The epidemiology of infection with hepatitis E virus (HEV) in Tepehuanos (a Mexican ethnic group living in rural areas) is largely unknown. This study aimed to determine the seroprevalence of and risk factors associated with HEV infection in Tepehuanos in Durango, Mexico, and to compare this seroprevalence with that in non-Tepehuanos.
Materials and methodsThrough a case–control seroprevalence study, we studied 146 Tepehuanos and 146 age- and gender-matched control subjects of the general population from rural settings. The frequency of anti-HEV IgG antibodies was determined using an enzyme-linked immunoassay. Bivariate and multivariate analyses were used to assess the association between seropositivity and socio-demographic, clinical and behavioral characteristics of the Tepehuanos.
ResultsIgG antibodies against HEV were found in 5 (3.4%; 95% CI: 1.1–7.8) of 146 Tepehuanos and in 46 (31.5%; 95% CI: 24.1–39.7) of 146 control subjects (OR=0.01; 95% CI: 0.0007–0.20; P<0.000001). Bivariate analysis showed that HEV seropositivity was associated with age, consumption of meat from goat, sheep, boar, turkey and pigeon, and concrete flooring at home. However, these variables were no longer significant when analyzed by logistic regression.
ConclusionsThis is first study on the epidemiology of HEV exposure in Tepehuanos. We demonstrated serological evidence of HEV infection in this ethnic group. The seroprevalence of HEV exposure in Tepehuanos is low as compared with that found in non-Tepehuano people living in rural Durango. Further studies to determine the risk factors associated with HEV exposure in Tepehuanos are needed.
Hepatitis E virus (HEV) is a single-stranded RNA virus [1] and is the sole member of the family Hepeviridae [2]. HEV is the most common cause of viral hepatitis in developed and developing countries [3,4]. It is estimated that 20 million cases of acute hepatitis occur every year [5]. Infections with HEV are mainly food- and water-borne [4]. However, transmission of HEV can also occur by blood transfusion [4,6], and solid organ transplantation [7]. HEV is found in a variety of animal species [2,8]. Consumption of raw or undercooked meat is involved in foodborne transmission of HEV [2,8]. Infections with HEV can cause outbreaks or sporadic cases of hepatitis [5]. Acute hepatitis E is most often mild and resolves spontaneously [5]. However, HEV infection can lead to chronic hepatitis and extrahepatic manifestations such as neurological and renal disease [6]. In addition, HEV infection can lead to cirrhosis in immunocompromised patients [3], and fulminant hepatic failure [7]. Infections with HEV in pregnant women can be severe with a high rate of mortality [5,8].
Mexico is a high-risk region for HEV infection [9]. In a study of subjects from 1 to 29 years old in this country, researchers found that age, type of community, and educational level were risk factors for HEV infection [10]. The epidemiology of HEV infection in Mexican populations in general and in ethnic groups in particular has been insufficiently studied. We are not aware of any study on the seroepidemiology of HEV exposure in Tepehuanos (indigenous people living in rural communities in northwestern Mexico). Tepehuanos live in poverty and have limited sanitation. We studied Tepehuanos because the epidemiology of HEV infection in this ethnic group is largely unknown, and they live in rural communities where HEV infection might exist due to a poor sanitation and limited health services for prevention, diagnosis and treatment of infectious diseases. This study aimed to determine: (1) the seroprevalence of anti-HEV IgG antibodies in Tepehuanos in Durango, Mexico; and (2) the association between HEV seropositivity and the socio-demographic, clinical and behavioral characteristics of Tepehuanos.
