Failures at any step in the hepatocellular carcinoma (HCC) surveillance process can result in HCC diagnostic delays and associated worse prognosis. We aimed to estimate the prevalence of surveillance failure and its associated risk factors in patients with HCC in Argentina, considering three steps: 1) recognition of at-risk patients, 2) implementation of HCC surveillance, 3) success of HCC surveillance.
MethodsWe performed a multi-center cross-sectional study of patients at-risk for HCC in Argentina seen between10.01.2018 and 10.30.2019. Multivariable logistic regression analysis was used to identify correlates of surveillance failure.
ResultsOf 301 included patients, the majority were male (74.8%) with a mean age of 64 years old. At the time of HCC diagnosis, 75 (25%) patients were unaware of their diagnosis of chronic liver disease, and only 130 (43%) patients were under HCC surveillance. Receipt of HCC surveillance was significantly associated with follow-up by a hepatologist. Of 119 patients with complete surveillance, surveillance failure occurred in 30 (25%) patients. Surveillance failure was significantly associated with alpha fetoprotein ≥20 ng/mL (OR 4.0, CI 95% 1.43−11.55).
ConclusionsHCC surveillance failure was frequent in all the evaluated steps. These data should help guide strategies to improve the implementation and results of HCC surveillance in our country.
Hepatocellular carcinoma (HCC) is the most prevalent primary liver cancer, currently representing the fourth cancer-related cause of death worldwide [1]. Approximately 90% of HCC cases arise in patients with pre-existing liver disease, such as chronic hepatitis B or cirrhosis from any etiology [2]. Liver transplantation rates due to HCC have increased significantly worldwide including Argentina, highlighting the growing burden of this disease [1,3].
The prognosis of HCC depends on its stage at diagnosis. When diagnosed at early stages, curative treatments such as resection, ablation, or liver transplantation are feasible with excellent survival rates [4]. HCC surveillance with an ultrasonography every six months with or without alpha fetoprotein measurements in at-risk patients has proven to be a cost-effective and critical intervention to reduce the mortality of HCC [5,6].
However, several studies have shown HCC surveillance is underused in clinical practice. Although seemingly simple, the HCC surveillance process includes several steps. The first is risk assessment, which includes the timely identification of at-risk patients who would benefit from inclusion in an HCC surveillance program [7]. The second step is the initiation and maintenance of HCC screening, with the aforementioned recommended tools and intervals. Finally, ultrasound must detect HCC at an early stage and a timely diagnostic evaluation should be performed [2,7]. Failure in any of these steps, such as under-recognition of at-risk individuals, underuse of HCC screening, or suboptimal screening test performance are responsible for delays in HCC diagnosis and worse prognosis of these patients [7].
Currently, there are scarce data regarding the frequency of failure in each step of HCC surveillance and its associated risk factors in Latin America, thus hindering the development of strategies to improve access and outcomes of HCC surveillance.
Therefore, we aimed to estimate the prevalence of surveillance failure and associated factors in a multi-center cohort of patients with HCC in Argentina.
2Patients and methods2.1Study population and designWe performed a multi-center cross-sectional study of consecutive patients in Argentina with a diagnosis of HCC between January 10th, 2018 and October 30th, 2019. All Units of Gastroenterology and/or Hepatology of Argentina were invited to participate under the endorsement of the Sociedad Argentina de Hepatología (S.A.H.E).
Participants were included if they had a diagnosis of HCC and fulfilled the definition of at-risk patients for HCC, i.e. those with clinical, imaging-based, or histological diagnosis of advanced fibrosis or cirrhosis, as well as patients with chronic hepatitis B infection with a Page-B score ≥10 points [8]. This population at-risk was defined according to the Argentinian guidelines for diagnosis and treatment of HCC [9]. Patients were excluded if information regarding HCC surveillance (dates and type of studies performed or study results) could not be obtained.
The diagnosis of HCC was determined by non-invasive criteria based on imaging findings on dynamic-MR and/or dynamic-CT consistent with LIRADS-5 [10]; or if compatible histological findings were met. Patients were suitable for inclusion in any stage of follow-up and treatment of HCC.
Data were collected from interrogation and complementary studies in a single visit with the patient after informed consent was obtained, using an ad hoc registration form with standardized options. Demographic, clinical, social determinants, variables related to the type of medical care, and variables related to HCC surveillance and diagnosis were collected. The study protocol underwent an Institutional Review Board and Ethics Committee revision and approval. Participants were required to sign informed consent prior to their enrollment. This research was conducted in accordance with the Declaration of Helsinki.
2.2Study outcome and variable definitions2.2.1OutcomesThe main outcome was to estimate the prevalence of HCC surveillance failure and associated factors in at-risk patients from Argentina. The process of surveillance was analyzed in three steps:
- 1
Recognition of at-risk patients for HCC, as defined above [7].
- 2
Implementation of HCC surveillance, defined as completion of liver ultrasound or dynamic imaging with HCC screening purposes with or without alpha-fetoprotein every 6 ± 2 months. It was defined as incomplete when patients were under HCC surveillance but the interval between the last image and HCC diagnosis was longer than 8 months [11].
- 3
Success of HCC surveillance, defined as of early stage HCC detection (i.e. within Milan criteria) among patients who completed surveillance [12]. Conversely, HCC surveillance failure was defined when patients who completed HCC surveillance but were diagnosed with HCC beyond Milan criteria [13].
- -
Advanced fibrosis or cirrhosis: clinical, imagen-based, or histological diagnosis.
- -
Decompensated cirrhosis: patients with a diagnosis of cirrhosis that developed ascites, encephalopathy, variceal bleeding, or jaundice before inclusion.
- -
Performance status: as defined by the Eastern Cooperative Oncology Group [14].
