Since 2004, pegylated interferon (P-IFN) in combination with ribavirin has become the optimal choice of therapy for chronic hepatitis C virus (HCV) infection. IFN α-2b suppresses HCV replication and restores elevated serum aminotransferase levels, leading to improvements in the histological changes in the livers of patients with chronic hepatitis C.1 Unfortunately, P-IFN has several adverse effects, including pneumonitis. This complication has been reported in the treatment of malignant diseases and CHC.2 We report a patient with interstitial pneumonitis thought to be caused by an IFN-based treatment in an unusual scenario of a patient with HCV-related Child-Pugh stage A cirrhosis, who experienced dyspnea, fever, and cough after 12 months of treatment with P-IFN α-2b. Her lung injury and pulmonary symptoms did not disappear despite discontinuation of IFN and the administration of corticosteroid. We concluded that the patient developed a fatal interstitial pneumonitis associated with P-INF α-2b therapy.
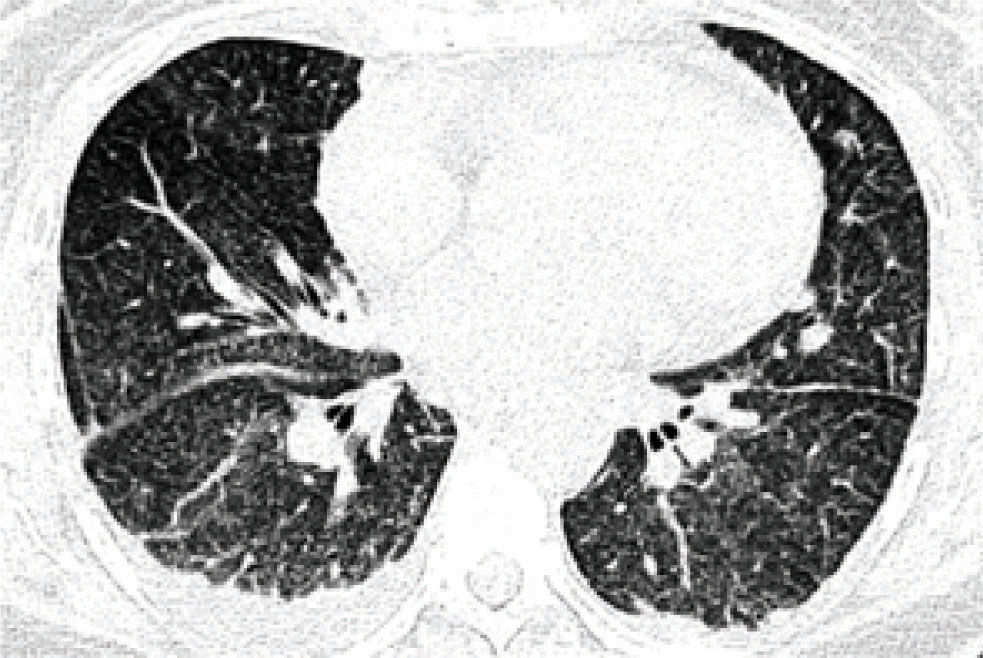
A 43-year-old woman with cirrhosis of the liver secondary to chronic infection with hepatitis C virus (HCV) genotype 1b was treated with a combination of pegylated interferon (P-IFN) α-2b (120, µg/week) plus ribavirin (800 mg/day) for the HCV infection. The patient responded slowly, achieving an undetectable viral load after six months. After 12 months of treatment, she began to experience an insidious generalized malaise, fatigue, progressive dyspnea, productive hyaline cough, occasional hemoptysis, fever (39 °C), and chills. On auscultation, crepitant rales were heard. Expectoration and blood cultures were negative. Table I shows the laboratory test results throughout her hospitalization. Chest radiography (Figure 1) showed images compatible with interstitial infiltration in both lungs. High-resolution computed tomography of the thorax (Figure 2andFigure 3) revealed a diffusely distributed, patchy, ground-glass opacity with a mosaic pattern, nodules, interstitial infiltration, and pleural effusion in both lungs.
