Zinc deficiency has been associated with poor prognosis in chronic liver disease. This systematic review and meta-analysis aimed to evaluate the role of zinc supplementation in the management of chronic liver diseases.
Materials and methodsWe searched MEDLINE, LILACS, EMBASE, and Cochrane CENTRAL databases from inception to August 2018. We included randomized controlled trials evaluating adult patients with chronic liver disease of any etiology receiving zinc supplementation. Studies with other designs or evaluating chronic conditions other than liver disease were excluded. Two reviewers independently screened and extracted data from eligible studies. Study quality was assessed using the Cochrane Collaboration's tool for assessing risk of bias in randomized studies.
ResultsOf 1315 studies screened, 13 were included. Six assessed chronic hepatitis C treatment, with a relative risk of 0.83 indicating no protective effect of zinc supplementation on the improvement of sustained virological response. Three evaluated response to hepatic encephalopathy treatment, with a relative risk of 0.66 indicating a favorable effect of zinc supplementation on clinical improvement of this condition. Of four studies evaluating the management of cirrhosis, two analyzed the effect of zinc supplementation on serum albumin levels, with no statistical difference between zinc and placebo groups.
ConclusionsClinical trials assessing zinc supplementation in liver diseases do not show benefits in terms of clinical improvement or disease halting. There are possible benefits of zinc supplementation on hepatic encephalopathy, however, this is based on limited evidence. This research question is still open for evaluation in larger, well-designed, clinical trials.
Zinc is an essential trace element and is involved in a wide variety of metabolic processes that play a significant role in immunity, which is the reason why zinc deficiency increases susceptibility to various diseases [1]. The liver is the main organ responsible for the zinc metabolism. Serum zinc levels are often reduced in patients with chronic liver disease, especially in cirrhosis and its complications, such as ascites, hepatic encephalopathy (HE), and hepatocellular carcinoma (HCC) [2]. On the other hand, zinc deficiency may alter hepatocyte functions and also immune responses in inflammatory liver diseases, consequently initiating a variety of metabolic abnormalities, including insulin resistance, hepatic steatosis, iron overload and HE in patients with chronic liver disease. Besides that, a decrease in albumin synthesis leads to zinc deficiency in patients with liver cirrhosis [3,4].
Data on the effects of zinc supplementation have been conflicting, and although zinc supplementation has been used to help alleviate the symptoms of chronic liver diseases and has shown some beneficial effects on metabolic abnormalities in experimental models and in patients with chronic liver disease, strong evidence about the beneficial effect of micronutrients supplementation in cirrhotic patients is not available, still being a matter of debate [1,4–12].
Therefore, we performed the present systematic review and meta-analysis to evaluate the role of zinc supplementation in the management of chronic liver diseases and their complications.
2Materials and methods2.1Guidelines and protocol registrationThis systematic review and meta-analysis was conducted following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [13]. The systematic review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration number CDR42018065028).
2.2Research questionThe present systematic review has a wide scope and it was based on the following research question, defined in terms of the PICO framework (Patients, Intervention, Comparator, Outcome) [13]: “What are the effects of zinc supplementation in addition to best liver-disease-specific practices in comparison to therapeutic strategies with no zinc supplementation for patients with any chronic liver condition in terms of any clinical, histological, biochemical, molecular or microbiological outcome?”.
2.3Eligibility criteriaRandomized controlled trials (RCTs) of adult patients (aged ≥18 years) with chronic liver disease of any etiology who received zinc supplementation alone (not combined with other supplements) were included. In the RCTs that fulfilled the eligibility criteria, the following outcomes of interest had been analyzed: sustained virological response to therapy in patients with chronic hepatitis C; clinical improvement of HE; and serum albumin in cirrhosis were included. Studies with other methodological designs, experimental studies, and those evaluating chronic conditions other than liver disease were excluded.
2.4Search strategyWe searched MEDLINE (via PubMed), LILACS, EMBASE, and Cochrane CENTRAL databases from inception to August 2018. No language restrictions were imposed. We used the following sets of search terms to search all databases: (“Liver Diseases” [Mesh] OR “Liver Cirrhosis” [Mesh] OR “Fatty Liver” [Mesh] OR “Hepatitis C” [Mesh] OR “Hepatitis C, Chronic” [Mesh] OR “Liver Neoplasms” [Mesh] OR “Adenoma, Carcinoma, Hepatocellular” [Mesh] AND “Zinc” [Mesh] OR “Zinc Sulfate” [Mesh]). The complete search strategy can be found in supplemental Table 1. Additionally, the reference lists of the retrieved articles were hand searched to detect other potentially eligible studies.
2.5Study selectionFor eligibility assessment, two independent reviewers (DD and JS) screened titles and abstracts, and full-text articles. If during title and abstract screening there was uncertainty as to whether a study met the eligibility criteria, the full text of the study was retrieved and examined in more detail. Disagreements between the two reviewers were resolved by consensus.
2.6Data extraction and assessment of risk of biasA specific form was developed for data extraction. The following data were extracted by two independent reviewers (DD and JS): study design, age and sex of the participants, study inclusion criteria, etiology of chronic liver disease, and formulation, dosage, and duration of zinc supplementation. The risk of bias was assessed using the Cochrane Collaboration's tool for assessing risk of bias in randomized studies [14]. The quality of studies was assessed in the following domains: generation of the random allocation sequence; allocation concealment; blinding of participants, health professionals, and outcome assessors; description of losses to follow-up; and selective outcome reporting. The risk of bias in each domain was rated as low, unclear, or high.
2.7Data synthesisRCTs were meta-analyzed by directly comparing studies evaluating intervention and control groups with similar characteristics and reporting the outcomes of interest. We calculated relative risks (RRs) for categorical outcomes and weighted mean differences for continuous outcomes. Heterogeneity was measured by the I2 test, and a random-effects model was used (I2 values >50%). Statistical analysis was performed using RevMan version 5.3.
