The purpose of this study was to confirm whether hepatitis B virus (HBV) infection and the levels of liver enzymes would increase the risk of prediabetes and diabetes mellitus (DM) in China.
Materials and methodsA total of 10,741 individuals was enrolled in this prospective cohort study. Cox regression analysis was used to calculate the Hazard ratios (HRs) to evaluate the relationships between HBV infection and the risk of DM and prediabetes. Decision trees and dose response analysis were used to explore the effects of liver enzymes levels on DM and prediabetes.
ResultsIn baseline population, HBV infection ratio was 5.31%. In non-adjustment model, the HR of DM in HBV infection group was 1.312 (95% CI, 0.529–3.254). In model adjusted for gender, age and liver cirrhosis, the HR of DM in HBV infection group were 1.188 (95% CI, 0.478–2.951). In model adjusted for gender, age, liver cirrhosis, smoking, drinking, the HR of DM was 1.178 (95% CI, 0.473–2.934). In model further adjusted for education, family income and occupation, the HR of DM was 1.230 (95% CI, 0.493–3.067). With the increases of levels of Alanine aminotransferase (ALT), Aspartate aminotransferase (AST) and Gamma-glutamyl transferase (GGT), the risk of prediabetes was gradually increasing (Pnon-linearity<0.05). There were dose-response relationships between ALT, GGT and the risk of DM (Pnon-linearity<0.05).
ConclusionsHBV infection was not associated with the risk of prediabetes and DM. The levels of liver enzymes increased the risk of prediabetes and DM.
Diabetes mellitus (DM) is a major public health problem and one of the fastest growing diseases in the world. The prevalence of DM worldwide in adults was 6.4%, with approximately 285 million patients. By 2030, the number of adults with DM in developing countries will increase by 69% [1]. China has the largest number of patients with DM in the world. In 2010, nearly 92.4 million adults in China suffered from DM, and the prevalence of DM was 11.6% [2,3]. At the same time, prediabetes is an important indicator to prevent DM and may develop into DM [4]. Prediabetes has become a public health problem with increasing concern in China. According to a national survey in 2013, 148.2 million adults suffered from prediabetes, and the prevalence of prediabetes in adult was 35.7% in China [2].
Similar to DM, hepatitis B virus (HBV) infection has also attracted much attention. HBV infection affected more than 2 billion people worldwide, of whom 350 million were chronic carriers of HBV, and more than 780,000 people died every year from diseases caused by HBV [5,6]. In China, the prevalence of HBsAg-positive in population aged 1–59 was 7.2% and in newborn born in 1992–2005 was 2.1% [7,8]. In recent years, accumulating studies have explored the association between HBV infection and the risk of DM, but this relationship remains unclear due to conflicting results illustrated in previous studies. A cross-sectional study conducted in North America in 2015 indicated that HBV infection related to an increased risk of DM [9], while a cohort study found that the risk of DM in patients with HBV infection did not increase [10].