- -
Residence at the time of diagnosis: the city and province of residence at diagnosis was registered. Patients were categorized according to their residence in the city of Buenos Aires. This is the country's capital and largest city, it has the largest concentration of third-level hospitals and hepatology centers in Argentina. Additionally, patients were grouped according to their region within the country as follows: Center region (Buenos Aires, Córdoba, Entre Ríos, Santa Fe, and the country's capital city, Ciudad Autónoma de Buenos Aires), Cuyo region (La Rioja, Mendoza, San Juan y San Luis), North East region (Chaco, Corrientes, Formosa and Misiones), North West region (Catamarca, Jujuy, Tucumán, Salta and Santiago del Estero) and Patagonia region (Chubut, La Pampa, Neuquén, Río Negro, Santa Cruz and Tierra del Fuego).
- -
Employment status: patients were considered as actively working if they had a stable job (employed or self-employed) without depending on welfare or other benefits for living expenses in the last 6 months prior to HCC diagnosis.
- -
Housing status: patients were considered to have cohabitants when they declared to live with another independent adult (whether related or not).
- -
Milan criteria: patients were considered inside Milan when they had a single tumor ≤5 cm in diameter or no more than three tumors ≤3 cm in diameter, without vascular invasion or distant metastasis [15].
- -
BCLC score: categorized according to liver function, tumor burden, and performance status in 5 stages from very early HCC (BCLC stage 0) to terminal stage (BCLC D) [16].
Sampling was consecutive. We estimated the sample size for two of the objectives.
We estimated the sample size required to evaluate the objective of identifying variables associated with HCC diagnosis without prior surveillance. For this purpose, we did a two-step approach. First, we calculated the sample size required to obtain a precise estimate for prevalence of this outcome. According to the literature, approximately 47% of the HCC are diagnosed in patients without surveillance [5]. To define a width of 12% (6% above and 6% below the point estimate), for the 95% confidence interval, a total of 266 patients were required. We then estimated the sample size for the objective of identifying variables associated with HCC surveillance failure, adopting the same approach. According to the literature, surveillance failure is present in approximately 20–28% of patients under complete surveillance [11,12]. Considering that only one half of patients will receive complete surveillance, a surveillance failure rate of 18% and defining a width of 12.4% (6.2% above and 6.2% below the point estimate), for the 95% confidence interval, a total of 296 patients should be included.
2.4Statistical analysisResults are presented as absolute numbers and percentages for categorical data, mean and standard deviation for normally distributed data, and median and interquartile ranges (IQR) for non-normally distributed data. We reported the prevalence with 95% confidence intervals of a) patients without prior recognition of chronic liver disease, b) surveillance implementation in at-risk patients and, c) HCC surveillance failure. To compare the characteristics between groups, we used t-test, Mann-Whitney, Chi-square or Fisher exact, depending on characteristics of the variables and underlying test assumptions. To identify factors associated with surveillance implementation we compared patients who underwent complete surveillance and those without any surveillance prior to HCC diagnosis; those with incomplete surveillance were excluded from this analysis due to heterogeneity of surveillance intervals. To identify variables associated with surveillance failure, we compared patients who had successful surveillance and those with surveillance failure. To identify variables associated with both outcomes, we performed multivariable logistic regression analyses, considering clinically important variables according to prior studies as well as those found to be significantly imbalanced in bivariate analyses [17]. We reported odds ratios with their 95% confidence intervals and considered a significance level of 5% to be statistically significant. All tests were two-tailed. The software used in this study was STATA, (StataCorp version 13).
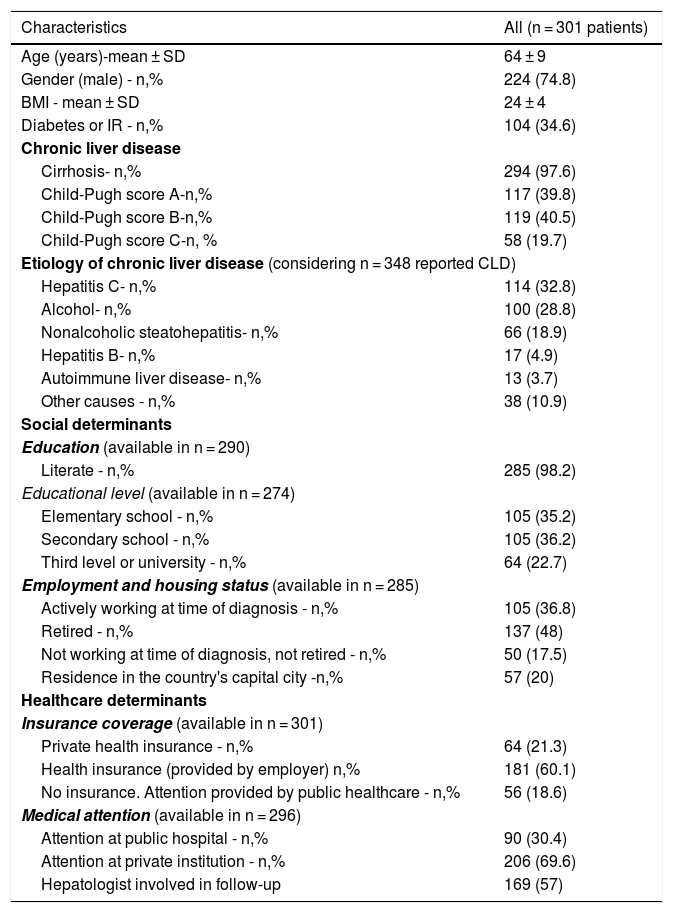
3Results3.1Demographic characteristicsOf 301 patients from 28 liver units in Argentina, the majority (74.8%) was male with a mean age of 64 years old. Nearly all (n = 294, 97.6%) patients had cirrhosis, with only 5 patients (1.7%) having advanced fibrosis and 2 (0.7%) hepatitis B with a PAGE-B score ≥10 points. Liver disease etiologies are detailed in Table 1. Most (n = 249, 82.7%) patients had one recognized etiology and 52 (17.3%) had two or more etiologies. Hepatitis C was the most common etiology (n = 114, 32.8%), of whom only 43 (37.7%) had achieved sustained virological response. Alcohol-related liver disease was reported in 100 (28.8%) patients, of whom 67 patients (67%) had more than 6 months of abstinence at the moment of HCC diagnosis. Only 17 (4.9%) patients had hepatitis B, of whom 12 (70.5%) were receiving antiviral treatment at inclusion. Mean MELD-Na was 14 ± 6 points and mean Child-Pugh score was 7 ± 2 points.