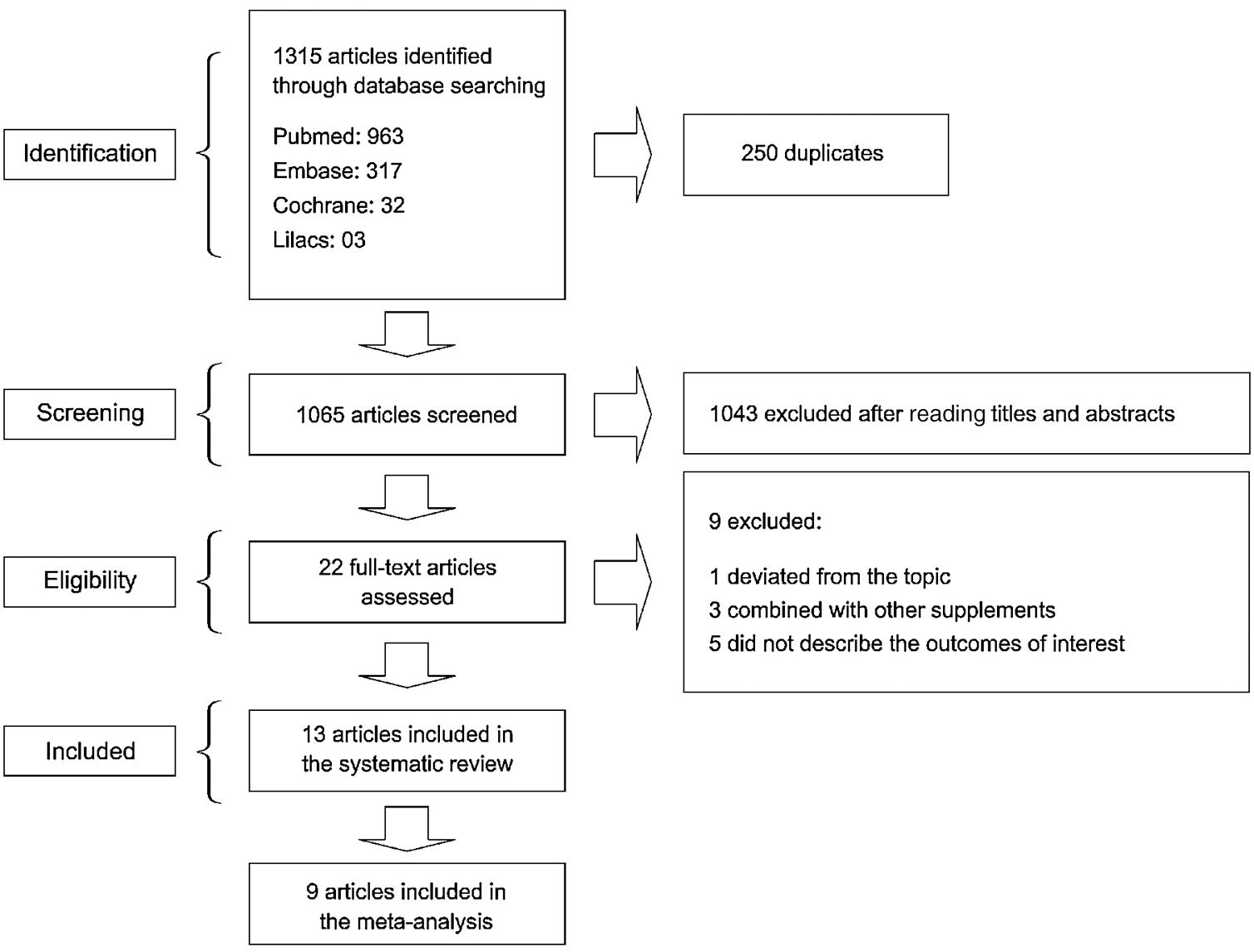
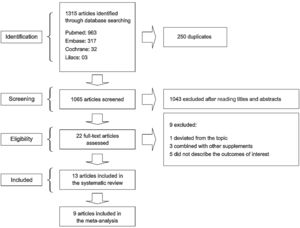
3ResultsOf 1315 studies identified through the search strategy, 22 were selected for full-text reading (Fig. 1). A total of nine studies were excluded: one study that deviated from the topic of interest; 3 studies that used zinc supplementation combined with other supplements; and five studies that failed to describe the outcomes of interest. As a result, 13 studies were included in the present systematic review [1,6,10,11,15–23].
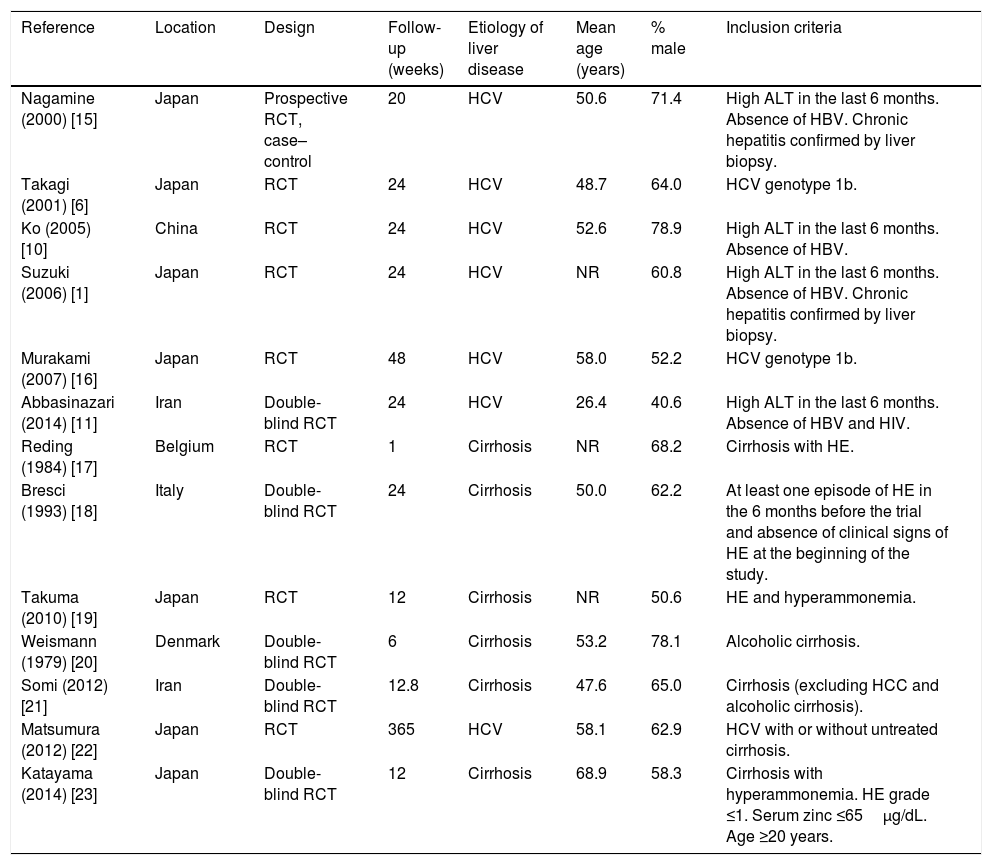
The characteristics of included studies are shown in Table 1[1,6,10,11,15–23]. Six studies (46%) assessed the treatment of chronic hepatitis C [1,6,10,11,15,16], three studies (23%) assessed the treatment of HE [17–19], and four studies (31%) assessed the management of cirrhosis [20–23]. In studies involving patients with cirrhosis, the stage of liver disease was determined by the Child–Pugh score. In most studies, participants were male patients with a mean age of 26.4–68.9 years.
