against HAV to the following groups: people who use drugs, people with an unstable housing situation, men who have sex with men, people who are currently o recently incarcerated and those with chronic liver disease (even without pre-vaccination serologic testing)19].
We found a surprisingly elevated rate of HIV and syphilis co-infection in our study cohort. The seroprevalence of HAV infection tends to be higher among HIV positive patients compared to those without HIV (15.1%–96.3%) with broad geographical variations. Higher seroprevalence of HAV in the HIV population has been reported in countries with low HAV endemicity and has been associated with oral-anal sex, number of sexual partners, older age, and injection drug use [21,22]. Syphilis has also been linked to HIV and HAV infection. In a large Taiwanese cohort study during a HAV outbreak in HIV positive patients, a recent syphilis infection was found to be associated with hepatitis A infection [23]. Similarly, in an analysis of a recent HAV outbreak in France, at least one concomitant sexually transmitted disease (syphilis, chlamydia, and/or gonococcal infection) was diagnosed in 54% of affected patients [24]. All these reports highlight the importance of testing for sexually transmitted diseases when diagnosing hepatitis A, to timely treat these conditions.
ConclusionOur study shows that young adults were predominantly affected by this HAV outbreak in Argentina during 2017–2018, particularly men who have sex with men. An elevated proportion of these patients were concomitantly diagnosed with sexually transmitted diseases. The associated significant public health impact, depicted by the high rate of hospitalization and new cases of fulminant hepatitis, suggests strategies should be undertaken to prevent further outbreaks, such as high-risk group immunization and sexual education.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors have no conflict of interest to declare.