Main characteristics of at-risk patients with HCC surveillance indication (n = 301).
| Characteristics | All (n = 301 patients) |
|---|---|
| Age (years)-mean ± SD | 64 ± 9 |
| Gender (male) - n,% | 224 (74.8) |
| BMI - mean ± SD | 24 ± 4 |
| Diabetes or IR - n,% | 104 (34.6) |
| Chronic liver disease | |
| Cirrhosis- n,% | 294 (97.6) |
| Child-Pugh score A-n,% | 117 (39.8) |
| Child-Pugh score B-n,% | 119 (40.5) |
| Child-Pugh score C-n, % | 58 (19.7) |
| Etiology of chronic liver disease (considering n = 348 reported CLD) | |
| Hepatitis C- n,% | 114 (32.8) |
| Alcohol- n,% | 100 (28.8) |
| Nonalcoholic steatohepatitis- n,% | 66 (18.9) |
| Hepatitis B- n,% | 17 (4.9) |
| Autoimmune liver disease- n,% | 13 (3.7) |
| Other causes - n,% | 38 (10.9) |
| Social determinants | |
| Education (available in n = 290) | |
| Literate - n,% | 285 (98.2) |
| Educational level (available in n = 274) | |
| Elementary school - n,% | 105 (35.2) |
| Secondary school - n,% | 105 (36.2) |
| Third level or university - n,% | 64 (22.7) |
| Employment and housing status (available in n = 285) | |
| Actively working at time of diagnosis - n,% | 105 (36.8) |
| Retired - n,% | 137 (48) |
| Not working at time of diagnosis, not retired - n,% | 50 (17.5) |
| Residence in the country's capital city -n,% | 57 (20) |
| Healthcare determinants | |
| Insurance coverage (available in n = 301) | |
| Private health insurance - n,% | 64 (21.3) |
| Health insurance (provided by employer) n,% | 181 (60.1) |
| No insurance. Attention provided by public healthcare - n,% | 56 (18.6) |
| Medical attention (available in n = 296) | |
| Attention at public hospital - n,% | 90 (30.4) |
| Attention at private institution - n,% | 206 (69.6) |
| Hepatologist involved in follow-up | 169 (57) |
Note: ∗: considering only patients with cirrhosis.
Only 57 patients (20%) resided in the city of Buenos Aires. When analyzed by regions, 179 (73.7%) resided in the Center region, 37 patients (15.2%) in the Northwest region, 13 patients (5.3%) in the Northeast region, and 7 patients (2.9%) in the Cuyo and Patagonia regions (data regarding residence was missing in 58 patients). Data regarding literacy was available in 290 patients. The majority (n = 285, 98.2%), were literate, with 58.9% having obtained higher education. When assessing employment status, 48% of patients were retired at the time of HCC diagnosis. The majority of patients (n = 245, 81.4%) had health insurance and 57% were followed by a hepatologist at least in the 6-month period prior to HCC diagnosis (Table 1).
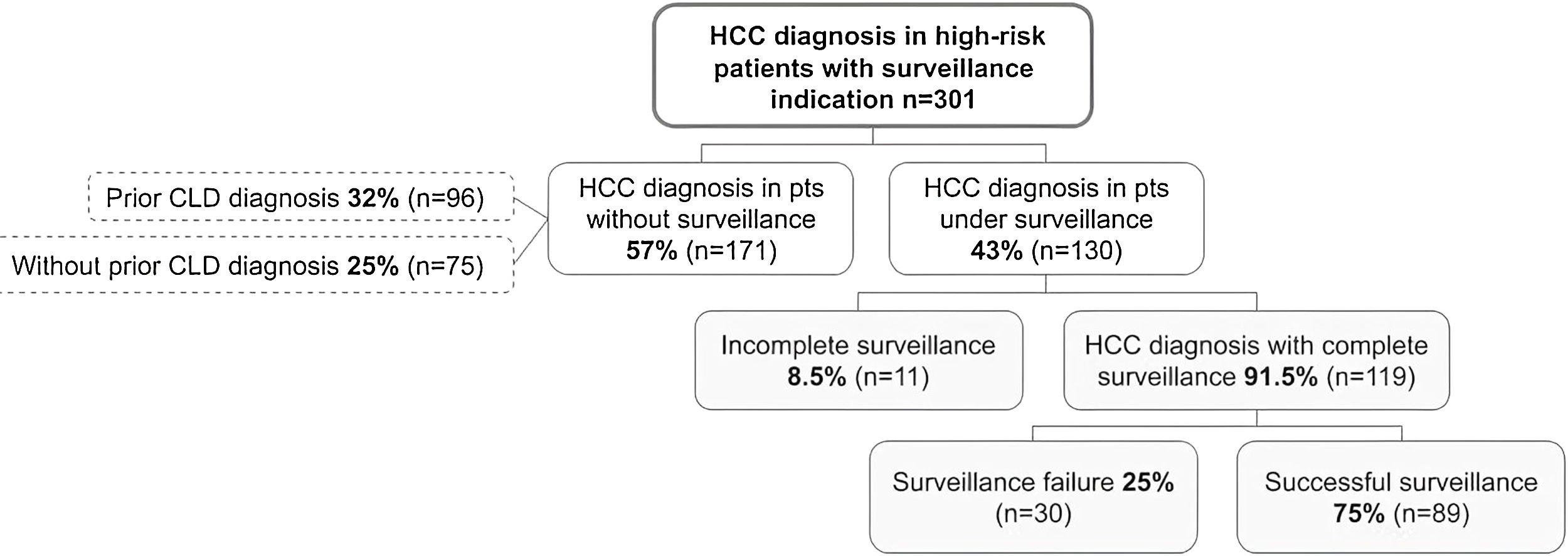
3.3Recognition of at-risk patients for HCCAt HCC diagnosis, 75 (24.9%, 95%CI 20–30%) patients were unaware of their diagnosis of chronic liver disease (Fig. 1). Of these, 72 (96%) patients had cirrhosis, 2 (2.7%) had advanced fibrosis and 1 patient (1.3%) had diagnosis of hepatitis B with indication for HCC surveillance. Regarding the severity of liver disease, 32 (44.4%) patients were classified as Child-Pugh A, 32 (44.4%) as Child-Pugh B and 8 (11.1%) as Child-Pugh C.