Characteristics of included studies.
| Reference | Location | Design | Follow-up (weeks) | Etiology of liver disease | Mean age (years) | % male | Inclusion criteria |
|---|---|---|---|---|---|---|---|
| Nagamine (2000) [15] | Japan | Prospective RCT, case–control | 20 | HCV | 50.6 | 71.4 | High ALT in the last 6 months. Absence of HBV. Chronic hepatitis confirmed by liver biopsy. |
| Takagi (2001) [6] | Japan | RCT | 24 | HCV | 48.7 | 64.0 | HCV genotype 1b. |
| Ko (2005) [10] | China | RCT | 24 | HCV | 52.6 | 78.9 | High ALT in the last 6 months. Absence of HBV. |
| Suzuki (2006) [1] | Japan | RCT | 24 | HCV | NR | 60.8 | High ALT in the last 6 months. Absence of HBV. Chronic hepatitis confirmed by liver biopsy. |
| Murakami (2007) [16] | Japan | RCT | 48 | HCV | 58.0 | 52.2 | HCV genotype 1b. |
| Abbasinazari (2014) [11] | Iran | Double-blind RCT | 24 | HCV | 26.4 | 40.6 | High ALT in the last 6 months. Absence of HBV and HIV. |
| Reding (1984) [17] | Belgium | RCT | 1 | Cirrhosis | NR | 68.2 | Cirrhosis with HE. |
| Bresci (1993) [18] | Italy | Double-blind RCT | 24 | Cirrhosis | 50.0 | 62.2 | At least one episode of HE in the 6 months before the trial and absence of clinical signs of HE at the beginning of the study. |
| Takuma (2010) [19] | Japan | RCT | 12 | Cirrhosis | NR | 50.6 | HE and hyperammonemia. |
| Weismann (1979) [20] | Denmark | Double-blind RCT | 6 | Cirrhosis | 53.2 | 78.1 | Alcoholic cirrhosis. |
| Somi (2012) [21] | Iran | Double-blind RCT | 12.8 | Cirrhosis | 47.6 | 65.0 | Cirrhosis (excluding HCC and alcoholic cirrhosis). |
| Matsumura (2012) [22] | Japan | RCT | 365 | HCV | 58.1 | 62.9 | HCV with or without untreated cirrhosis. |
| Katayama (2014) [23] | Japan | Double-blind RCT | 12 | Cirrhosis | 68.9 | 58.3 | Cirrhosis with hyperammonemia. HE grade ≤1. Serum zinc ≤65μg/dL. Age ≥20 years. |
ALT, alanine aminotransferase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HE, hepatic encephalopathy; HIV, human immunodeficiency virus; μg/dL, micrograms per deciliter; NR, not reported; RCT, randomized controlled trial.
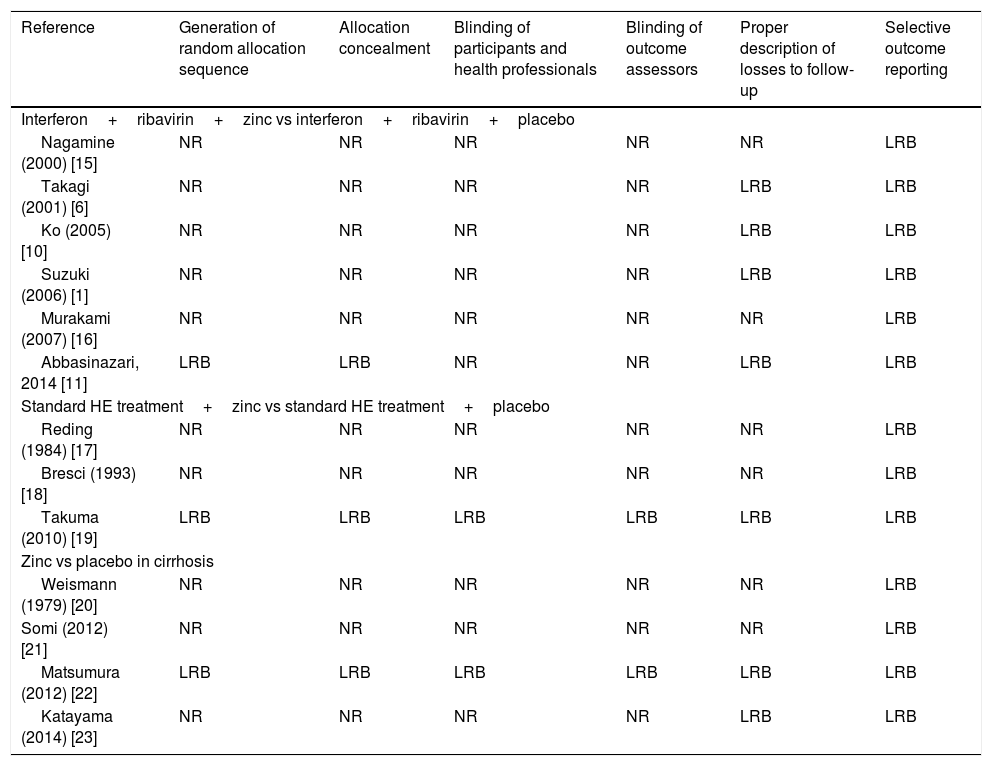
Table 2[1,6,10,11,15–23] shows a summary of the risk of bias of included studies based on Cochrane Collaboration's tool. Most studies were rated as having a low risk of bias for selective outcome reporting and description of losses to follow-up. However, except for the studies of Takuma et al. [19] and Matsumura et al. [22], the overall quality of studies was considered low, since most of them did not describe all the required parameters.