The metabolism of glucose is mainly carried out in the liver. Impaired liver function can affect normal glucose metabolism, and even lead to impaired glucose tolerance or hepatic DM. Liver enzymes, including ALT, AST and GGT, are essential for evaluating liver function [11]. Some studies have shown that ALT and GGT can be used as indicators to predict DM [12,13]. And AST was a risk factor for DM and IFG [14]. Although several studies have focused on the association between liver enzymes and the risk of DM, most studies were based on hospitals and lacked prospective cohort studies with large sample groups. Moreover, in China, a country with a large number of patients with HBV infection and DM, there is still a lack of prospective cohort studies based on large sample populations. Therefore, we examined the effects of HBV infection and liver enzymes on the risk of DM and prediabetes in Jinchang cohort in China.
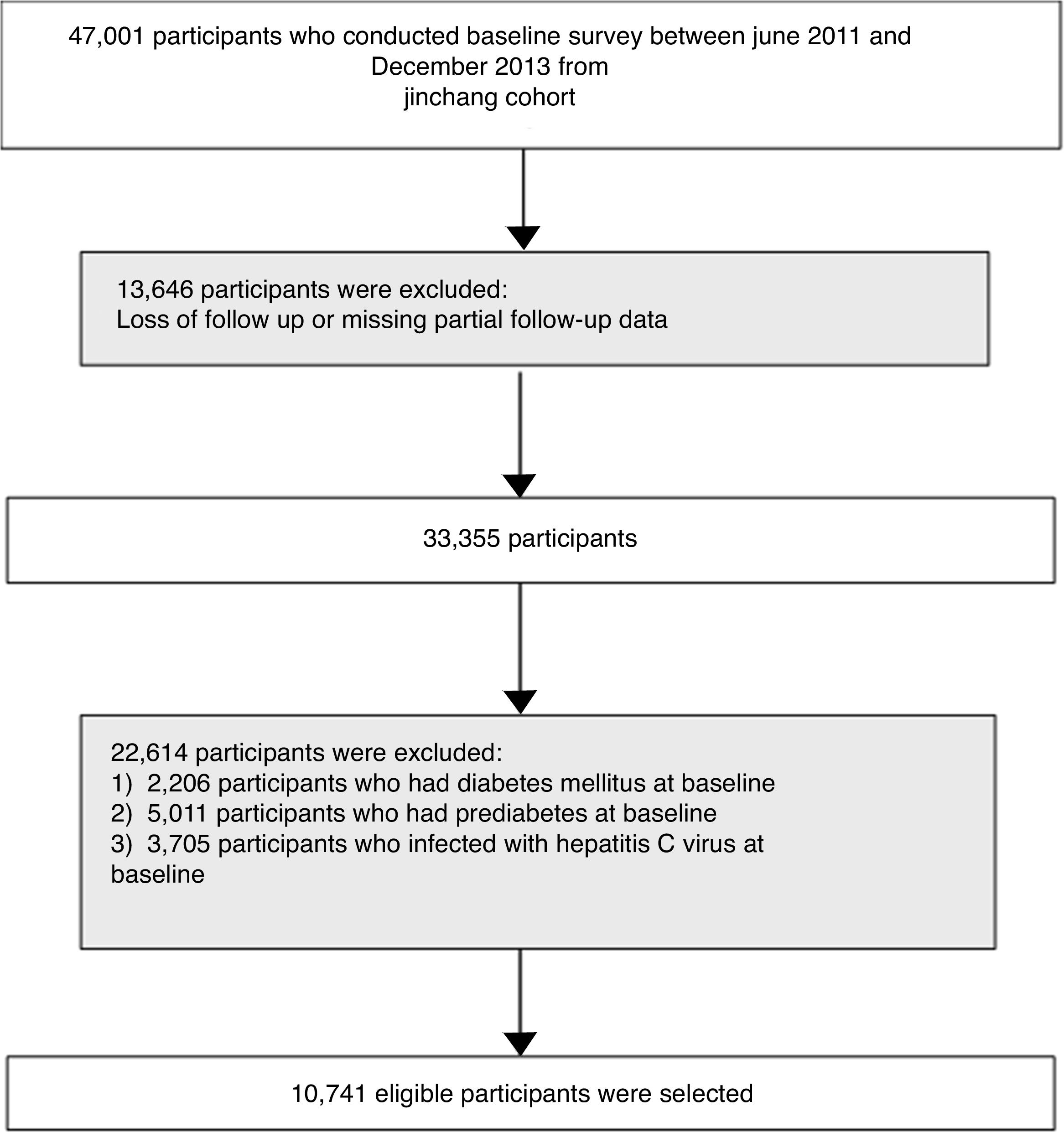
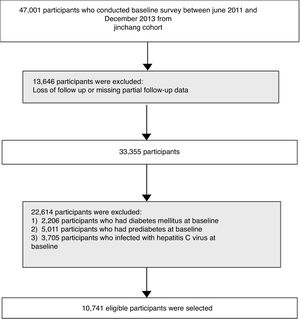
2Materials and methods2.1Study populationThe flow diagram of participants selection is shown in Fig. 1. This study was based on data from the China Metal Exposure Workers Cohort Study (Jinchang Cohort). Briefly, the baseline survey of the Jinchang Cohort Study began in June 2011 and ended in December 2013. And the follow-up survey was completed in December 2015. A total of 33,355 participants was followed up for an average follow-up of 2.2 years. In follow-up data, patients with hepatitis C infection, DM and prediabetes in baseline were excluded, a total of 10,741 participants was finally included. All participants had signed written informed consent and the study protocol was approved by the Ethical Committees of Workers’ Hospital of the Jinchang industry and the Ethical Committees of the Public Health School of Lanzhou University.
2.2Data collectionWe collected several types of data in this study, including questionnaire data obtained from in-person interviews and clinical data from physical and biochemical examinations. The physical examination was conducted by clinicians at the Worker's Hospital of the Jinchang Industry after completing the in-person interview. Fasting Plasma Glucose (FPG) testing was carried out by professionals from the hospital. All participants were measured for FPG once at the beginning of the baseline and again at the end of follow-up. A total of 12ml of venous blood was collected from each physical examination individual and place into three 4ml EDTA anticoagulant tubes. The blood samples of each individual were kept in a special plastic bag with a laboratory checklist, ID number and name. FPG (YZB/Shanghai, 1801-40-2010) was measured in the morning by automatic biochemical analyzer (Hitachi, 7600-020, Kyoto, Japan). Other biochemical examinations were also measured by automatic biochemical analyzer (Hitachi, 7600-020, Kyoto, Japan), including HBsAg (YZB/Shanghai, 2130-2012), ALT (YZB/Shanghai, Shanghai, China, 0586-40-2009), AST (YZB/Shanghai, 0588-40-2009) and GGT (YZB/Shanghai, 1798-40-2014).
2.3Definitions of diagnosesDM and prediabetes were defined according to the American Diabetes Association 2019 criteria [15]. DM was defined as a self-reported diagnosis or the presence of suspected DM symptoms and FPG≥7.0mmol/L, and self-reported patients were required to provide medicals certificate previously issued by medical professionals. Prediabetes was defined as participants who have no DM but have FPG levels of 5.6–6.9mmol/L. HBV infection was diagnosed by doctors of the Workers’ Hospital of the Jinchang industry based on blood tests of five indicators of HBV. HBsAg-positive were defined as HBV infection. ALT, AST and GGT were measured by ultraviolet and visible spectrophotometry, ALT>40U/L, AST>40U/L, and GGT>50U/L was abnormal.