3.4Implementation of HCC surveillanceOnly 130 patients were under any HCC surveillance (complete or incomplete) at the time of HCC diagnosis, with a prevalence of 43.2% (95%CI 37–48%). Surveillance with ultrasonography was used in 77 (59.2%) patients, whereas ultrasonography combined with alpha-fetoprotein was used in 46 (35.4%) patients. In only 7 (5.4%) patients CT- or MRI-based surveillance was utilized. Of the 130 patients under surveillance, the majority of patients, (n = 119, 91.5%) received complete HCC surveillance. The remaining 11 patients (8.5%) had incomplete surveillance, with 5 being considered non-adherent by their treating physicians—2 cases due to lack of perceived surveillance importance, 1 case due to forgetting appointments, 1 case due to financial barriers and 1 case due to ongoing drug abuse.
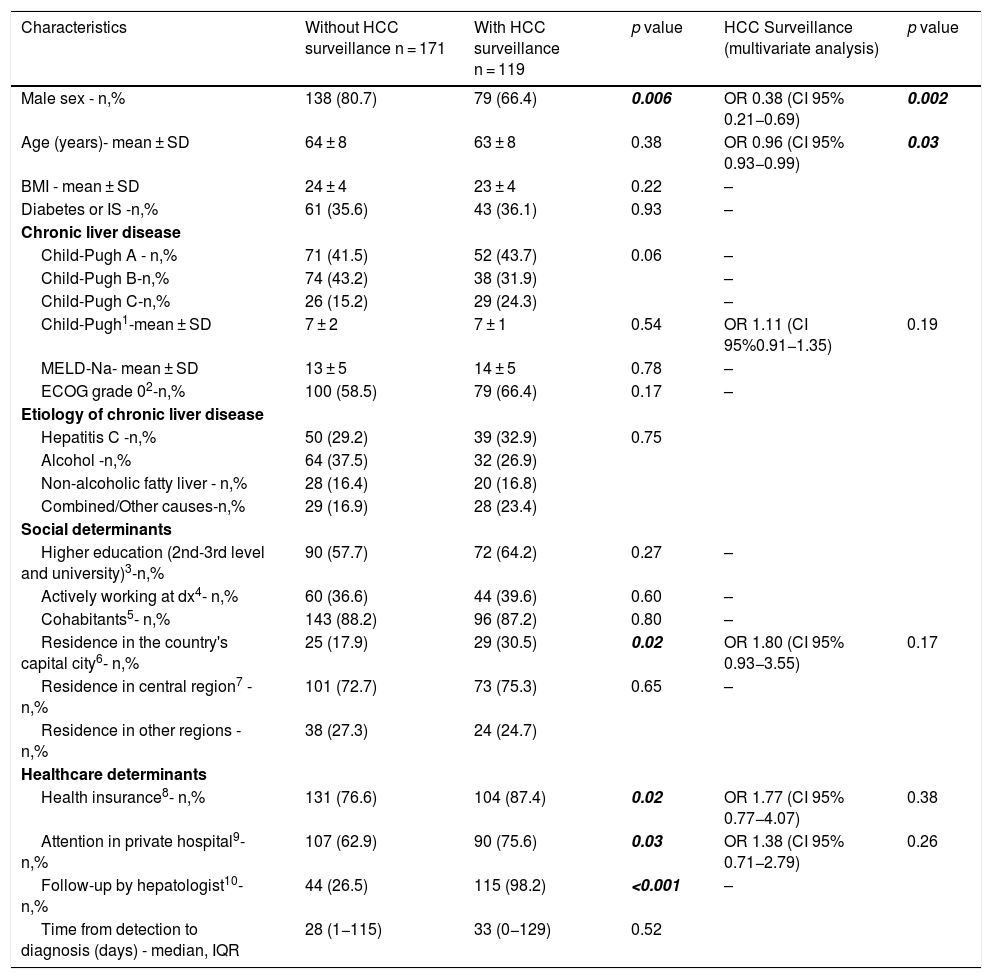
When comparing patients with or without the implementation of HCC surveillance at time of HCC diagnosis (Table 2), males were more likely to be diagnosed outside of surveillance at the time of HCC diagnosis. On the contrary, patients who resided in the city of Buenos Aires, patients with health insurance and those who received medical care in a private hospital were more frequently under surveillance at the time of HCC diagnosis. As shown in Table 2, almost all patients who were followed by a hepatologist were under surveillance at the time of HCC diagnosis, so the crude association between these two variables was very high. This situation might be explained by the fact that HCC surveillance is a highly established practice among hepatologists, and therefore the definition of both variables is similar and might contain similar information. For this reason, follow-up by a hepatologist was not included in the multivariate analyses. In multivariable logistic regression, only male gender and older age were independently associated with lower odds of HCC surveillance implementation at the time of HCC diagnosis.