Risk of bias of included studies (Cochrane Collaboration's tool).
| Reference | Generation of random allocation sequence | Allocation concealment | Blinding of participants and health professionals | Blinding of outcome assessors | Proper description of losses to follow-up | Selective outcome reporting |
|---|---|---|---|---|---|---|
| Interferon+ribavirin+zinc vs interferon+ribavirin+placebo | ||||||
| Nagamine (2000) [15] | NR | NR | NR | NR | NR | LRB |
| Takagi (2001) [6] | NR | NR | NR | NR | LRB | LRB |
| Ko (2005) [10] | NR | NR | NR | NR | LRB | LRB |
| Suzuki (2006) [1] | NR | NR | NR | NR | LRB | LRB |
| Murakami (2007) [16] | NR | NR | NR | NR | NR | LRB |
| Abbasinazari, 2014 [11] | LRB | LRB | NR | NR | LRB | LRB |
| Standard HE treatment+zinc vs standard HE treatment+placebo | ||||||
| Reding (1984) [17] | NR | NR | NR | NR | NR | LRB |
| Bresci (1993) [18] | NR | NR | NR | NR | NR | LRB |
| Takuma (2010) [19] | LRB | LRB | LRB | LRB | LRB | LRB |
| Zinc vs placebo in cirrhosis | ||||||
| Weismann (1979) [20] | NR | NR | NR | NR | NR | LRB |
| Somi (2012) [21] | NR | NR | NR | NR | NR | LRB |
| Matsumura (2012) [22] | LRB | LRB | LRB | LRB | LRB | LRB |
| Katayama (2014) [23] | NR | NR | NR | NR | LRB | LRB |
HE, hepatic encephalopathy; LRB, low risk of bias; *NR, not reported.
*Not reported items are assumed to confer a high risk of bias for that particular item.
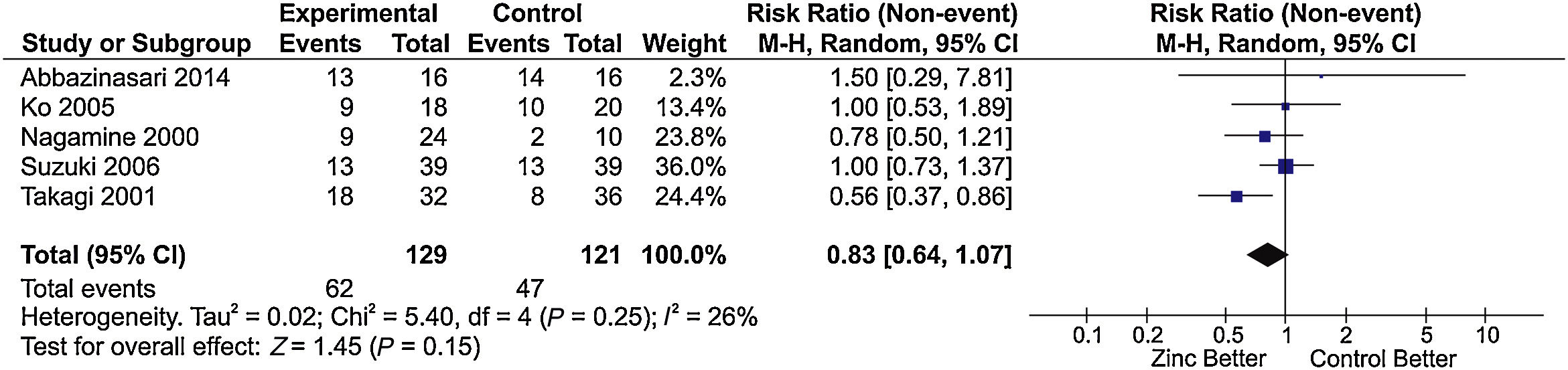
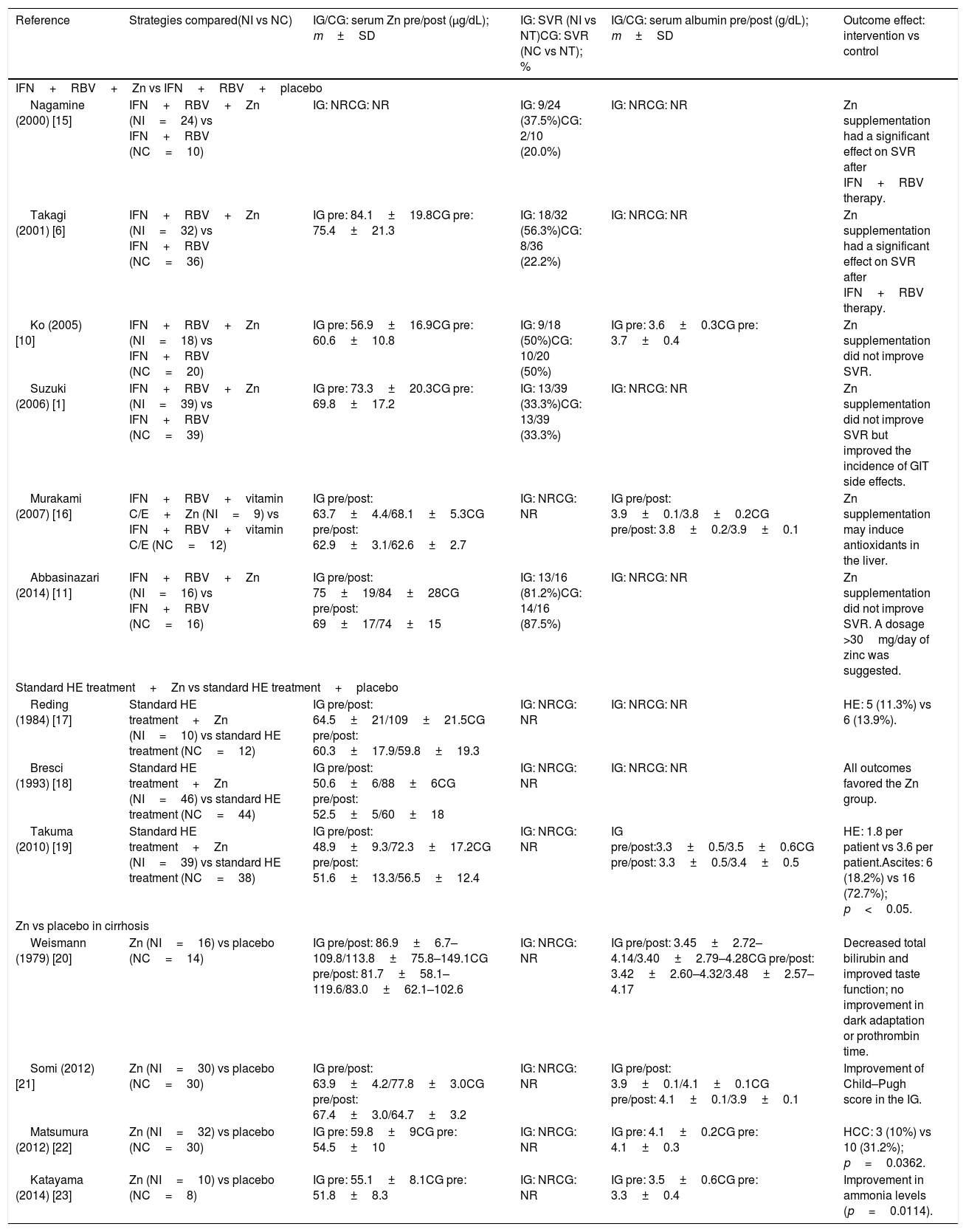
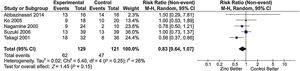
The main results of the included studies are shown in Table 3[1,6,10,11,15–23]. Six studies [1,6,10,11,15,16] compared zinc supplementation vs no supplementation in patients with chronic hepatitis C treated with interferon plus ribavirin. Of these, five had sufficient information for inclusion in the meta-analysis for sustained virological response. An RR of 0.83 was estimated (95% confidence interval [CI], 0.64–1.07; I2=26%), indicating absence of a protective effect of zinc supplementation on the improvement of sustained virological response (Fig. 2).
Main results of included studies.
| Reference | Strategies compared(NI vs NC) | IG/CG: serum Zn pre/post (μg/dL); m±SD | IG: SVR (NI vs NT)CG: SVR (NC vs NT); % | IG/CG: serum albumin pre/post (g/dL); m±SD | Outcome effect: intervention vs control |
|---|---|---|---|---|---|
| IFN+RBV+Zn vs IFN+RBV+placebo | |||||
