Malnutrition among patients with chronic liver disease (CLD) is a common complication with significant prognostic implications for patients with liver cirrhosis. Micronutrient deficiency has been associated with an increased risk of hepatic decompensation and is an independent risk factor for mortality among cirrhotic patients. Micronutrient deficiencies in patients with CLD include zinc, vitamin A, vitamin D and selenium. This review article aims to evaluate the literature to date on the complications of zinc deficiency in patients with CLD. A management algorithm for zinc replacement has also been proposed.
Malnutrition is a common complication seen in patients with chronic liver disease (CLD), affecting more than 50% of those with cirrhosis [1]. Malnutrition has significant prognostic implications for patients with cirrhosis, being associated with an increased risk of hepatic decompensation (ascites, spontaneous bacterial peritonitis (SBP), hepatic encephalopathy (HE) and variceal bleeding), and being an independent risk factor for mortality [2,3]. Micronutrient deficiency is commonly seen in patients with CLD [4], and, in recent times there has been a renewed interest in evaluating the clinical effects of zinc deficiency in patient with CLD. The cause zinc of deficiency in patients with CLD appears to multifactorial, with intake, absorption, transport, storage and excretion of zinc all being affected [5]. There has been an increase in the literature evaluating the potential benefits of zinc supplementation in patients with liver cirrhosis, with a particular focus on HE, hepatocellular carcinoma (HCC), and the benefits in improving patients’ health related quality of life. This is the first paper to critically review these studies and to outline recommended regimens for zinc replacement and monitoring.
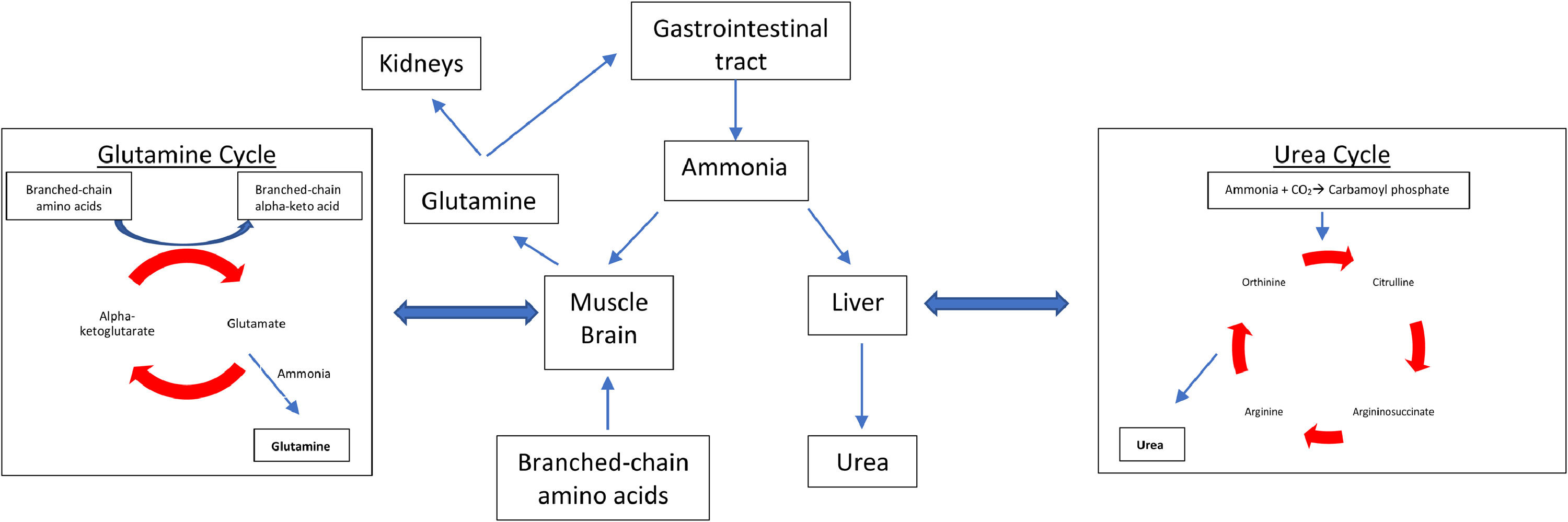
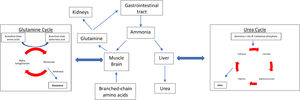
2Role and function of zincZinc is an essential micronutrient which is required for many important bodily functions. It has a critical role in cellular metabolism and exerts antioxidant and anti-inflammatory effects [6]. It also plays a significant role in the determination and regulation of apoptosis [7]. In the normally functioning liver, zinc is used in many enzymatic reactions such as in the activation of ornithine transcarbamylase and glutamate dehydrogenase, in the urea cycle and the glutamine synthetase cycle respectively (Fig. 1) [8]. The antioxidant benefits of zinc have been linked to the enzyme superoxide dismutase, which requires zinc for its activation. Zinc deficiency has been associated with superoxide dismutase inactivity which can in turn lead to an increase in reactive oxygen species [9]. Zinc additionally has crucial anti-inflammatory effects which are thought to be mediated by its role in the clearance of extracellular adenosine triphosphate and subsequent adenosine generation [10]. Other essential enzymes which require zinc include DNA polymerase, RNA polymerase, and alkaline phosphatase, which may account for the effects of zinc on liver function and its potential inhibitory role in cancer pathogenesis [11].