Factors associated with the implementation of HCC surveillance in patients diagnosed with HCC (n = 290).
| Characteristics | Without HCC surveillance n = 171 | With HCC surveillance n = 119 | p value | HCC Surveillance (multivariate analysis) | p value |
|---|---|---|---|---|---|
| Male sex - n,% | 138 (80.7) | 79 (66.4) | 0.006 | OR 0.38 (CI 95% 0.21−0.69) | 0.002 |
| Age (years)- mean ± SD | 64 ± 8 | 63 ± 8 | 0.38 | OR 0.96 (CI 95% 0.93−0.99) | 0.03 |
| BMI - mean ± SD | 24 ± 4 | 23 ± 4 | 0.22 | – | |
| Diabetes or IS -n,% | 61 (35.6) | 43 (36.1) | 0.93 | – | |
| Chronic liver disease | |||||
| Child-Pugh A - n,% | 71 (41.5) | 52 (43.7) | 0.06 | – | |
| Child-Pugh B-n,% | 74 (43.2) | 38 (31.9) | – | ||
| Child-Pugh C-n,% | 26 (15.2) | 29 (24.3) | – | ||
| Child-Pugh1-mean ± SD | 7 ± 2 | 7 ± 1 | 0.54 | OR 1.11 (CI 95%0.91−1.35) | 0.19 |
| MELD-Na- mean ± SD | 13 ± 5 | 14 ± 5 | 0.78 | – | |
| ECOG grade 02-n,% | 100 (58.5) | 79 (66.4) | 0.17 | – | |
| Etiology of chronic liver disease | |||||
| Hepatitis C -n,% | 50 (29.2) | 39 (32.9) | 0.75 | ||
| Alcohol -n,% | 64 (37.5) | 32 (26.9) | |||
| Non-alcoholic fatty liver - n,% | 28 (16.4) | 20 (16.8) | |||
| Combined/Other causes-n,% | 29 (16.9) | 28 (23.4) | |||
| Social determinants | |||||
| Higher education (2nd-3rd level and university)3-n,% | 90 (57.7) | 72 (64.2) | 0.27 | – | |
| Actively working at dx4- n,% | 60 (36.6) | 44 (39.6) | 0.60 | – | |
| Cohabitants5- n,% | 143 (88.2) | 96 (87.2) | 0.80 | – | |
| Residence in the country's capital city6- n,% | 25 (17.9) | 29 (30.5) | 0.02 | OR 1.80 (CI 95% 0.93−3.55) | 0.17 |
| Residence in central region7 - n,% | 101 (72.7) | 73 (75.3) | 0.65 | – | |
| Residence in other regions -n,% | 38 (27.3) | 24 (24.7) | |||
| Healthcare determinants | |||||
| Health insurance8- n,% | 131 (76.6) | 104 (87.4) | 0.02 | OR 1.77 (CI 95% 0.77−4.07) | 0.38 |
| Attention in private hospital9- n,% | 107 (62.9) | 90 (75.6) | 0.03 | OR 1.38 (CI 95% 0.71−2.79) | 0.26 |
| Follow-up by hepatologist10-n,% | 44 (26.5) | 115 (98.2) | <0.001 | – | |
| Time from detection to diagnosis (days) - median, IQR | 28 (1−115) | 33 (0−129) | 0.52 | ||
Note: 1 (available in n = 272); 2 (available in n = 289); 3 (available in = 267); 4 (available in n = 275); 5 (available in n = 272); 6 (available in n = 236); 7 (available in n = 236); 8 (available in n = 289); 9 (available in n = 289); 10 (available in n = 283).