| Nagamine (2000) [15] | IFN+RBV+Zn (NI=24) vs IFN+RBV (NC=10) | IG: NRCG: NR | IG: 9/24 (37.5%)CG: 2/10 (20.0%) | IG: NRCG: NR | Zn supplementation had a significant effect on SVR after IFN+RBV therapy. |
| Takagi (2001) [6] | IFN+RBV+Zn (NI=32) vs IFN+RBV (NC=36) | IG pre: 84.1±19.8CG pre: 75.4±21.3 | IG: 18/32 (56.3%)CG: 8/36 (22.2%) | IG: NRCG: NR | Zn supplementation had a significant effect on SVR after IFN+RBV therapy. |
| Ko (2005) [10] | IFN+RBV+Zn (NI=18) vs IFN+RBV (NC=20) | IG pre: 56.9±16.9CG pre: 60.6±10.8 | IG: 9/18 (50%)CG: 10/20 (50%) | IG pre: 3.6±0.3CG pre: 3.7±0.4 | Zn supplementation did not improve SVR. |
| Suzuki (2006) [1] | IFN+RBV+Zn (NI=39) vs IFN+RBV (NC=39) | IG pre: 73.3±20.3CG pre: 69.8±17.2 | IG: 13/39 (33.3%)CG: 13/39 (33.3%) | IG: NRCG: NR | Zn supplementation did not improve SVR but improved the incidence of GIT side effects. |
| Murakami (2007) [16] | IFN+RBV+vitamin C/E+Zn (NI=9) vs IFN+RBV+vitamin C/E (NC=12) | IG pre/post: 63.7±4.4/68.1±5.3CG pre/post: 62.9±3.1/62.6±2.7 | IG: NRCG: NR | IG pre/post: 3.9±0.1/3.8±0.2CG pre/post: 3.8±0.2/3.9±0.1 | Zn supplementation may induce antioxidants in the liver. |
| Abbasinazari (2014) [11] | IFN+RBV+Zn (NI=16) vs IFN+RBV (NC=16) | IG pre/post: 75±19/84±28CG pre/post: 69±17/74±15 | IG: 13/16 (81.2%)CG: 14/16 (87.5%) | IG: NRCG: NR | Zn supplementation did not improve SVR. A dosage >30mg/day of zinc was suggested. |
| Standard HE treatment+Zn vs standard HE treatment+placebo | |||||
| Reding (1984) [17] | Standard HE treatment+Zn (NI=10) vs standard HE treatment (NC=12) | IG pre/post: 64.5±21/109±21.5CG pre/post: 60.3±17.9/59.8±19.3 | IG: NRCG: NR | IG: NRCG: NR | HE: 5 (11.3%) vs 6 (13.9%). |
| Bresci (1993) [18] | Standard HE treatment+Zn (NI=46) vs standard HE treatment (NC=44) | IG pre/post: 50.6±6/88±6CG pre/post: 52.5±5/60±18 | IG: NRCG: NR | IG: NRCG: NR | All outcomes favored the Zn group. |
| Takuma (2010) [19] | Standard HE treatment+Zn (NI=39) vs standard HE treatment (NC=38) | IG pre/post: 48.9±9.3/72.3±17.2CG pre/post: 51.6±13.3/56.5±12.4 | IG: NRCG: NR | IG pre/post:3.3±0.5/3.5±0.6CG pre/post: 3.3±0.5/3.4±0.5 | HE: 1.8 per patient vs 3.6 per patient.Ascites: 6 (18.2%) vs 16 (72.7%); p<0.05. |
| Zn vs placebo in cirrhosis | |||||
| Weismann (1979) [20] | Zn (NI=16) vs placebo (NC=14) | IG pre/post: 86.9±6.7–109.8/113.8±75.8–149.1CG pre/post: 81.7±58.1–119.6/83.0±62.1–102.6 | IG: NRCG: NR | IG pre/post: 3.45±2.72–4.14/3.40±2.79–4.28CG pre/post: 3.42±2.60–4.32/3.48±2.57–4.17 | Decreased total bilirubin and improved taste function; no improvement in dark adaptation or prothrombin time. |