Ammonia metabolism pathways. In patients without chronic liver disease ammonia is metabolised by the liver (urea cycle) and in the skeletal system (glutamine synthetase) in a 1:1 ratio. With chronic liver disease and zinc deficiency, the urea cycle is unable to adequately metabolise ammonia, therefore more ammonia is detoxified in the skeletal system by the glutamine cycle.
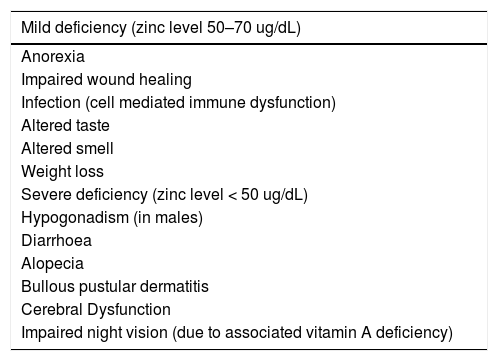
Serum zinc deficiency can lead to a wide variety of clinical manifestations, including gastrointestinal consequences (dysgeusia, anorexia, appetite loss), dermatological effects (alopecia, dermatitis), and other effects including impaired wound healing and hypogonadism in males. Of particular importance to individuals with CLD are impairments in cellular immune function, cellular apoptosis and cerebral function. The correlation between plasma zinc level and clinical symptoms, has been established in the general population, with plasma zinc levels between 50 and 70 ug/dL associated with mild symptoms, and levels less than 50 ug/dL correlating with severe symptoms (Table 1) [12]. Many of these clinical manifestations are well described clinical manifestations in patients with CLD. However, the accuracy of serum zinc measurement in CLD is questionable as inflammatory processes and hypoalbuminemia can artificially lower serum zinc levels. Furthermore, zinc exerts its main physiological functions within the body's cells and only a small amount of the body's total zinc is measurable in serum. However, a number of studies have demonstrated that decreases in hepatic and blood cell levels of zinc do in fact parallel serum levels in various liver diseases [13]. Despite these questions regarding the accuracy of serum zinc measurement in patients with cirrhosis, a reduction in serum albumin and zinc is likely to significantly impair the body's capacity to transport zinc between cells which is a tightly regulated homeostatic process.
Complications of zinc deficiency.
The liver plays an essential role in zinc homeostasis, being the main organ involved in zinc metabolism. Zinc deficiency has been well described in patients with CLD, with papers as early as the 1950’s describing altered zinc homeostasis in these patients [14]. As such, there have been several earlier reviews evaluating nutrient deficiencies in patients with cirrhosis, which have highlighted the importance of zinc supplementation in this cohort [15]. With advancing liver disease, zinc deficiency has been shown to increase and mirror the severity of fibrosis [16]. Zinc deficiency is also seen more frequently in certain aetiologies of CLD including hepatis C, cholestatic liver disease and alcohol related liver disease [17], however, studies to date are yet to conclude whether zinc supplementation leads to improved clinical outcomes in patient's with differing underlying liver diseases.