2.4Statistical analysisOur research is a prospective cohort study. Statistical data analysis was performed using the SPSS (version 22.0; SPSS, Chicago, Illinois, United States) and STATA (version 12.0; StataCorp LP, College Station, TX, USA). All analyses were two-sided, and the test level was based on α=0.05. The continuous data were expressed as mean±standard deviation (SD) and categorical data were expressed as number or percent. The incidence of categorical variables was tested by chi-square test. Cox regression analysis was used to calculate the Hazard ratios (HRs) before and after adjustment to quantitatively evaluate the relationship between HBV infection and risk of DM and prediabetes. Decision trees were used to analyze the effects of liver enzymes and other factors on DM and prediabetes. The dose-response relationship between liver enzymes and the risk of DM and prediabetes mellitus were analyzed by stratified analysis.
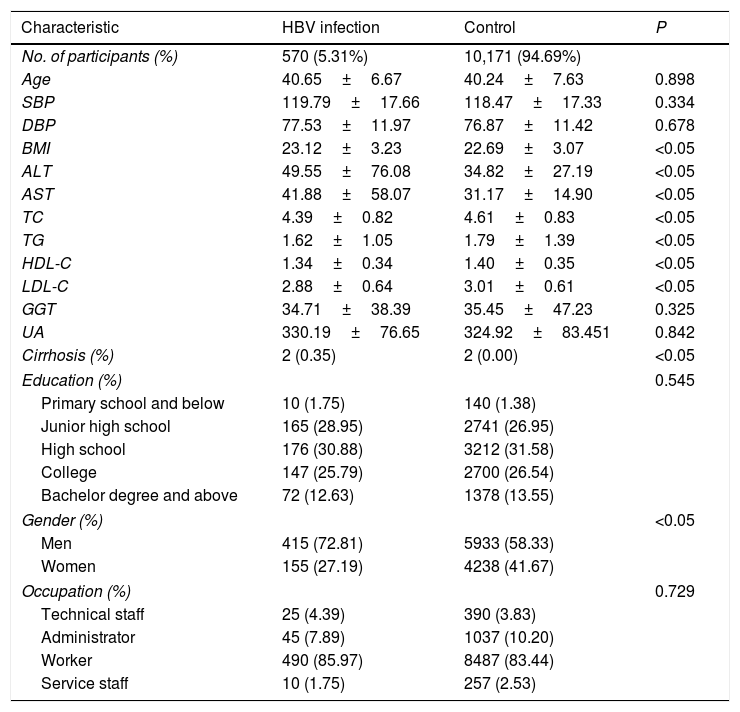
3ResultsThe baseline demographic data of 10,741 individuals in Jinchang cohort are shown in Table 1. In baseline population, HBV infection ratio was 5.31%. The mean age (SD) of the HBV infection group was 40.65±6.67 and the control group was 40.24±7.63. BMI, ALT, AST, TC, TG, HDL-C, LDL-C and cirrhosis were significantly different between the HBV infection group and control group (P<0.05). The education background of the individuals in this cohort was mainly middle school, high school and college degrees. The main occupational composition of this cohort was workers, followed by administrators.
Demographic characteristics of Jinchang Cohort baseline population in cohort analysis.
| Characteristic | HBV infection | Control | P |
|---|---|---|---|
| No. of participants (%) | 570 (5.31%) | 10,171 (94.69%) | |
| Age | 40.65±6.67 | 40.24±7.63 | 0.898 |
| SBP | 119.79±17.66 | 118.47±17.33 | 0.334 |
| DBP | 77.53±11.97 | 76.87±11.42 | 0.678 |
| BMI | 23.12±3.23 | 22.69±3.07 | <0.05 |
| ALT | 49.55±76.08 | 34.82±27.19 | <0.05 |
| AST | 41.88±58.07 | 31.17±14.90 | <0.05 |
| TC | 4.39±0.82 | 4.61±0.83 | <0.05 |
| TG | 1.62±1.05 | 1.79±1.39 | <0.05 |
| HDL-C | 1.34±0.34 | 1.40±0.35 | <0.05 |
| LDL-C | 2.88±0.64 | 3.01±0.61 | <0.05 |
| GGT | 34.71±38.39 | 35.45±47.23 | 0.325 |
| UA | 330.19±76.65 | 324.92±83.451 | 0.842 |
| Cirrhosis (%) | 2 (0.35) | 2 (0.00) | <0.05 |
| Education (%) | 0.545 | ||
| Primary school and below | 10 (1.75) | 140 (1.38) | |
| Junior high school | 165 (28.95) | 2741 (26.95) | |
| High school | 176 (30.88) | 3212 (31.58) | |
| College | 147 (25.79) | 2700 (26.54) | |
| Bachelor degree and above | 72 (12.63) | 1378 (13.55) | |
| Gender (%) | <0.05 | ||
| Men | 415 (72.81) | 5933 (58.33) | |
| Women | 155 (27.19) | 4238 (41.67) | |
| Occupation (%) | 0.729 | ||
| Technical staff | 25 (4.39) | 390 (3.83) | |
| Administrator | 45 (7.89) | 1037 (10.20) | |
| Worker | 490 (85.97) | 8487 (83.44) | |
| Service staff | 10 (1.75) | 257 (2.53) | |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein; TC, serum total cholesterol; TG, triglyceride; UA, uric acid.
Notes: P value by t test for continuous variables and chi-square test for categorical variables. Data are means (standard deviation), medians (interquartile range) or percentages.