| Somi (2012) [21] | Zn (NI=30) vs placebo (NC=30) | IG pre/post: 63.9±4.2/77.8±3.0CG pre/post: 67.4±3.0/64.7±3.2 | IG: NRCG: NR | IG pre/post: 3.9±0.1/4.1±0.1CG pre/post: 4.1±0.1/3.9±0.1 | Improvement of Child–Pugh score in the IG. |
| Matsumura (2012) [22] | Zn (NI=32) vs placebo (NC=30) | IG pre: 59.8±9CG pre: 54.5±10 | IG: NRCG: NR | IG pre: 4.1±0.2CG pre: 4.1±0.3 | HCC: 3 (10%) vs 10 (31.2%); p=0.0362. |
| Katayama (2014) [23] | Zn (NI=10) vs placebo (NC=8) | IG pre: 55.1±8.1CG pre: 51.8±8.3 | IG: NRCG: NR | IG pre: 3.5±0.6CG pre: 3.3±0.4 | Improvement in ammonia levels (p=0.0114). |
CG, control group; g/dL, grams per deciliter; GIT, gastrointestinal tract; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy; IFN, (conventional or pegylated) interferon; IG, intervention group; m±SD, mean and standard deviation; μg/dL, micrograms per deciliter; NC, number of patients in the control group; NI, number of patients in the intervention group; NR, not reported; NT, total number of patients; RBV, ribavirin; SVR, sustained virological response; Zn, zinc.
Meta-analysis of the ‘sustained virological response’ outcome* in studies comparing treatment of chronic hepatitis C with IFN plus RBV associated with zinc supplementation vs placebo.
Chi2, chi-squared; CI, confidence interval; df, degrees of freedom; I2, I-squared; IFN, interferon; M–H, Mantel–Haenszel; P, p-value; RBV, ribavirin; Tau2, tau-squared; Z, z-score.
*The forest plot represents the risk of non-occurrence of sustained virological response.
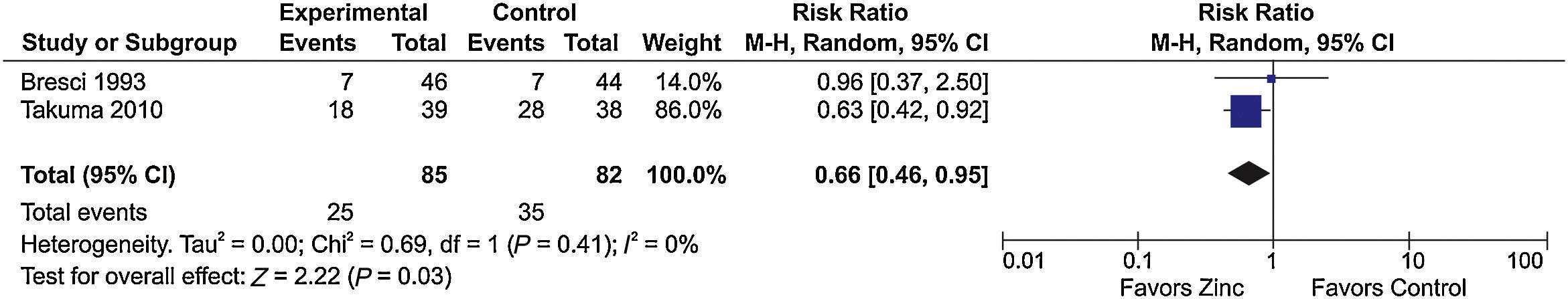
Three studies [17–19] evaluating the response to HE treatment compared zinc supplementation associated with standard therapy vs standard therapy alone. Of these, two had sufficient information for inclusion in a meta-analysis that showed a favorable effect of zinc supplementation on the clinical improvement of HE (RR=0.66; 95% CI, 0.46–0.95; I2=0%) (Fig. 3).
Meta-analysis of the ‘improvement of hepatic encephalopathy’ outcome in studies comparing zinc supplementation vs placebo for the treatment of hepatic encephalopathy.
Chi2, chi-squared; CI, confidence interval; df, degrees of freedom; I2, I-squared; M–H, Mantel–Haenszel; P, p-value; Tau2, tau-squared; Z, z-score.