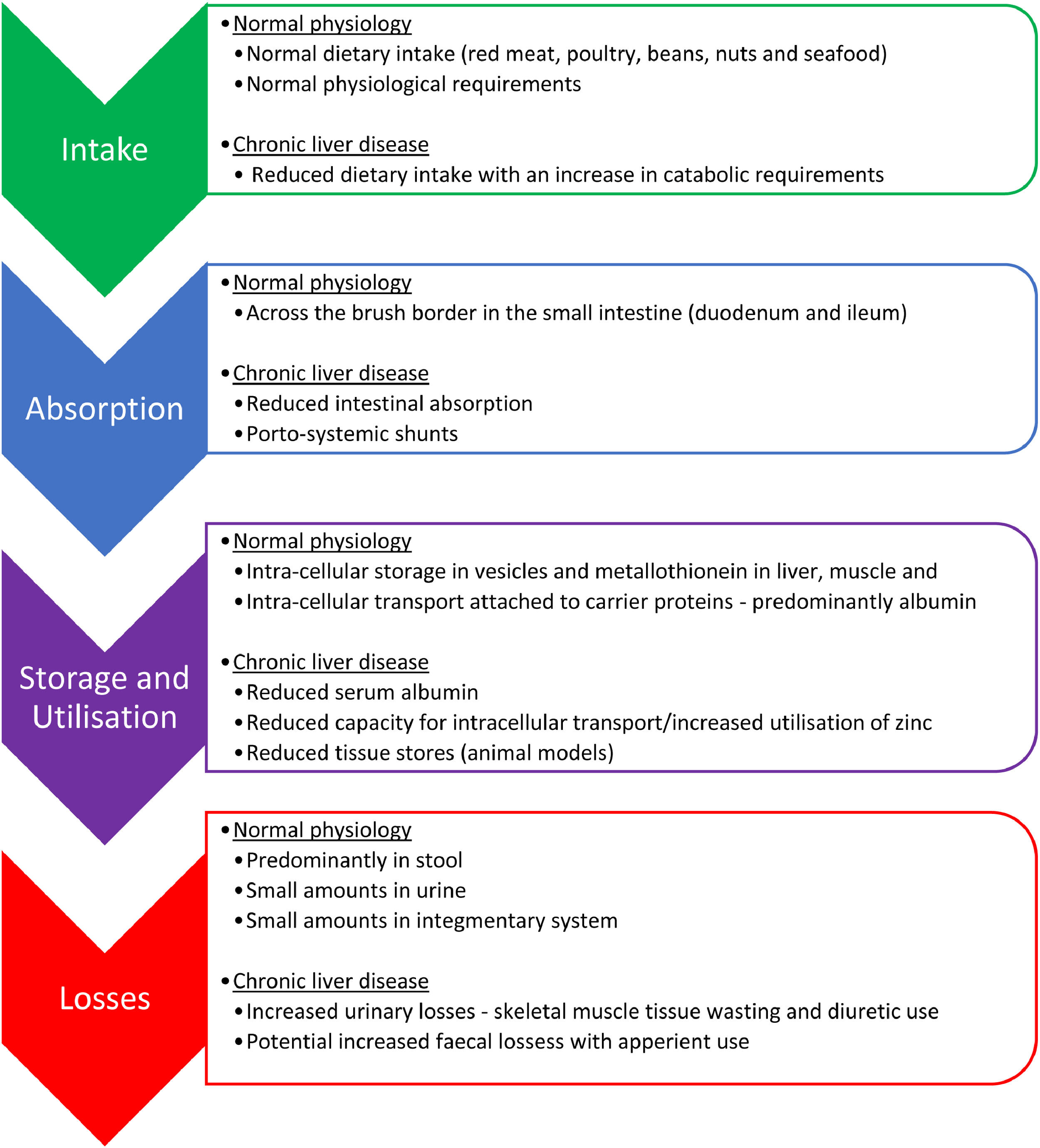
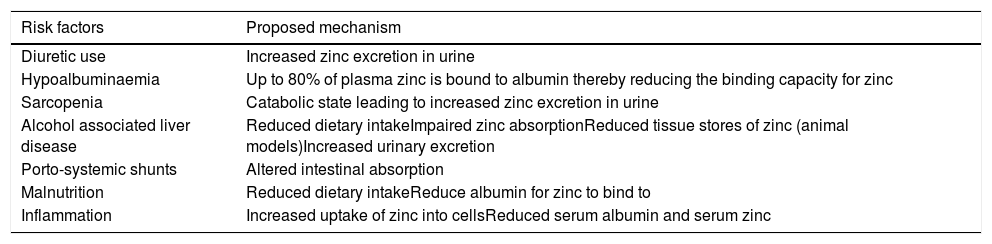
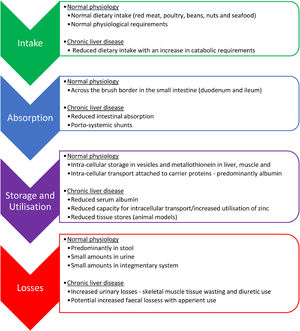
The main mechanisms behind zinc deficiency in cirrhotic patients include reduced nutritional intake, increased hepatic utilization and increased urinary losses (Fig. 2, Table 2). A low serum albumin, which is seen in advanced liver disease, malnutrition, fluid overload and inflammation, can contribute to a reduction in the measurement of serum zinc because in plasma, approximately 80% of zinc is bound to albumin which acts as an essential carrier protein [18]. With advancing liver disease and zinc deficiency, disorders of nitrogen metabolism are exacerbated. In particular, the reduced capacity of the liver to metabolise ammonia in the urea cycle, leads to an increased need for ammonia to be metabolised in skeletal muscle via glutamine-synthetase which requires branched-chain amino acids (BCAA) for this process (Fig. 1) [19]. This results in an increased utilization of BCAA, which are themselves needed as an essential component of protein synthesis and for the proper functioning of transmitting signals needed for protein synthesis [20]. This reduction in BCAA also leads to reduced albumin synthesis [21].