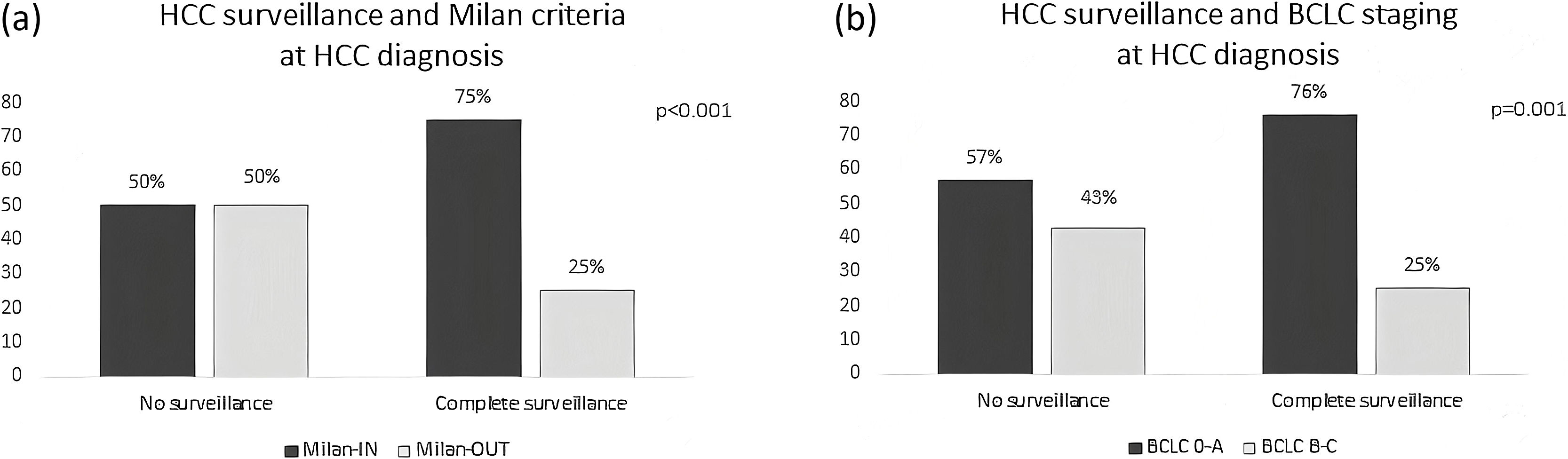
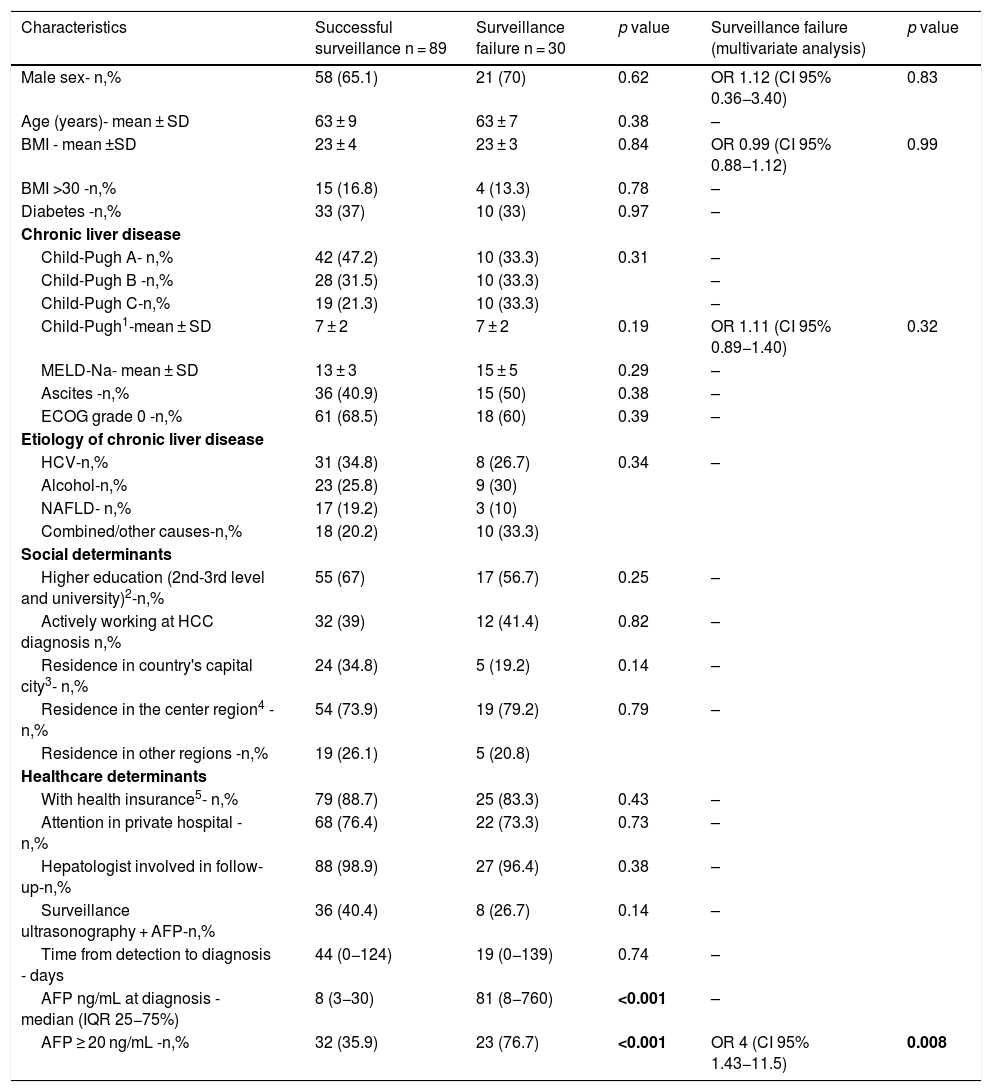
Of the 301 included patients, 194 (64.4%) were detected at a very early or early stage (BCLC stages 0-A) and 180 patients (59.8%) detected within Milan criteria at diagnosis. Among the 119 patients who had complete HCC surveillance, surveillance failure occurred in 30 (25.2%, 95%CI 18–33%) patients (Fig. 1). Patients under complete surveillance were significantly more likely to be diagnosed within Milan criteria than those without surveillance (75% vs. 50%, p < 0.001), (Fig. 2A). Similarly, patients under complete surveillance were significantly more likely to be diagnosed in BCLC 0-A stages than those without surveillance (76% vs. 57%, p = 0.001) (Fig. 2B). In multivariable analysis, only AFP ≥ 20 ng/mL at HCC diagnosis was significantly higher in patients with surveillance failure when compared to those with successful surveillance (Table 3).