2Materials and methods2.1Study design and Tepehuanos casesWe performed a case–control study of 146 Tepehuanos (cases) and 146 age- and gender-matched subjects of the general population (controls) in rural Durango, Mexico. The study was performed from 2006 to 2014 in public health care centers and hospitals (Hospital Integral del Mezquital, Hospital General de Durango, Centros de Salud de Santa Clara, Villa Montemorelos and San Dimas). Several strategies to recruit participants were used: (1) we asked communities leaders, municipal authorities, and medical authorities in clinics to provide information about the project in their settings and to invite people to participate; (2) we looked for participants at their homes; and (3) we looked for participants in hostels. Blood sampling was performed mainly in health care centers or hospitals, and in a few cases at homes and hostels. The origin of the Tepehuanos was rural communities in the Mezquital municipality (23°28′22″N 104°24′40″W) in the northern Mexican state of Durango. Inclusion criteria for the Tepehuanos were: (1) subjects of Tepehuano ethnicity; (2) 15 years and older; and (3) who accepted to participate in the survey. Gender, occupation, and socioeconomic status were not restrictive criteria for enrollment. Tepehuano ethnicity was considered to those who identify themselves as Tepehuanos and speak the Tepehuano language. In total, 62 males and 84 females were included in the study. Age in Tepehuanos varied from 15 to 89 (mean: 30.65±16.38) years. Inclusion criteria for the control subjects were: (1) non-Tepehuanos of the general population from rural Durango; (2) 15 years and older; and 3) who accepted to participate in the survey. Non-Tepehuanos control subjects were enrolled in rural communities of the municipalities of Santa Clara, Durango, and San Dimas in Durango State. Control subjects were selected at random and included 62 males and 84 females. Age in controls varied from 18 to 78 (mean: 32.23±13.76) years. Gender was similar in cases and controls (P=1.0). Age in Tepehuanos was comparable to that found in non-Tepehuanos (P=0.37 by the Student's t test). In addition, age in female cases and controls, and in male cases and controls was also comparable (P=0.061, and P=0.051, respectively by the Mann–Whitney U test).
2.2General characteristics of TepehuanosGeneral (socio-demographic, clinical, and behavioral) characteristics of the Tepehuanos were obtained by an in-person interview. The socio-demographic characteristics birthplace, age, sex, occupation, and educational and socio-economic levels were recorded. Clinical features included presence of any disease, frequent headache, history of lymphadenopathy, blood transfusion, or surgery. The obstetric history of female Tepehuanos was also obtained. Behavioral information included type of meat consumed (boar, pork, turkey, chicken, beef, lamb, goat, venison, rabbit, or other), consumption of raw or undercooked meat, consumption of ham, salami or sausages, consumption of unwashed raw vegetables or fruits, untreated water or unpasteurized milk. Furthermore, information about animal contacts, foreign traveling, frequency of eating out of home (in restaurants or fast food outlets) and type of flooring at home was obtained.
2.3Laboratory testsA blood sample was obtained from each participant. Blood was centrifuged, and serum was obtained and frozen at −20°C until analyzed. Serum samples were analyzed for detection of anti-HEV IgG antibodies by the commercially available enzyme immunoassay kit “AccuDiag™ HEV IgG ELISA” (Diagnostic Automation Inc., Woodland Hills, CA, USA). According to information provided in the kit, this immunoassay has a sensitivity of 99.8% and a specificity of 99.8%. Tests were performed according to the instructions provided by the manufacturer.
2.4Statistical analysisWe analyzed the data with the aid of the software SPSS version 20 and Epi Info version 7. We calculated the sample size using the following values: a 95% two-sided confidence level, a power of 80%, a 1:1 ratio of cases and controls, and a 6.3% outcome in unexposed group [11]. The result of this calculation was 121 cases and 121 controls. We compared the age in cases and controls using the Student's t test, and the Mann–Whitney U test. For comparison of the HEV IgG seropositivity rate in cases and controls, we used the McNemar's paired test. The association between the HEV IgG seropositivity rate and the characteristics of Tepehuanos was initially assess using the Pearson's chi-squared test or the two-tailed Fisher's exact test (for small values). Then, socio-demographic, housing, and behavioral variables of the Tepehuanos with a P value<0.05 obtained in the bivariate analysis were additionally analyzed by binary regression analysis with the Enter method. Odds ratio (OR) and 95% confidence interval were calculated, and statistical significance was considered when a P value<0.05 was obtained.