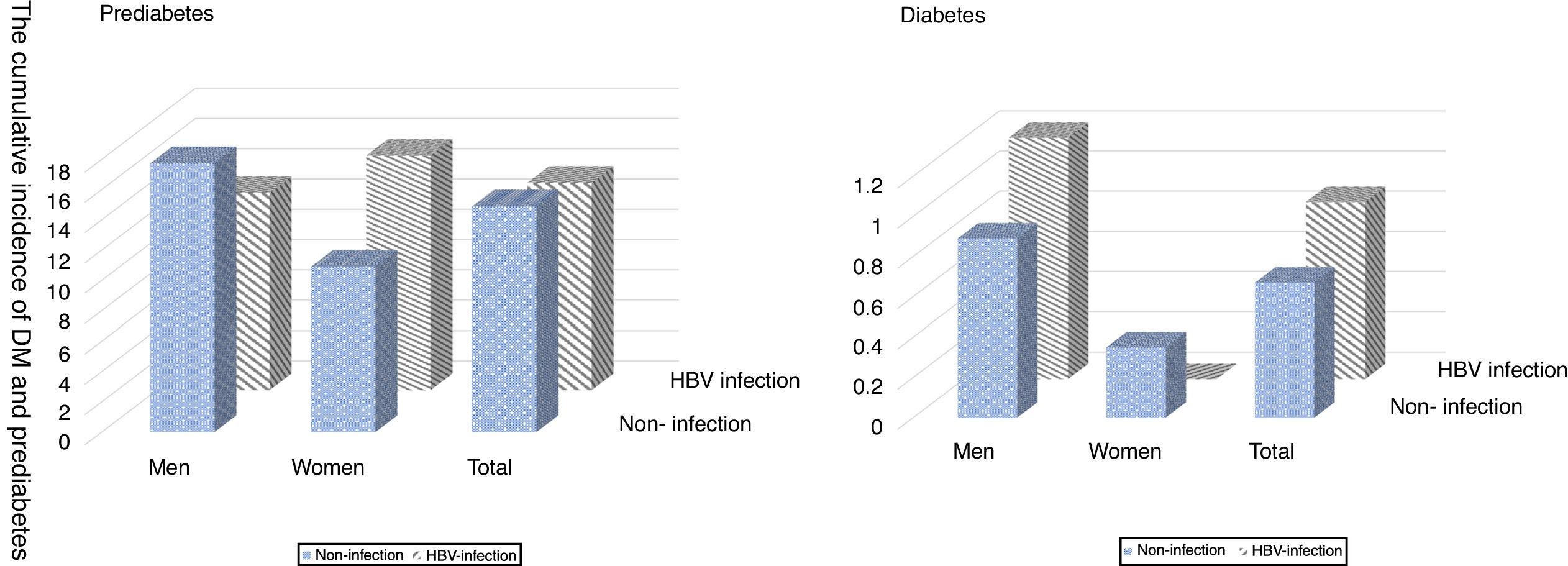
The cumulative incidence of DM and prediabetes in this cohort are presented in Fig. 2, the incidence of prediabetes was 13.68% in individuals with HBV infection and 14.89% in individuals without infection. In men group, the incidence of prediabetes in non-HBV infection group was statistically higher than HBV infection (17.73% vs. 13.01%, P<0.05). But no significant difference was found in women group. Compared to participants without HBV infection, the incidence of DM in men, women, and the total population were not statistically significant, which was 0.89%, 0.35% and 0.67% respectively.
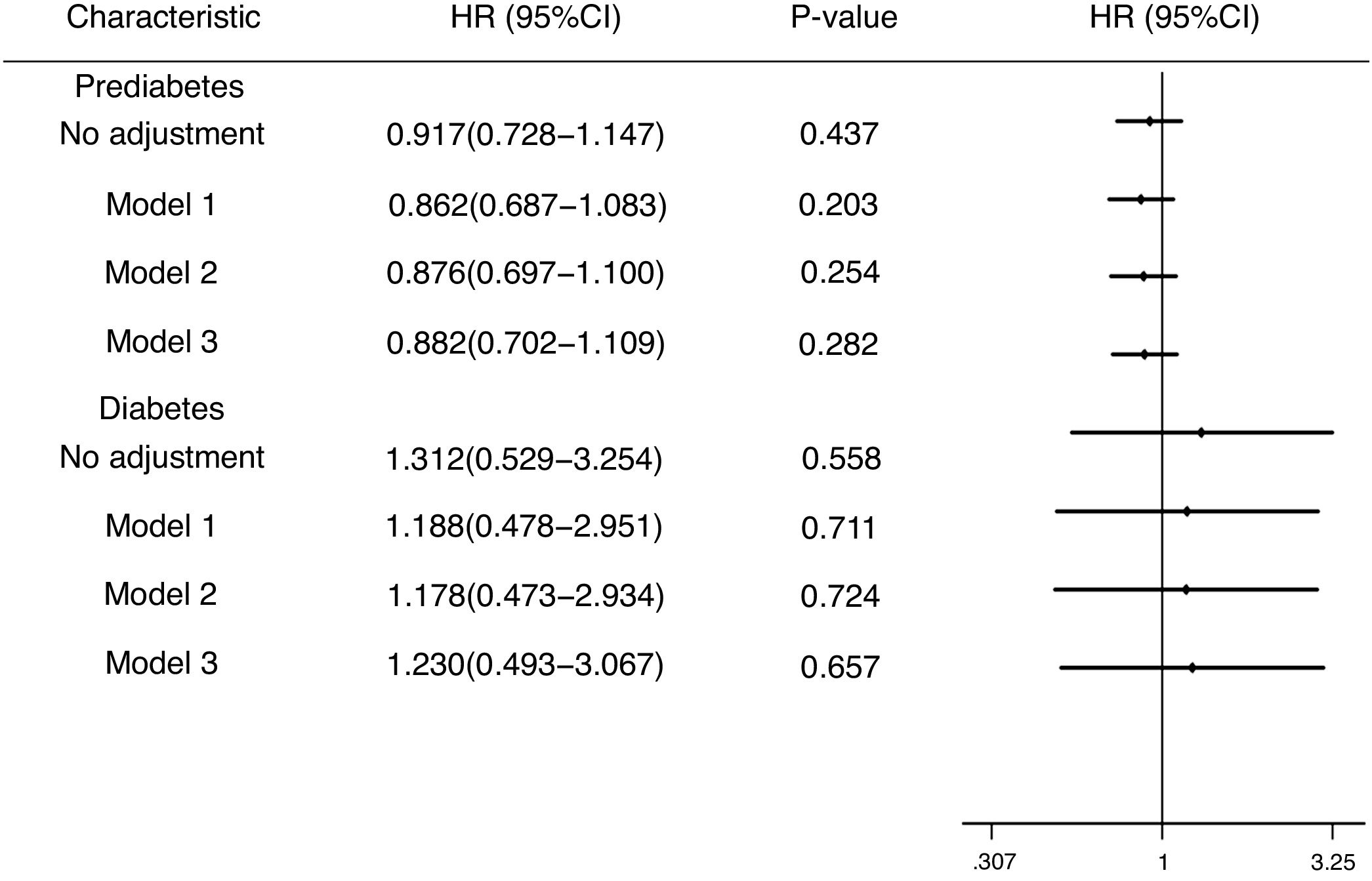
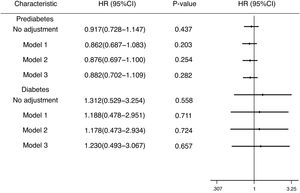
Fig. 3 expresses the HRs of known risk factors for incidence of DM and prediabetes in HBV infection group. Possible confounding variables as covariates were included to COX regression analysis. In no adjusted model, compared to individuals without HBV infection, the HR of DM