Zinc deficiency in chronic liver disease.
Reduced dietary intake of zinc in cirrhotic patients is multifactorial. An increase in inflammatory cytokines contribute to reduced appetite and early satiety, which is further exacerbated by the presence of ascites, hepatosplenomegaly and a build-up of bilirubin in the blood. Furthermore, typical dietary intake is poor with the displacement of food with alcohol in alcoholic liver disease and the symptoms of dysgeusia can often lead to an avoidance of protein rich foods. Intestinal absorption of zinc may also be altered due to portal hypertension associated systemic shunts [22] and is compounded by alcohol associated impairment in absorption. Increased nutritional requirements and periods of fasting during hospital admissions can worsen these issues during episodes of acute illness [23].
Serum zinc levels decrease in acute phase response to inflammation, infection, and trauma. During inflammatory events, cytokine activity mediates zinc transportation into the cells to where it is utilized for protein synthesis, neutralization of free radicals and to prevent microbial invasion. Within the liver, there is also increased hepatic uptake of zinc, with upregulation of zinc transporters occurring in hepatocytes in response to inflammatory cytokines leading to the accumulation of zinc bound to metallothionein in the liver [24]. Metallothionein is proposed to play an essential role in intra-cellular zinc homeostasis by tracking zinc through the cells and distributing zinc to proteins [25].
Increased urinary losses of zinc are commonly seen in cirrhotic patients, particularly, in patients with ascites who have severe muscle wasting due to their catabolic state and in those patients who have regular diuretic use, where zinc excretion has been shown to increase by up to 60% [26,27]. Hypoalbuminemia also increases the potential for urinary losses due to increased potential for glomerular filtration of zinc unbound to albumin.
Sarcopenia is a progressive decline in muscle mass and function which affects between 30% and 70% of patients with CLD [28], and zinc deficiency has been identified as an independent risk factor for sarcopenia in patients with CLD [29]. While age related sarcopenia can be seen as part of the physiological process of ageing, patient's with CLD are at risk of sarcopenia at any age. The pathogenesis of sarcopenia in patients with CLD is poorly understood, but it is thought to relate to protein-energy malnutrition and other metabolic and hormonal disorders seen in CLD, ultimately leading to an imbalance in muscle formation and breakdown [30]. Sarcopenia has been associated with serious adverse health outcomes in patients with CLD including increased episodes of decompensated liver disease, reduced quality of life and increased mortality [31].
4.1Hepatic encephalopathyHE is a common neurological complication seen in patients with CLD, affecting up to 30% of patients. It results in a reversible decline in cognitive function and reduced health related quality of life [32]. Decreasing serum ammonia and related nitrogenous by-products, have been shown to reduce the effects of HE. The most common treatment to date is lactulose, which aims to reduce ammonia absorption, and has been proven to reduce serum ammonia levels in minimal HE; however, lactulose does not help to detoxify ammonia once it is absorbed, thereby potentially reducing its benefit in more advanced HE [33,34]. Zinc is an essential co-factor needed for several enzymatic reactions involved in ammonia metabolism. These reactions occur within the liver, where ammonia is converted to urea via ornithine transcarbamylase and in skeletal muscle, where ammonia is metabolised to glutamic acid via glutamine synthetase. Zinc deficiency has been shown to impair the activity of both these enzymes [35,36].