2.5Ethical aspectsAll subjects were informed about the aims, purposes, and procedures of the survey. Participants enrolled the study voluntarily and provided a written informed consent. The Ethical Committee of the Faculty of Medicine and Nutrition in Durango City, Mexico approved this study.
3ResultsIgG antibodies against HEV were found in 5 (3.4%; 95% CI: 1.1–7.8) of the 146 Tepehuanos and in 46 (31.5%; 95% CI: 24.1–39.7) of the 146 control subjects. Tepehuanos had a significantly lower seroprevalence of HEV exposure than controls (OR=0.01; 95% CI: 0.0007–0.19; P<0.000001). Stratification by sex in cases and controls showed a lower seroprevalence (P=0.00001) of HEV infection in male Tepehuanos (4/62: 6.5%; 95% CI: 0.18–15.7) than in male controls (25/62: 40.3%; 95% CI: 28.1–53.6). Female Tepehuanos showed a lower seroprevalence (P=0.000001) of HEV infection (1/84: 1.2%; 95% CI: 0.00–6.5) than female controls (21/84: 25.0%; 95% CI: 16.2–35.6). Mean age of seropositive Tepehuanos was 50.0±16.17 (range: 27–67; 95% CI: 29.92–70.08) years whereas mean age of seropositive controls was 39.91±14.58 (range: 18–69; 95% CI: 35.58–44.25) years. No difference in the mean age of HEV seropositive cases and controls was found (P=0.70).
Concerning the sociodemographic characteristics of Tepehuanos, bivariate analysis showed that seroprevalence of HEV exposure increased with age (P=0.02), whereas other characteristics including sex, occupation, educational level, and socio-economic status showed no association with HEV seropositivity.
None of the clinical characteristics of Tepehuanos including presence of any disease, frequent headache, history of lymphadenopathy, blood transfusion, or surgery, and obstetric history of female Tepehuanos showed an association with HEV seropositivity.
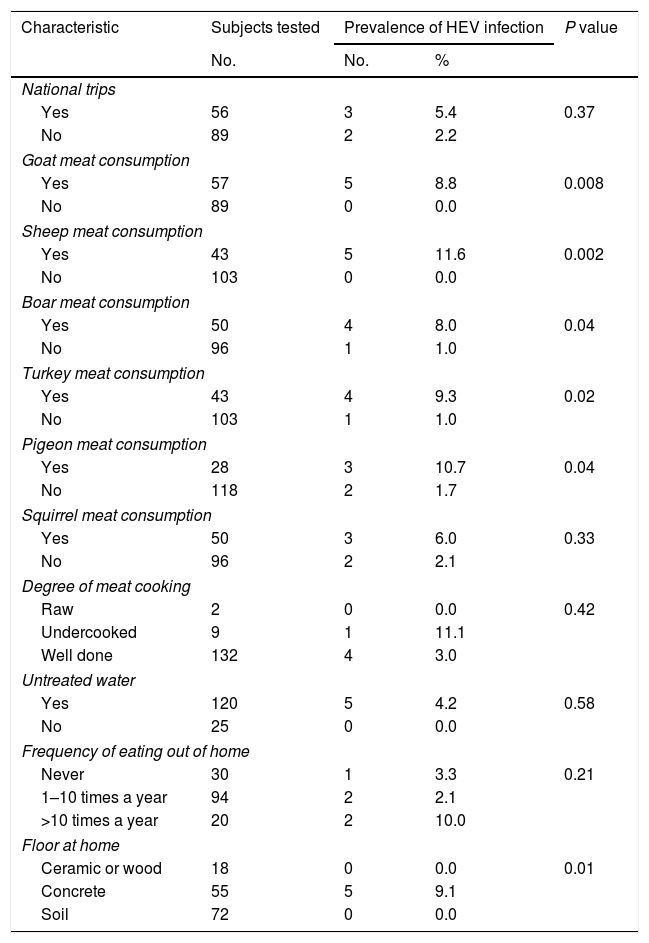
Of the behavioral characteristics of Tepehuanos, bivariate analysis showed that consumption of meat from goat, sheep, boar, turkey and pigeon were associated (P<0.05) with HEV seropositivity. In addition, bivariate analysis showed that the variable concrete flooring at home was associated (P<0.05) with HEV seropositivity. Other behavioral variables including contact with animals, consumption of raw or undercooked meat, consumption of meat other than those from goat, sheep, boar, turkey and pigeon, consumption of ham, salami or sausages, unwashed raw vegetables or fruits, untreated water or unpasteurized milk, foreign traveling, and frequency of eating out of home showed no association with HEV seropositivity. A correlation of HEV exposure and a selection of variables of Tepehuanos is shown in Table 1.