in HBV infection group was 1.312 (95% CI, 0.529–3.254). In model adjusted for gender, age and cirrhosis, the HR of DM in HBV infection group were 1.188 (95% CI, 0.478–2.951). In model adjusted for gender, age, cirrhosis, smoking, drinking, the HR of DM in HBV infection group was 1.178 (95% CI, 0.473–2.934). In model further adjusted for education, family income and occupation, the HR of DM in HBV infection group was 1.230 (95% CI, 0.493–3.067). However, these results were not statistically significant. In prediabetes group, in no adjusted model, the HR was 0.917(95% CI, 0.728–1.147). In model 1, the HR in prediabetes group was 0.862(95% CI, 0.687–1.083). In mode 2, the HR was 0.876 (95% CI, 0.697–1.100). In model 3, the HR of prediabetes in HBV infection group was 0.882 (95% CI, 0.702–1.109). Similar to DM, the above results were not statistically significant.
The hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of diabetes mellitus and prediabetes in differing populations. Model 1: Adjusted for gender, age, cirrhosis; Model 2: Adjusted for gender, age, cirrhosis, smoking, drinking; Model 3: Adjusted for gender, age, cirrhosis, smoking, drinking, education, family income, occupation.
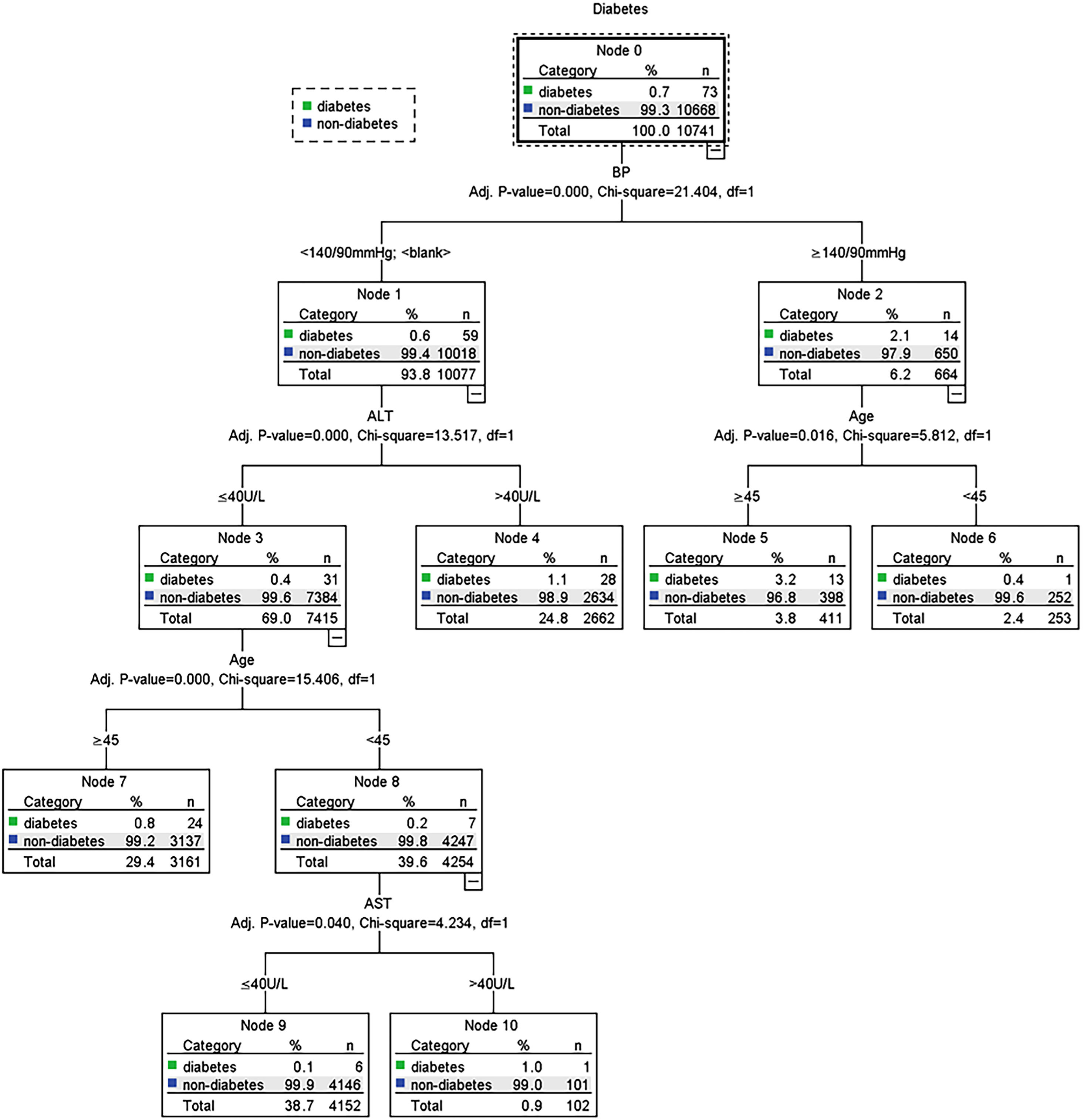
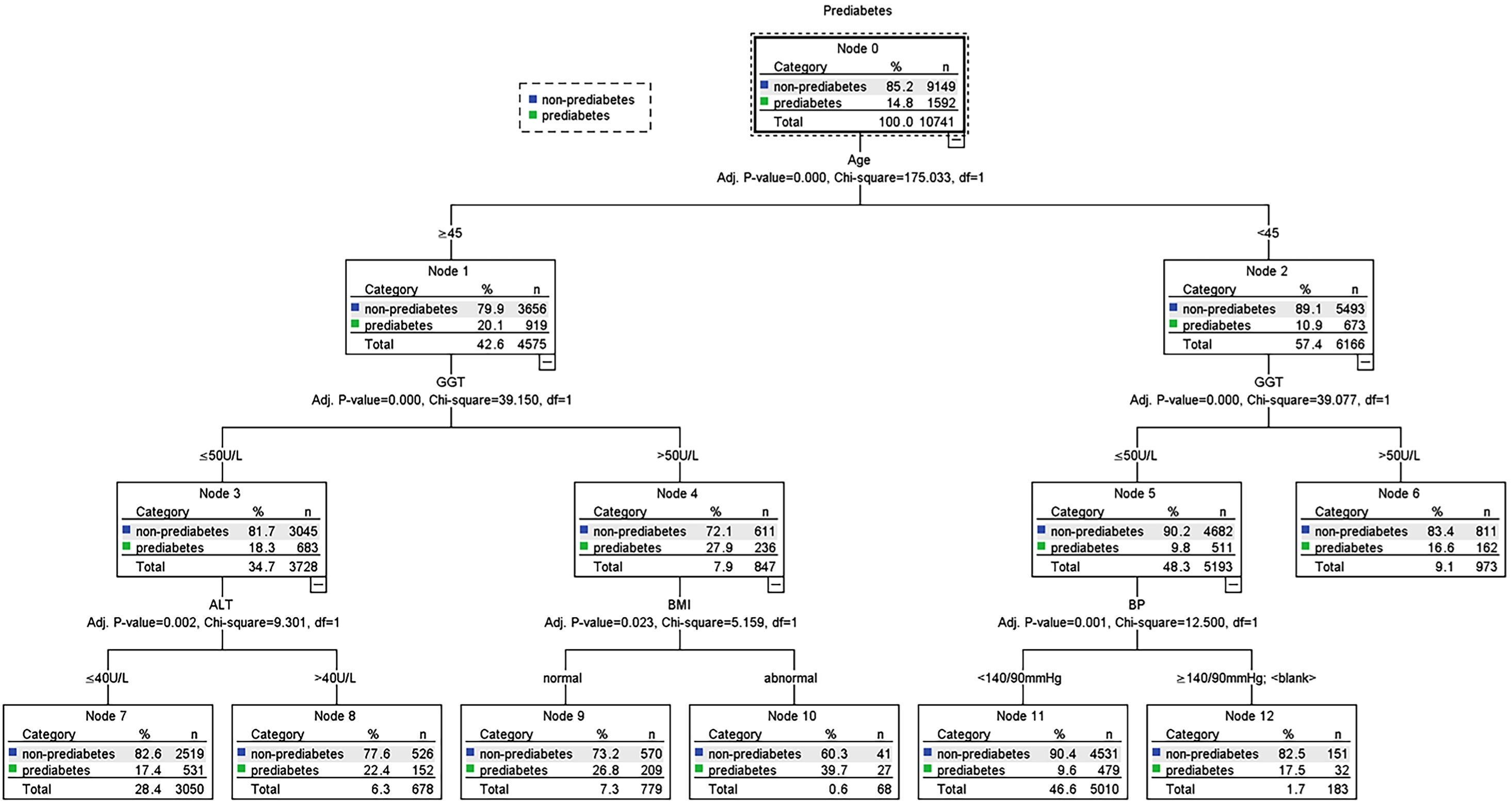
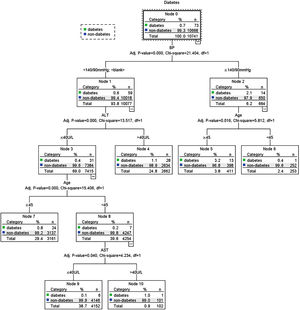
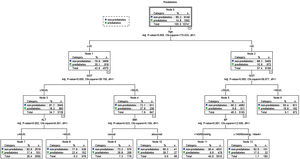
Possible risk factors of DM and prediabetes along with liver enzymes were included in the decision tree model. Fig. 4 shows the influencing factors of prediabetes in Jinchang cohort population. In the decision tree model, age was the most important factor in prediabetes incidence. BMI and BP were vital for prediabetes incidence. ALT and GGT also influenced the prediabetes incidence, these results were statistically significant (P<0.05). Fig. 5 shows the influencing factors of DM in Jinchang cohort. BP was the most important factor in DM incidence. Age, ALT and AST were important factors for DM incidence (P<0.05).