There have been three good quality meta-analyses evaluating the benefit of zinc supplementation in patients with HE. Chavez-Tapia (2013) [37] looked at a total of four randomized controlled trials incorporating 233 patients. These trials compared the benefit of oral zinc supplementation with no intervention, placebo or other management for HE. They reported a significant improvement in the number connection test results across three of their studies. Their other outcome of interest was the recurrence rate of HE: two studies reported a trend towards lower recurrence rate in the zinc group; however, given the small overall sample size, this did not reach statistical significance. The authors additionally set out to evaluate the benefits of zinc replacement on quality of life, but this was not measured robustly in the majority of studies analyzed and was therefore not able to be adequately evaluated.
A later meta-analysis by Shen (2019) [38] evaluated a total of four trials incorporating 247 patients. They analyzed the effects of combination therapy with zinc and regular lactulose use in patients with low grade HE (Westhaven criteria ≤ grade 2). Their primary outcome was the degree of HE as assessed by clinical evaluation or psychometric tests (number connection test and digital symbol test). Their secondary outcome of interest were serum ammonia levels, adverse events and healthcare utilization measures, including hospital length of stay and admission cost. They found that the combination of zinc replacement with regular lactulose over a period of three to six months led to a significant improvement in the patients’ performance in the number connection test when compared to lactulose alone in patients with low grade HE. However, they did not find any significant difference between combination therapy and lactulose therapy alone in other measures of HE. There were several significant limitations of this analysis, including the sample size, exclusion of patients with more advanced HE (Westhaven criteria ≥ grade 3), and the significant heterogeneity between studies with respect to zinc replacement regimes. The most recent meta-analysis by Diglio (2020) [39] evaluated HE results from only two studies, with 167 patients comparing zinc supplementation versus placebo. They also found an improvement in HE in cirrhotic patients receiving zinc supplementation.
Despite three meta-analyses in this area in recent times there still remains a paucity of high quality synthesized data available to clinicians in this area, due to the small number of randomized controlled trials and the heterogeneity of the studies. The randomized control trial by Takuma (2010) [32] accounted for the majority of the positive results seen in these meta-analyses. In this study, 79 cirrhotic patients with HE were randomized to receive 225 mg of zinc in addition to standard of care (protein restricted diet, and lactulose) vs standard of care alone for a 6-month period. They concluded that zinc supplementation is effective in the management of HE, finding a significant reduction in HE grade by Westhaven criteria (p = 0.03) and blood ammonia levels (p = 0.01) in the zinc treatment group.
Additional limitations of these studies included either the prescription of a low protein diet (0.8–1 g/kg of protein a day), or not specifying dietary protein intake. High protein diets of 1.2–1.5 g/kg/day are recommended in liver cirrhosis and during periods of HE [40,41]. Sources of protein from animal products are generally high in zinc and highly bioavailable. If the above studies aimed to meet dietary protein requirements through food fortification or oral nutrition support products, an additional ∼6.7–11.6 mg of highly bioavailable zinc could have been consumed by participants. This poses the question of whether lower doses of zinc supplementation could have achieved similar results seen in these studies.
4.2Hepatocellular carcinomaHCC remains the most common type of primary liver cancer in patients with CLD. Despite fairly universal guidelines on surveillance and management options for patients with HCC, the pathogenesis remains a clinically important research area. Early laboratory based studies have demonstrated a significant reduction in zinc levels in hepatoma tissue by up to 50% compared to surrounding normal parenchyma [42], and an association between zinc depletion and increased p53 mRNA in hepatoma cells. This increase is also associated with a mutant wild-type form of p53, thereby potentially reducing its DNA binding capacity and its beneficial tumour suppression activity [43].
There have been several clinical studies evaluating the role of zinc in the pathogenesis of HCC. The largest of these by Fang (2019) [44], was a prospective study evaluating 989 patients with incident HCC. They evaluated serum zinc levels, serum copper levels and the ratio of serum copper to serum zinc levels. The rationale of this study was that serum copper/serum zinc homeostasis was associated with the development and progression of several different cancers [45,46] and increased overall cancer mortality [47]. The primary outcomes of interest were liver cancer specific survival (LCSS) and overall survival (OS). They found a positive association between the copper to zinc ratio and LCSS (HR 1.31; 95% CI 0.89–1.92, p > 0.05) and OS (HR 1.43; 95% CI 0.99–2.08, p > 0.05), however, this association did not reach statistical significance, and was only evident in the high serum copper to zinc (>1.38) versus low serum copper to zinc (< 0.87) subgroups. No overall significant association between serum zinc level and LCSS or OS in the overall cohort was found. Subgroup analysis did identify significant positive associations between increasing serum zinc levels and better LCSS and OS in patients with alpha feto-protein greater than 400 ng/ml, and also patients with advanced stage HCC (Barcelona Clinic Liver Cancer stage B-C) [48]. A significant relationship between high serum copper levels and poorer LCSS and OS was identified. Serum copper levels were shown to increase in patients with HCC with advancing Child Pugh scores and a raised C-reactive protein > 3.0 mg/L [49,50], however, these findings must be interpreted with caution as it may just reflect a higher incidence of HCC in patients with progressively worsening liver disease, which is well described [51]. The main limitations of this paper was that copper and zinc levels were only measured on one occasion at diagnosis, and there was a lack of data describing whether patients were receiving supplementation at this time.