Bivariate analysis of selected variables of Tepehuanos and seropositivity to hepatitis E virus in Durango, Mexico.
| Characteristic | Subjects tested | Prevalence of HEV infection | P value | |
|---|---|---|---|---|
| No. | No. | % | ||
| National trips | ||||
| Yes | 56 | 3 | 5.4 | 0.37 |
| No | 89 | 2 | 2.2 | |
| Goat meat consumption | ||||
| Yes | 57 | 5 | 8.8 | 0.008 |
| No | 89 | 0 | 0.0 | |
| Sheep meat consumption | ||||
| Yes | 43 | 5 | 11.6 | 0.002 |
| No | 103 | 0 | 0.0 | |
| Boar meat consumption | ||||
| Yes | 50 | 4 | 8.0 | 0.04 |
| No | 96 | 1 | 1.0 | |
| Turkey meat consumption | ||||
| Yes | 43 | 4 | 9.3 | 0.02 |
| No | 103 | 1 | 1.0 | |
| Pigeon meat consumption | ||||
| Yes | 28 | 3 | 10.7 | 0.04 |
| No | 118 | 2 | 1.7 | |
| Squirrel meat consumption | ||||
| Yes | 50 | 3 | 6.0 | 0.33 |
| No | 96 | 2 | 2.1 | |
| Degree of meat cooking | ||||
| Raw | 2 | 0 | 0.0 | 0.42 |
| Undercooked | 9 | 1 | 11.1 | |
| Well done | 132 | 4 | 3.0 | |
| Untreated water | ||||
| Yes | 120 | 5 | 4.2 | 0.58 |
| No | 25 | 0 | 0.0 | |
| Frequency of eating out of home | ||||
| Never | 30 | 1 | 3.3 | 0.21 |
| 1–10 times a year | 94 | 2 | 2.1 | |
| >10 times a year | 20 | 2 | 10.0 | |
| Floor at home | ||||
| Ceramic or wood | 18 | 0 | 0.0 | 0.01 |
| Concrete | 55 | 5 | 9.1 | |
| Soil | 72 | 0 | 0.0 | |
None of the variables with P<0.05 obtained by bivariate analysis (age, consumption of meat from goat, sheep, boar, turkey and pigeon, and concrete flooring at home) showed an association with HEV seropositivity by logistic regression analysis.
4DiscussionDespite that HEV infection is responsible of morbidity and mortality worldwide, the seroepidemiology of HEV exposure has been poorly studied. Infection with HEV has been linked to fulminant disease, chronic liver damage and extrahepatic manifestations [12]. Information on the seroprevalence of and risk factors associated with HEV infection in Latin America is still limited [13]. In addition, the study of HEV infection in ethnic groups is scanty. This study aimed to determine the seroprevalence of HEV exposure and the sociodemographic, clinical, and behavioral characteristics of Tepehuanos associated with HEV exposure. We found a low (3.4%) seroprevalence of HEV infection in Tepehuanos, and this seroprevalence was significantly lower than that (31.5%) found in an age- and gender-matched control group of non-Tepehuano people living in rural communities in Durango State. The low seroprevalence of HEV exposure found in Tepehuanos was unexpected because they have a number of known risk factors for infection with HEV including consumption of untreated water [11], meat [8], and raw vegetables [14]. In addition, Tepehuanos use to hunt wild animals for obtaining meat, and consumption of meat from some wild animals has been associated with HEV infection. For instance, consumption of wild boar was associated with acute hepatitis E in Germany [14]. Infections with HEV have been demonstrated in deer, rabbit [15], rat [16], and hare [17]. Tepehuanos also rise farm animals, and contact with farm animals was associated with HEV infection among hunters in Poland [18].