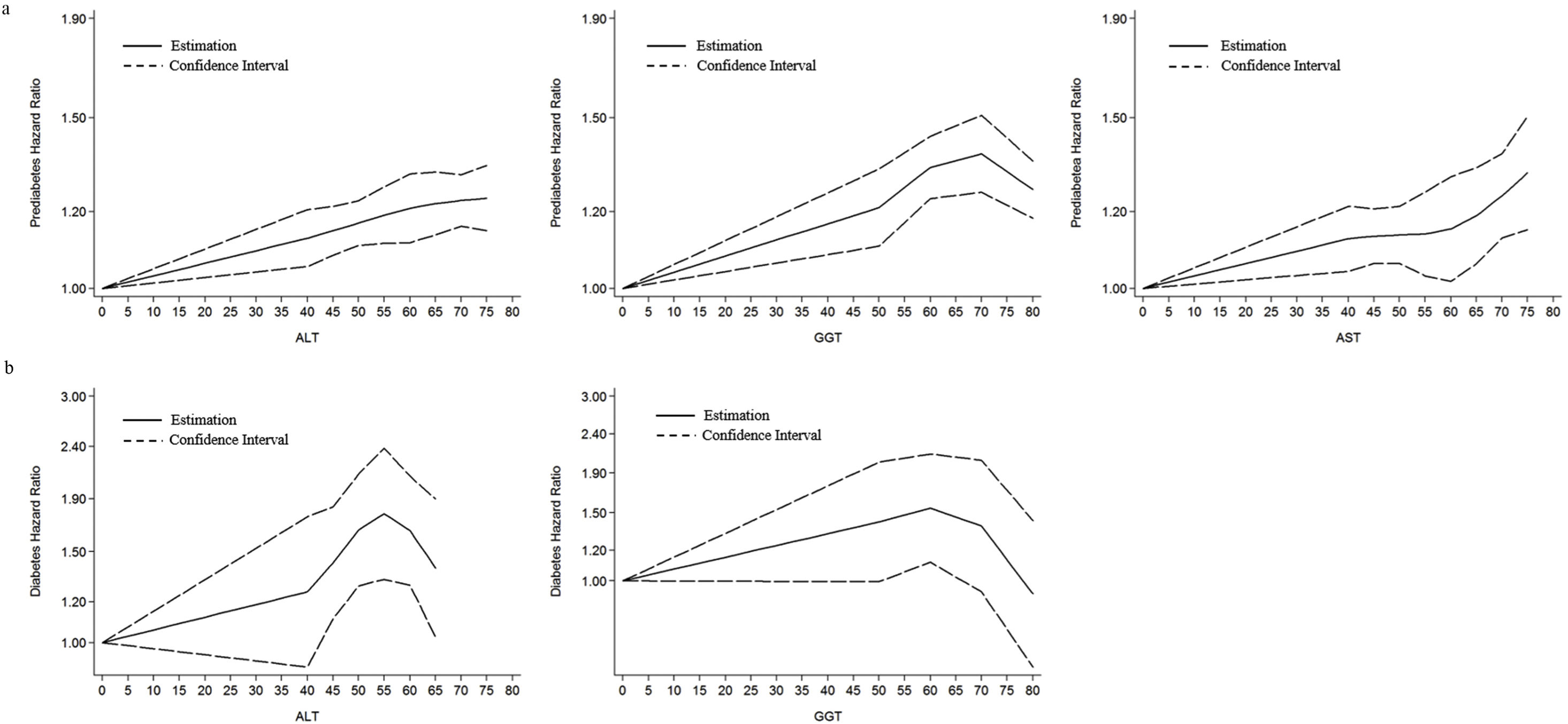
Dose-response relationship between ALT, AST and GGT and the risk of prediabetes in Jinchang cohort population are shown in Fig. 6a, as the levels of ALT, AST and GGT increased, the risk of DM increased gradually, and there was a certain positive nonlinear correlation (Pnon-linearity<0.05). Fig. 6b shows the results of the analysis of the dose-response relationship between ALT, GGT and the risk of DM. In our cohort, with the increases of the levels of ALT and GGT, the risk of DM increased, with a slight decrease in ALT values of 55 and GGT values of 60. Overall, ALT, GGT and risk of DM were positively nonlinear dose-response (Pnon-linearity<0.05).