Factors associated with surveillance failure in patients with HCC diagnosis (n = 119).
| Characteristics | Successful surveillance n = 89 | Surveillance failure n = 30 | p value | Surveillance failure (multivariate analysis) | p value |
|---|---|---|---|---|---|
| Male sex- n,% | 58 (65.1) | 21 (70) | 0.62 | OR 1.12 (CI 95% 0.36−3.40) | 0.83 |
| Age (years)- mean ± SD | 63 ± 9 | 63 ± 7 | 0.38 | – | |
| BMI - mean ±SD | 23 ± 4 | 23 ± 3 | 0.84 | OR 0.99 (CI 95% 0.88−1.12) | 0.99 |
| BMI >30 -n,% | 15 (16.8) | 4 (13.3) | 0.78 | – | |
| Diabetes -n,% | 33 (37) | 10 (33) | 0.97 | – | |
| Chronic liver disease | |||||
| Child-Pugh A- n,% | 42 (47.2) | 10 (33.3) | 0.31 | – | |
| Child-Pugh B -n,% | 28 (31.5) | 10 (33.3) | – | ||
| Child-Pugh C-n,% | 19 (21.3) | 10 (33.3) | – | ||
| Child-Pugh1-mean ± SD | 7 ± 2 | 7 ± 2 | 0.19 | OR 1.11 (CI 95% 0.89−1.40) | 0.32 |
| MELD-Na- mean ± SD | 13 ± 3 | 15 ± 5 | 0.29 | – | |
| Ascites -n,% | 36 (40.9) | 15 (50) | 0.38 | – | |
| ECOG grade 0 -n,% | 61 (68.5) | 18 (60) | 0.39 | – | |
| Etiology of chronic liver disease | |||||
| HCV-n,% | 31 (34.8) | 8 (26.7) | 0.34 | – | |
| Alcohol-n,% | 23 (25.8) | 9 (30) | |||
| NAFLD- n,% | 17 (19.2) | 3 (10) | |||
| Combined/other causes-n,% | 18 (20.2) | 10 (33.3) | |||
| Social determinants | |||||
| Higher education (2nd-3rd level and university)2-n,% | 55 (67) | 17 (56.7) | 0.25 | – | |
| Actively working at HCC diagnosis n,% | 32 (39) | 12 (41.4) | 0.82 | – | |
| Residence in country's capital city3- n,% | 24 (34.8) | 5 (19.2) | 0.14 | – | |
| Residence in the center region4 -n,% | 54 (73.9) | 19 (79.2) | 0.79 | – | |
| Residence in other regions -n,% | 19 (26.1) | 5 (20.8) | |||
| Healthcare determinants | |||||
| With health insurance5- n,% | 79 (88.7) | 25 (83.3) | 0.43 | – | |
| Attention in private hospital - n,% | 68 (76.4) | 22 (73.3) | 0.73 | – | |
| Hepatologist involved in follow-up-n,% | 88 (98.9) | 27 (96.4) | 0.38 | – | |
| Surveillance ultrasonography + AFP-n,% | 36 (40.4) | 8 (26.7) | 0.14 | – | |
| Time from detection to diagnosis - days | 44 (0−124) | 19 (0−139) | 0.74 | – | |
| AFP ng/mL at diagnosis - median (IQR 25−75%) | 8 (3−30) | 81 (8−760) | <0.001 | – | |
| AFP ≥ 20 ng/mL -n,% | 32 (35.9) | 23 (76.7) | <0.001 | OR 4 (CI 95% 1.43−11.5) | 0.008 |
Note: 1 (available in n = 113); 2 (available in n = 118); 3 (available in n = 108); 4 (available in n = 97); 5 (available in n = 118).
This multi-center cohort study analyzed the pitfalls of HCC surveillance in Argentina throughout the entire process: risk assessment, implementation of surveillance, and performance of surveillance to detect HCC at an early stage. The main findings of this study are the following: a) 25% of patients were unaware of their diagnosis of advanced chronic liver disease at the time of HCC diagnosis, b) only 43% of at-risk patients were under surveillance; and c) surveillance failure for early detection occurred in 25% of patients. Follow-up by a hepatologist was the strongest correlate for implementation of HCC surveillance, whereas male sex and increasing age were associated with underuse of HCC surveillance. Surveillance failure was associated with higher AFP values.
The first necessary step for improving HCC surveillance utilization is accurate identification of patients at risk for HCC. Underdiagnosis of cirrhosis and/or chronic liver disease ranged between 23–50% in a large US series [18,19]; however, there is scarce information from Latin America. Very recently, a large retrospective database analysis from Brazil that included patients with HCC and at least one record in the public system from 2011 until 2016 reported that 86% of patients were unaware of having a chronic liver disease prior to HCC diagnosis [20]. In our study cohort, only 25% of patients were diagnosed with HCC and cirrhosis concomitantly. These rates are similar to those reported in US series, and significantly lower than those reported in Brazil, probably due to the methodology for data acquisition.
The implementation of HCC surveillance is globally underused. Two recent meta-analyses including studies with worldwide distribution reported that surveillance for HCC is only implemented in 24–37% of the patients [21,22]. These rates differ by geographic region, with higher surveillance rates reported in South America (only one study included, 47%) and lower rates in Oceania, Africa and North America (29, 30 and 31% respectively) [21]. In our study cohort, 43% of patients were under surveillance at the time of HCC diagnosis, similar to previous studies. Underuse of HCC surveillance has been associated with several patient-related factors, such as increased liver disease severity, black race, alcohol consumption, or alcohol- and non-alcoholic liver disease related cirrhosis [22,23]. We failed to find an association with factors related to the etiology or severity of liver disease. However, we found that older age and male sex were associated with underuse of surveillance. These factors have been scarcely found significant by other authors [24,25]. Lower surveillance rates in older patients may reflect the perception of physicians of lower likelihood to access curative therapy, whereas male patients are reported to be less likely to attend clinic visits and undergo cancer screening [26].