The 2018 pivotal paper by Hosui et al. [52] was a retrospective cohort study which set out to evaluate the effects of long term zinc supplementation on death, liver failure and HCC development in patients with CLD. Their study was the first to evaluate long term supplementation (up to 3 years) and to attempt to stratify the incidence rates of events by serum zinc supplementation. Supplementation with 60–120 mg/day of zinc daily for at least 6 months, resulted in a significantly lower incidence of HCC and events (death, liver cancer or liver failure) at 3 years compared to patients who did not receive zinc supplementation. This was found despite the zinc supplementation group having more advanced liver disease (Child-Pugh-B and C) and also being of older age [53]. Further subgroup analysis demonstrated that patients reaching zinc concentrations above 70 ug/dl, achieved significantly lower cumulative incidence of event rates and HCC.
4.3Spontaneous bacterial peritonitisSBP is a common cause of decompensation in patients with CLD and can be associated with significant morbidity and mortality. The effect of zinc deficiency in the pathogenesis of SBP has been evaluated in two recent studies. Hanna (2019) [54], performed a cross sectional analysis of 306 patients with liver cirrhosis who underwent inpatient diagnostic paracentesis for exclusion of SBP. 79 participants (25.8%) had SBP, and they found that these patients had a significantly lower serum zinc level (49.11 + -11.84 vs 79.27 + -9.58 ug/dl, P < 0.001)) compared to those patients who did not have SBP. Subgroup analysis concluded that a serum zinc concentration below 70.5 ug/dl predicted SBP in patients with cirrhosis with a sensitivity of 94.9% and a specificity of 84.1%.
Similar results were seen in the earlier study by Mohammad (2016) [55], who performed a cross sectional study of 176 cirrhotic patients with ascites. 54 participants (31%) had SBP and they found zinc deficiency to be an independent risk factor for the development of SBP (p = 0.001) at a level < 60 ug/dl. These results need to be interpreted with a degree of caution, as patients with cirrhosis have been shown to have underlying immune dysfunction which may predispose them to infections [56].
4.4Quality of lifeMeasuring quality of life as part of a wider management strategy in managing CLD has led to improved patient benefits including reducing hospital admissions, length of admissions and improved engagement with healthcare services. This is a challenging management area in patients with liver cirrhosis.
There have only been a limited number of studies evaluating health-related quality of life in patients with CLD and its relationship to serum zinc concentration. Nishikawa et al. (2019) [57] conducted a retrospective analysis of 322 patients with CLD (122 of which had cirrhosis) and evaluated participants’ serum zinc level against validated health related quality of life tools, including the Beck Depression Inventory-2nd edition (BDI-II), the Pittsburgh Sleep Quality Index Japanese version (PSQI-J) and the Short Form Health Survey (SF-36). Higher BDI-II and PSQI-J scores were associated with lower serum zinc levels reflecting higher depression scores and poorer sleep quality in this cohort. The SF-36 score, in particular its components for physical function, role physical and physical component summary score, all significantly correlated with serum zinc levels, and this significant result was maintained across the cohort on subgroup analysis, irrespective of age, degree of CLD and aetiology of liver disease. The main limitation of this study was its retrospective study design, and therefore lacked important patient data regarding zinc supplementation, and clinical complications such as HCC and HE. Despite its limitations, this study has identified quality of life as an important factor for future studies in this area.