4DiscussionIn this prospective cohort study, we did not find that HBV infection could increase the risk of prediabetes and DM. We found there was no significant difference in the incidence of prediabetes and DM with HBV infection, compared with non-HBV infection. These results indicated that HBV infection may not be a risk factor for the DM among Jinchang cohort in China. In addition, our study demonstrated that the liver enzymes were important factors in the incidence of prediabetes and DM, and there were dose-response relationships between them.
The association between HBV infection and DM has been controversial. In a 10-year cohort study of 1233 people, HBV infection did not increase the incidence of DM compared with the control group, and HBV carrier was not associated with the incidence of DM [16]. Similarly, a cohort study based on Alaska native persons for more than 20 years showed that HBV infection has no effect on DM development [10]. However, a cross-sectional study conducted in Korea in 2017 indicated that the prevalence of DM in HBV infection was higher than that with non-HBV infection [17]. A study in Hong Kong discovered that HBV carrier in pregnant women can lead to DM [18]. However, these positive studies have ignored that HBV infection can cause liver damage, especially cirrhosis. The liver plays an important role in glucose homeostasis, in the case of liver disease, glucose homeostasis damage and DM may be prone to occur [19]. Therefore, the association between HBV infection and DM may be due to HBV-associated liver disease rather than the HBV itself [20,21]. In this study, the number of patients with cirrhosis was small, only 4 cases, and we adjusted for cirrhosis in the COX regression to avoid this bias. In addition, almost all of these studies were retrospective case-control studies or cross-sectional studies, and these studies may be biased. This prospective cohort study based on a large sample population to proof the relationship between HBV infection and prediabetes and DM, and the results demonstrated that HBV infection did not increase the risk of prediabetes and DM.
ALT, AST, and GGT are generally considered to be indicators of liver damage due to steatosis and inflammatory responses [11,14,22]. Elevated levels of serum AST, ALT and GGT reflect excessive deposition of fat in the liver. Excessive lipid accumulation in hepatocytes leads to hepatic insulin resistance, which leads to DM [23]. A meta-analysis in 2014 have found that GGT has a strong correlation with DM [24]. A cross-sectional study included 96 patients also found that GGT increased the risk of DM [25]. A study suggested that ALT increased the risk of DM, with a HR (95% CI) of 1.96 (1.15–3.33) [26]. Liu et al. [27] reported that ALT increased the prevalence of DM which was 2.99 times the risk of normal people. A cohort study in Guangzhou, China presented that ALT affected the development of DM, while AST did not affect DM [28]. However, Miyatake et al. suggested that AST also had an effect on blood glucose elevation [29].
These studies focused on whether liver enzymes were associated with DM, however, they did not notice the relationship between liver enzymes and prediabetes. Additionally, previous studies did not analyze dose-response relationship between liver enzymes and risk of DM and prediabetes. Our study made up for this deficiency and found that ALT, GGT were important factors in DM development. Moreover, these three liver enzymes are associated with the development of prediabetes. As the levels of liver enzymes increased, the risk of prediabetes and also increased. The risk of DM increased with the levels of ALT and GGT, and showed a first increase and then a decrease, which might be related to changes in behavioral factors in patients with liver enzymes that were too high in this population.
In conclusion, we found that HBV infection was not associated with the risk of prediabetes and the risk of DM in this large population study. HBV infection did not increase the risk of DM and of prediabetes. The levels of liver enzymes increased the risk of prediabetes and DM. Our studies add to the growing body of evidence suggesting that HBV infection is not an independent risk factor for DM and liver enzymes are important factors that affect DM development and prediabetes.AbbreviationsALT
alanine aminotransferase
ASTaspartate aminotransferase
BMIbody mass index
BPblood pressure
DBPdiastolic blood pressure
DMdiabetes mellitus
FPGfasting plasma glucose
GGTgamma-glutamyl transferase
HBVhepatitis B virus
HDL-Chigh-density lipoprotein
HRhazard ratio
IFGimpaired fasting glucose
LDLlow-density lipoprotein
SBPsystolic blood pressure
TCserum total cholesterol
TGtriglyceride
UAuric acid
FundingThis work was supported by Belt and Road Special Project of Lanzhou University (2018ldbrzd008), and National Natural Science Foundation of China (no. 81673248), and National Major Infectious Disease Project-Follow-up Study of High-risk Population Cohort of Liver Cancer in Gansu Province (no. 2018ZX10732202). All sponsors provided assistance during the maintenance of this work.
Conflict of interestThe authors have no conflicts of interest to declare.
We thank all the participants for their collaboration in this study and the professors at the Key Laboratory of Preclinical Study for New Drugs of Gansu Province and Institute of Epidemiology and Biostatistics, School of Public Health of Lanzhou University for their efforts in the collection and management of epidemiologic data. In addition, the authors also appreciate the Jinchuan Nonferrous Metals Corporation for their cooperation.