The seroprevalence of HEV infection found in Tepehuanos is comparable with a low (6.7%) seroprevalence of HEV infection found in 150 Mennonites in Durango, Mexico [19]. The low HEV seroprevalence in Tepehuanos and Mennonites might be due to a low contamination of the water they consume. Furthermore, the seroprevalence of HEV infection found in Tepehuanos is similar to the one (5.7%) found in pregnant women in rural Durango [20]. In contrast, the seroprevalence found in Tepehuanos is lower than the one (36.6%) reported in general population in rural Durango [21]. However, this comparison should be interpreted with care since differences in age and gender in subjects among the studies exist. It is not clear why Tepehuanos have a low seroprevalence of HEV infection. It is possible that contamination of drinking water and environment by HEV is low in the mountainous region where Tepehuanos live. Some habits and housing conditions of Tepehuanos may help them to avoid HEV infection; for instance, they usually well cook their meals including meat; and they do not have a water pipe system that provides them water at home from a common water well. Contamination of a water well in a community may lead to HEV dissemination to houses through the water pipe system. On the other hand, control subjects of the present study were recruited in communities with water pipe systems that supply them water at home. It is likely that differences in the water supply system among cases and controls may have contributed for the statistically significant difference in the seroprevalence of HEV infection.
In the Americas context, the seroprevalence of HEV exposure in Tepehuanos is similar to those reported in rural populations in central Brazil (3.4%) [22], Bolivia (6.3%) [23], and Amerindians in Venezuela (5.4%) [24], but is lower than the 12.9% seroprevalence found in rural settlements in the Amazon Basin of Brazil [25], and the 17% seroprevalence reported in Araucanian Indians in Chile [26]. It is not clear why these population groups have a higher seroprevalence of HEV than Tepehuanos. Although these populations groups live in rural areas, differences in the seroprevalences could be due to differences in age, water supply, and cooking habits among groups.
We searched for risk factors associated with HEV seropositivity in Tepehuanos. Bivariate analysis showed that age, consumption of meat from goat, sheep, boar, turkey and pigeon, and concrete flooring at home were associated with HEV seropositivity. However, further analysis using logistic regression showed no association between these variables and HEV seropositivity. Further studies to determine the risk factors associated with HEV infection in Tepehuanos should be conducted. In a study of general population in rural Durango, associations between HEV exposure and increasing age, consumption of untreated water, and availability of water at home were found [21].
This study had the limitation of a very low number of seropositive cases among Tepehuanos. This fact impacted on the power to obtain statistical significance of risk factors for HEV infection in the logistic regression analysis. In addition, samples were tested with a single immunoassay, and discrepancies between anti-HEV-IgG prevalences using two immunoassays have been reported [27,28].
5ConclusionsThis is first study on the epidemiology of HEV exposure in Tepehuanos. We demonstrated serological evidence of HEV infection in this ethnic group. The seroprevalence of HEV exposure in Tepehuanos is low as compared with that found in non-Tepehuanos people living in rural Durango. Further studies to determine the risk factors associated with HEV exposure in Tepehuanos are needed.
AbbreviationsHEV hepatitis E virus immunoglobulin G odds ratio confidence interval Statistical Package for the Social Sciences
The authors have no conflicts of interest to declare.
This study was financially supported by Juárez University of Durango State, Mexico. The sponsor had no role in collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the manuscript for publication.