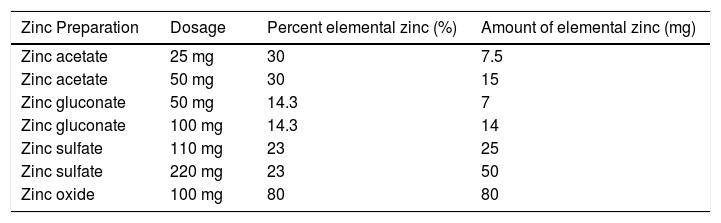
5Zinc replacement5.1Formulation and dosingThere are several different types of zinc formulations commercially available in Australia. The most commonly used for zinc replacement are zinc sulphate or zinc acetate (Table 3). The bioavailability of zinc differs between oral zinc supplements with the highest bioavailability in zinc bound to amino acids such as aspartate, cysteine and histidine, followed by zinc chloride, sulphate and acetate with the lowest bioavailability in zinc oxide [58].
The optimal dosing and duration of supplementation still remains a relatively challenging issue. Several papers have explored the concept of targeting serum zinc concentrations, which may provide a useful treat to target alternative, with a serum zinc concentration of greater than 70 ug/dl described by Hosui et al. (2018) [52] and a similar target of greater than 72 ug/dl described by Takamatsu (2013) [59], to aid in the suppression of liver fibrosis. The 2007 paper by Hayashi [60] used 600 mg of zinc sulphate (138 mg of elemental zinc) when serum zinc levels < 50 ug/dl and 200 mg (46 mg of elemental zinc) when serum zinc levels were 50–70 ug/dl for a duration of 150–180 days.
Dietary intake of zinc is also an important consideration, with a recommended dietary intake of 14 mg/day for men and 8 mg/day for women with vegetarians requiring ∼50% higher intake due to the reduced bioavailability of zinc from plant based sources compared to animal based sources. Food sources which are high in zinc include oysters, red meat, crab, pork, hard cheese, wheat bran, nuts and seeds. An upper limit of 40 mg/day for adult women and men from food and supplement sources is recommended by the Australian Nutrient Reference values due to reported adverse events associated with chronic intake of supplemental zinc [61].
Interestingly, the combination of BCAA and zinc supplementation has also been evaluated in two small studies to date with mixed results. One study found that the combination of BCAA and zinc supplementation resulted in a significant reduction in the serum ammonia level in the combination group; while the second study evaluated BCAA vs combination BCAA and zinc replacement in patients with Child Pugh C cirrhosis [62]. Interestingly, they concluded that zinc supplementation itself does not improve HCC cancer-free survival, however, cancer free survival rates were improved if a serum zinc level of > 80 ug/dl was seen pre-treatment and at 12 months [63]. Despite both studies being of interest, these studies had small cohort sizes and their results must be interpreted with caution. It does however appear that combination therapy should be an interesting focus for future studies in this area.
5.2Tolerability/safety/durationSome patients describe common side effects such as nausea and a metallic taste in their mouth, however, overall oral zinc supplementation is relatively well tolerated. Despite this, zinc toxicity can still occur, and has been described in both an acute and chronic form. Mild symptoms such as gastric discomfort have been described in doses from 0.8 mg/kg/day of elemental zinc in as early as 6 weeks [64]. Acute toxicity can result in predominantly gastrointestinal side effects including anorexia, dyspepsia, nausea, vomiting and diarrhoea. Administrating zinc pre meals has been described as a useful strategy to reduce nausea. Acute ingestion of 570 mg of elemental zinc has been shown to result in severe nausea and vomiting within 30 min of ingestion [65]. Chronic toxicity has been described with doses of 150–450 mg of elemental zinc, with potential side effects including reduced copper absorption, decreased immune function and reduced high density lipoprotein [66,67]. Long term zinc supplementation over a number of years, has been associated with a significant increase in hospitalization rates for genito-urinary conditions, which was seen in an age-related macular degeneration study, however, the underlying pathogenesis remains uncertain [68]. In this study patients were given 100 mg of zinc oxide (80 mg of zinc) for six years on average, which was significantly longer than many other studies, including the studies undertaken in patients with CLD. Interestingly, in this study, patients were all pre-emptively supplemented with 2 mg daily of oral copper.
Zinc reacts with several commonly used medications including antibiotics, particularly quinolones and tetracyclines. The interaction occurs in the gastrointestinal tract, resulting in reduced absorption of both zinc and the antibiotic. To minimize this potential interaction, it is recommended that the administration of the antibiotic occurs at least two hours prior or four to six hours post the administration of the zinc supplement [69]. Zinc can also interact with penicillamine, a chelator used in Wilson's Disease, and it is recommended that zinc is not taken within two hours before or after penicillamine.