Additionally, different health-care related factors, such as lack of an automated screening process, lack of health insurance, limited time in clinic, or limited time for correct ultrasound performance have been reported as barriers to HCC surveillance implementation [23,27]. Conversely, follow-up by a hepatologist/gastroenterologist is one of the most consistent factors associated with the implementation of HCC surveillance [22]. When US hepatologists and gastroenterologists were surveyed regarding their surveillance practices, 98% endorsed and practiced semi-annual HCC surveillance in patients with cirrhosis [28]. In contrast, when US primary care providers were surveyed, only 45% of them screened patients with cirrhosis for HCC [29]. These reports correlate with HCC surveillance in practice. HCC surveillance implementation was found to vary widely depending on the study setting in an aforementioned meta-analysis, with utilization rates in gastroenterology and hepatology clinics approaching 75% compared to as low as <10% in large population-based cohorts [22]. In our study, follow-up by a hepatologist had a very high correlation with the implementation of HCC surveillance (98%), whereas the implementation of surveillance by other specialties was extremely low (26%), thus mirroring the impact of the treating physician as reported in other countries.
HCC surveillance has been associated with higher rates of detection at early stage (whether considering Milan criteria or BCLC staging) and thus, higher access to curative treatment [30]. Despite the fact that available data regarding the survival benefit of surveillance derives mainly from observation studies [23], semi-annual surveillance is broadly endorsed in at-risk patients since there are no strategies proven to be superior for early HCC diagnosis. In the present cohort, patients under complete HCC surveillance were significantly times more likely to be diagnosed with early HCC whether considering Milan criteria or BCLC staging. Patients with HCC detected at an early stage had received surveillance ultrasound and AFP in 43% of cases, compared to only 28% in patients with surveillance failure. These results are consistent with a meta-analysis including over 15.000 patients, that reported patients under HCC surveillance were nearly two-times more likely to be diagnosed at an early HCC stage [30].
However, ultrasound-based surveillance is also known to be at risk of surveillance failure, with many patients still detected at a late stage. In fact, the sensitivity of ultrasound and AFP for early HCC detection was only 63% in a recent systematic review [31]. Surveillance failure has been reported in 20–38% of at-risk patients [11,12,32]. Severity of underlying disease (Child-Pugh score B–C) and elevated AFP level have been independently associated with surveillance failure in a recent prospective European cohort of HCC diagnosis in at-risk patients [12]. Other variables found to be associated are parenchymal macronodularity [33], diabetes, male gender, and obesity [11,13,34]. In our study cohort, surveillance failure occurred in 25% of patients. This is lower than previously reported rates in Argentina, which may be related mainly to differences used for surveillance failure [32]. We identified AFP level as the only determinant for surveillance failure, with an odds ratio of 4 when considering a 20 ng/mL cut-off – the optimal cut-off point for the screening strategy in prior studies [35].
To our knowledge, this is the first study considering social and healthcare related determinants regarding HCC surveillance implementation and success in Latin America. The main strengths of this study are the broad variety of the study cohort characteristics that reflects the true heterogeneity of the country's population, including patients with different underlying etiologies, disease severity, city and region of residence, educational status, type of insurance, health-care center and provider, and type of surveillance strategy. We analyzed all the steps involved in the HCC surveillance process, from the identification of at-risk patients to the success after the application of bi-annual screening, an approach yet to be described in Latin American cohort.
The main weakness of the present study is related to patient inclusion, since only centers and physicians that chose to participate were included (in comparison to patient inclusion from large databases). Furthermore, only specialists (gastroenterologists and hepatologists) participated in the present study, thus constituting a selection bias that may overestimate the proportion of patients with surveillance implementation. Additionally, patients who lacked complete information regarding their HCC surveillance or diagnosis studies were excluded, thus creating a selection bias.
5ConclusionThe present study illustrates several pitfalls in the HCC surveillance process: 25% of patients are unaware of their risk for HCC, only 43% of at-risk patients are under surveillance at time of HCC diagnosis, and surveillance fails to detect HCC at an early stage in 25% of patients. These failures are concerning given the association between HCC surveillance and early detection, with this strategy being associated with 2.3–3 times more chances to diagnose HCC in early stages. These data highlighting failures in each step of the surveillance process, and its associated factors, should help guide strategies to improve the implementation and results of HCC surveillance in our country.AbbreviationsHCC
hepatocellular carcinoma
AFPAlpha fetoprotein
BCLCBarcelona Clinic Liver Cancer
IQRinterquartile ranges
MELDModel for End Stage Liver Disease
CTcomputed tomography
MRImagnetic resonance imaging
USUnited States
Financial supportThis study was awarded with a scholarship from the Sociedad Argentina de Hepatología (S.A.H.E).
Conflict of interestThe authors have no conflicts of interest to declare.
We thank the valuable participation of the following collaborators: Sebastián Paredes (Hospital de Alta Complejidad Juan D. Perón, Formosa), María del Valle Aubone (Hospital Dr Guillermo Rawson, San Juan), Juan Sordá, Jorge Daruich, Esteban Gonzalez Ballerga (Hospital de Clínicas José de San Martín (UBA), CABA), Natalia Ratusnu (Hospital Regional Gob. Ernesto Campos, Ushuaia, Tierra del Fuego), Antonela Ferrari, Juana Pascual (Hospital Centenario, Rosario, Santa Fe), Jose Martínez (Sanatorio Boratti, Posadas, Misiones), María Elena Pawluk (Consultorio privado, Resistencia, Chaco), Silvina Yantorno (Fundación Favaloro, CABA), Daniela Basso (Gastromed, Río Cuarto, Córdoba), Jesica Soto (Hospital de Clínicas José de San Martín, CABA) Diego Piombino (Hospital de Emergencias Dr Clemente Álvarez, Rosario, Santa Fe), Ana Calabria (Hospital Francisco J Muñiz, CABA), Gabriela Ruffillo, Graciela B. Landeira (Hospital Nacional Prof. Alejandro Posadas, El Palomar), Cecilia Haller (Gastroenterología, Puerto Madryn, Chubut), Yamila Martinez Artola (Sanatorio Julio Mendez, CABA), María Pía Raffa (Sanatorio Sagrado Corazón, CABA).