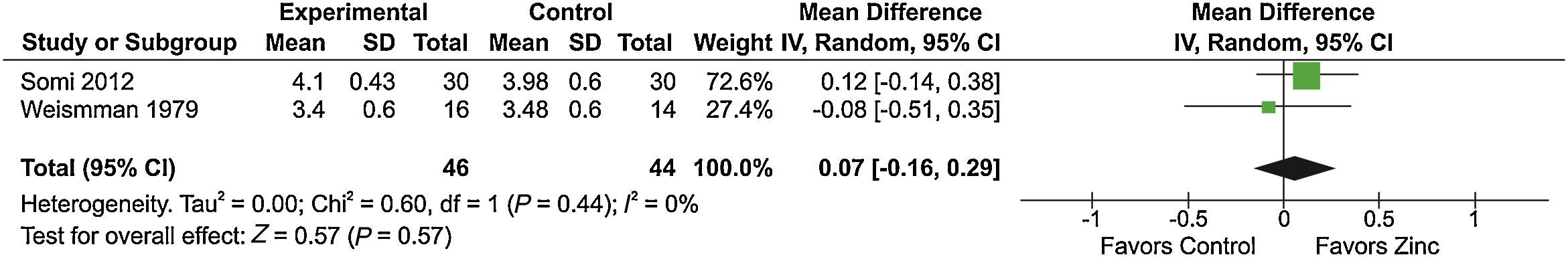
Of the four studies [20–23] comparing zinc supplementation vs placebo in cirrhosis, two [20,21] analyzed the effect of zinc supplementation on serum albumin levels and had sufficient information for inclusion in a meta-analysis. However, the meta-analysis of these studies showed no significant difference between the groups (Fig. 4). Of note, these two studies [20,21] reported serum albumin levels at the end of treatment, but standard deviations were not provided. For the study of Somi et al. [21], we calculated final standard deviation values for the zinc and control groups based on standard error values. The study of Weismann et al. [20] did not provide sufficient information to allow algebraic recalculation of standard deviations, which were then imputed from the highest standard deviation value observed in the study of Somi et al. [21] by meta-analysis-level imputation [24].
Meta-analysis of the ‘serum albumin level’ outcome in studies comparing zinc supplementation vs placebo for the treatment of cirrhosis-related outcomes.
Chi2, chi-squared; CI, confidence interval; df, degrees of freedom; I2, I-squared; IV, interval variable; P, p-value; SD, standard deviation; Tau2, tau-squared; Z, z-score.
Furthermore, zinc supplementation was associated with reduced progression to HCC (p=0.0362) [22], improvement in the Child–Pugh score (p=0.001) [21], improvement in bilirubin levels (p≤0.005) [20], lower incidence of ascites (p=0.08) [19], and decrease in ammonia levels (p=0.0114) [23]. However, it was not possible to meta-analyze the results of these studies.
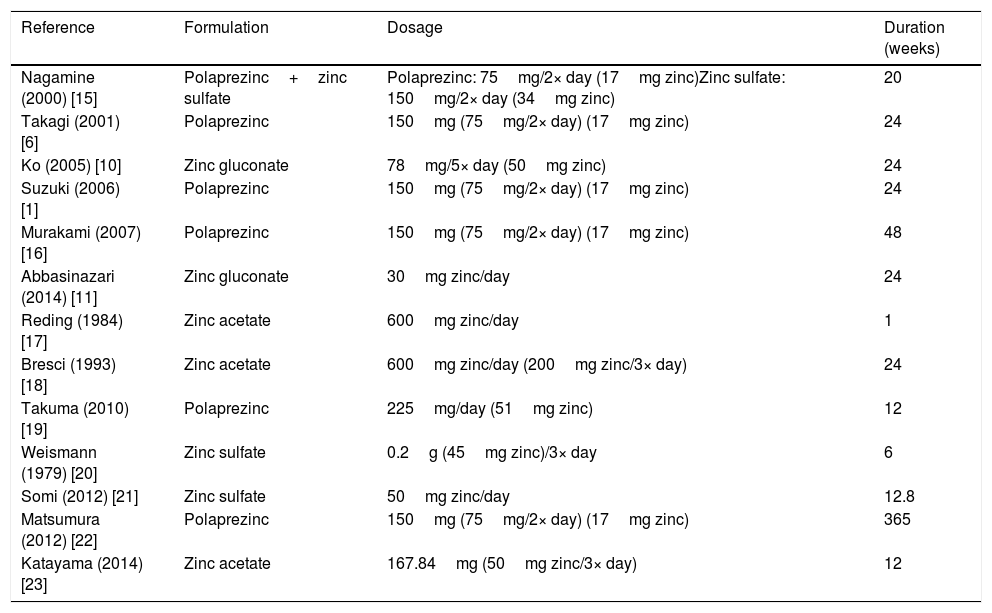
The formulation, dosage, and duration of zinc supplementation varied among studies (Table 4) [1,6,10,11,15–23]. Six studies [1,6,15,16,19,20] used polaprezinc, three studies [15,20,21] used zinc sulfate, three studies [17,18,23] used zinc acetate, and two studies [10,11] used zinc gluconate. Dosage ranged from 45 to 200mg of zinc per day. The duration of treatment ranged from one to 365 weeks.
Formulation, dosage, and duration of zinc supplementation in the included studies.
| Reference | Formulation | Dosage | Duration (weeks) |
|---|---|---|---|
| Nagamine (2000) [15] | Polaprezinc+zinc sulfate | Polaprezinc: 75mg/2× day (17mg zinc)Zinc sulfate: 150mg/2× day (34mg zinc) | 20 |
| Takagi (2001) [6] | Polaprezinc | 150mg (75mg/2× day) (17mg zinc) | 24 |
| Ko (2005) [10] | Zinc gluconate | 78mg/5× day (50mg zinc) | 24 |
| Suzuki (2006) [1] | Polaprezinc | 150mg (75mg/2× day) (17mg zinc) | 24 |
| Murakami (2007) [16] | Polaprezinc | 150mg (75mg/2× day) (17mg zinc) | 48 |
| Abbasinazari (2014) [11] | Zinc gluconate | 30mg zinc/day | 24 |
| Reding (1984) [17] | Zinc acetate | 600mg zinc/day | 1 |
| Bresci (1993) [18] | Zinc acetate | 600mg zinc/day (200mg zinc/3× day) | 24 |
| Takuma (2010) [19] | Polaprezinc | 225mg/day (51mg zinc) | 12 |
| Weismann (1979) [20] | Zinc sulfate | 0.2g (45mg zinc)/3× day | 6 |
| Somi (2012) [21] | Zinc sulfate | 50mg zinc/day | 12.8 |
| Matsumura (2012) [22] | Polaprezinc | 150mg (75mg/2× day) (17mg zinc) | 365 |
| Katayama (2014) [23] | Zinc acetate | 167.84mg (50mg zinc/3× day) | 12 |
In this systematic review and meta-analysis of 13 RCTs comparing zinc supplementation vs no supplementation in patients with chronic liver disease, we found an association of zinc supplementation with clinical improvement of HE, but no effect was observed on the improvement of sustained virological response to therapy in patients with chronic hepatitis C. To our knowledge, this is the first systematic review to address the relationship of zinc supplementation and variable outcomes in patients with liver diseases of different etiologies.