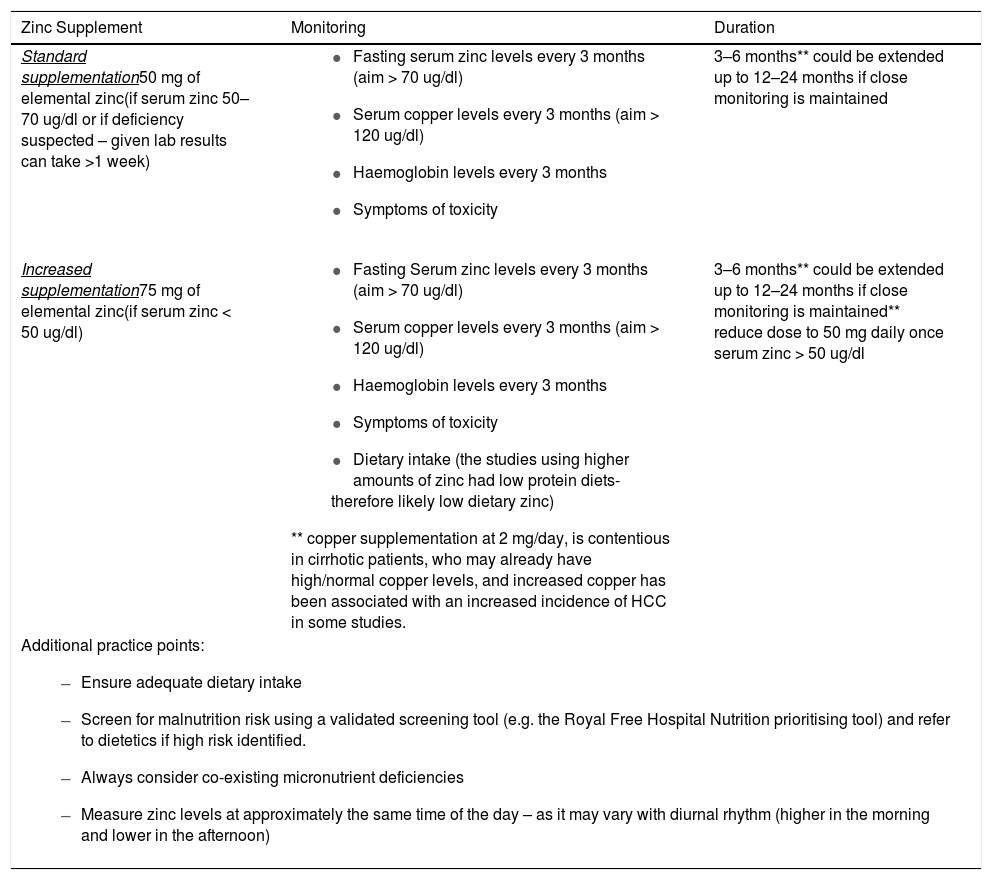
5.3Summary of zinc replacement recommendationsWe have developed the following zinc replacement protocol which is used at our tertiary referral center (Table 4). This protocol has been developed based on our critical review of the aforementioned studies in this paper, followed by an extensive consultative process involving all authors also incorporating their clinical experience at our tertiary center. The resultant zinc replacement protocol is the result of the consensus opinion reached from all authors during this consultative process. We acknowledge there is a degree of heterogeneity in the literature to date with regards to optimal dosing and duration, which highlights the importance of this review and the need to evaluate the validity of this protocol in future prospective studies in this area.
Recommended regimen for zinc replacement.
This paper highlights the importance of zinc deficiency and its replacement in patients with liver cirrhosis. There have been many potential beneficial effects of zinc replacement described, including a reduced incidence of HCC, a reduction in the severity and clinical impact of HE, a reduced incidence of SBP and an improvement in patient's quality of life. The strongest evidence to date for zinc use in patients with liver cirrhosis is in the management of HE, whereas HCC risk reduction remains unconfirmed at present.
Despite the promising benefits described from zinc supplementation in the literature to date in cirrhotic patients, there are still many important questions which require further evaluation. In particular establishing the optimum dosage of zinc required to improve clinical outcomes and to treat deficiency when adequate dietary intake is accounted for, as well as defining the optimum duration of treatment and monitoring regimens for zinc supplementation. We recommend future studies include standardized protein intake and measure malnutrition status as these are important factors which impact serum zinc levels and function. Treat to target dosing offers a more personalized approach and may prove beneficial in future studies. We believe these clinical questions lend themselves as pivotal questions for future studies in this area.
FundingNone.