The increasing number of patients with chronic liver diseases worldwide highlights the importance of treatments that can improve the management of such diseases by positively influencing prognosis. The RCTs included in this systematic review evaluated the ability of zinc supplementation to potentiate treatment efficacy in patients with hepatitis C [1,6,10,11,15,16], to improve clinical manifestations related to HE [17–19], and to improve clinical conditions (e.g., serum albumin and ammonia levels, Child–Pugh score) or halt disease progression (e.g., progression to HCC) [20–23].
Yuasa et al. [25], examining the effect of zinc on hepatitis C virus (HCV) replication in vitro, demonstrated that zinc may play an important role as a negative genome regulator, suggesting that zinc supplementation may be an approach to the development of future strategies for the treatment of chronic hepatitis C. Six studies included in the present systematic review evaluated the effect of adding zinc to interferon plus ribavirin therapy on the treatment of chronic hepatitis C [1,6,10,11,15,16], especially because zinc supplementation is a low-cost intervention associated with a reduction in the side effects of therapy with interferon and ribavirin. However, no improvement was reported in sustained virological response within 6 months of treatment, except for two studies [6,15]. A decrease in serum zinc concentration usually occurs during the course of interferon plus ribavirin therapy, especially in patients responsive to treatment. An explanation for this phenomenon is the increased demand for zinc associated with cytokine-mediated immune responses [16].
The improvement in HCV-related chronic liver disease associated with zinc supplementation can be explained by the anti-inflammatory effect that zinc exerts on the liver in these patients, represented by decreased aspartate aminotransferase and alanine aminotransferase levels and reduced iron overload [26]. However, conflicting results regarding the efficacy of zinc in HCV treatment may be explained by differences in the formulation, dosage, and duration of zinc supplementation. We believe that dosage should not be standardized for chronic diseases, but rather tailored to individual body composition and albumin levels, since zinc binds to albumin for transportation and is stored in its active form in skeletal muscle [27].
Three studies included in the present systematic review evaluated the response to HE treatment associated with zinc supplementation [17–19], which showed promise as a useful addition to HE treatment. According to the results of these studies, there was an improvement in HE grade in 55–60% of patients, along with improvements in serum ammonia levels, Child–Pugh score, and neuropsychological tests. For an abnormality to be considered significant in the pathogenesis of HE, three requirements must be met: it must be demonstrated in association with HE; HE must appear once the abnormality is induced; and HE must improve once the abnormality is corrected [17]. Two of these studies were meta-analyzed and demonstrated a therapeutic effect of zinc supplementation on HE by meeting at least two of these requirements.
On the other hand, a systematic review assessing the role of zinc in the treatment of HE in patients with cirrhosis concluded that, although oral zinc supplementation improved performance on psychometric tests, there was no evidence of improvement for other clinical or biochemical outcomes [28]. The studies evaluating zinc supplementation in cirrhosis that were included in the present systematic review [20–23] reported improvements in some clinical and laboratory parameters (serum albumin and ammonia levels, Child–Pugh score, total bilirubin, and taste function), as well as reduced progression to HCC. It was not possible to meta-analyze the results for all the outcomes, only for serum albumin level; however, no difference was found in the results.
Zinc supplementation in patients with cirrhosis produces metabolic effects that can help improve liver function, HE, and nutritional status. Somi et al. [21], using low-dose zinc sulfate supplementation (50mg/day for 12.8 weeks), showed clinical improvement of non-alcoholic cirrhosis. Matsumura et al. [22] corroborated these findings by showing that administration of 150mg polaprezinc per day for 365 weeks to patients with liver cirrhosis reduced the incidence of HCC compared with the control group (not administered polaprezinc). Likewise, Katayama et al. [23], in patients with liver cirrhosis receiving zinc acetate supplementation (167.84mg/day for 12 weeks), observed improvement in ammonia levels (p=0.0114), also favoring zinc supplementation.
The present systematic review and meta-analysis has limitations. First, despite a comprehensive database search, we did not actively searched unpublished or “gray literature” studies, and only a few studies were eligible for inclusion. As a justificative, we understand that, if the quality of the studies evaluated was low, the search in the “gray literature” would be futile and would not add relevant information to the present study. Second, many of the included studies have limitations, such as lack of consensus on the type of formulation, dosage, and duration of zinc supplementation. Third, studies evaluating the role of zinc as a catalyst for HCV treatment have been conducted in different decades, and the metabolic mechanism involved is unclear. Also, we found that the oldest studies had less standardized reporting, with confusing description of interventions or controls. Moreover, information on zinc preparation or dose unit was not always available in a clear and unambiguous way [17,18,20]. Finally, some studies did not report the means of some variables of interest or did not analyze some variables that are commonly used for liver function assessment [17,18].
In conclusion, clinical trials assessing zinc supplementation in liver diseases do not show benefits in terms of clinical improvement or disease halting. The current systematic review indicates possible benefits of zinc supplementation on hepatic encephalopathy, however, even this finding is based on just two trials with substantial risk of bias. This research question is still open for evaluation in larger, well-designed, clinical trials.AbbreviationsHCC hepatocellular carcinoma hepatic encephalopathy hepatitis C virus Preferred Reporting Items for Systematic Reviews and Meta-analyses randomized controlled trial relative risk
The authors have no financial relationships relevant to this article to disclose.
Conflict of interestThe authors have no conflicts of interest to declare.