Laboratory findings during hospitalization.
| Time (days) | 1 | 4 | 7 | 10 | 13 | 16 | 19 |
|---|---|---|---|---|---|---|---|
| WBC (cells/μL) | 2,900 | 6,500 | 8,100 | 6,800 | 4,500 | 7,300 | 3,200 |
| PLT (cells/μL) | 7,000 | 5,000 | 4,000 | 3,000 | 6,000 | 3,000 | 6,000 |
| PT (s) | 11.2 | 11.3 | 12.3 | 19.6 | 17.3 | 14 | 15.4 |
| TB (mg/dL) | 1.1 | 1.2 | 2.2 | 5.8 | 12.2 | 17.2 | 26.7 |
| DB (mg/dL) | 0.4 | 0.6 | 1.2 | 4.0 | 8.4 | 12.7 | 18.8 |
| ALT (U/L) | 67 | 65 | 63 | 1,144 | 942 | 168 | 221 |
| AST (U/L) | 80 | 122 | 228 | 4,167 | 2,250 | 275 | 696 |
| ALP (U/L) | 329 | 298 | 472 | 560 | 463 | 304 | 157 |
| γ-GTP (U/L) | 363 | 385 | 415 | 379 | 325 | 193 | 117 |
| LDH (U/L) | 351 | 324 | 775 | 4,665 | 1,575 | 875 | 1,117 |
| DD (ng/mL) | 9,803 | 5,079 | 4,298 | 12,187 | 9,327 | 8,157 | 8,401 |
| Fibr (mg/dL) | 255 | 229 | 200 | 165 | 0 | 89 | 150 |
| CRP Mg/L | 68 | 82 | 96 | 136 | 94.2 | 62 | 85.8 |
| Ammonia (μg/dL) | 86 | 104 |
WBC = white blood cells, PLT = platelets, PT = prothrombin time, TB = total bilirubin, DB = direct bilirubin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, ALP = alkaline phosphatase, γ-GTP = gamma glutamyl transpeptidase, LDH = lactate dehydrogenase, DD = D dimer, Fibr = fibrinogen, RCP = C reactive protein
The patient was diagnosed with pulmonary failure resulting from interstitial pneumonitis and received mechanical ventilation support and steroid therapy with methylprednisolone (one dose of 125 mg) and prednisone (50 mg per day) for 12 days, with gradual reduction until withdrawal. Refractory thrombocytopenia persisted despite multiple platelet transfusions. The patient later developed ventilator-dependent lung injury, pulmonary infection, compartmental abdominal syndrome, and sepsis. Although hemodynamic, pulmonary, and antibiotic supports were given, the patient continued to deteriorate and developed severe progressive cholestatic liver dysfunction. She received plasmapheresis for five consecutive days, but finally died from acute cholestatic hepatitis.
DiscussionDespite its great efficacy in the treatment of chronic HCV infection, IFN α has a number of adverse effects, including a flu-like syndrome, leukopenia, thrombocytopenia, depression, seizures, thyroid dysfunction, alopecia, and the activation of oral lichen planus.2 Reported pulmonary toxicities, including sarcoidosis, bronchiolitis obliterans organizing pneumonia, pleural effusion, interstitial pneumonitis, and the exacerbation of bronchial asthma, occur rarely and develop after a long period of treatment.3,4
Pulmonary toxicity is a rare but potentially fatal adverse effect of IFN α treatment for chronic hepatitis C. Cell-mediated pneumonitis associated with IFN α therapy is strongly related to the accumulated dose and is highly reversible. It is a rare adverse effect, with an incidence of about 0.4%.2 Eighteen cases of interstitial pneumonitis associated with various types of IFN a treatment for chronic hepatitis C have been reported. Natural IFN a was used in six of these cases, conventional IFN α-2b in nine, pegylated IFN α-2a in one, and pegylated IFN α-2b in two.2
The effects of IFN α include antiviral activity, growth regulation, inhibition of angiogenesis, regulation of cell differentiation, enhancement of the expression of major histocompatibility complex antigens, enhancement of the activity of natural killer cells and cytotoxic T lymphocytes,2 and the increased expression of class I and II human leukocyte antigens.5 These effects are considered to be partly responsible for the development of the autoantibodies and autoimmune reactions reported both during and after IFN therapy.6
Several pathophysiological mechanisms have been proposed to explain the lung damage caused by IFN. These mechanisms center on the known immunomodulatory activity of IFN. The proposed mechanisms for the activity and pulmonary toxicity associated with IFN include the inhibition of suppressor T cells, the enhancement of cytotoxic T cells, the induction of proinflammatory cytokines, and the exaggerated release of fibrinogenic cytokines, such as platelet-derived growth factor and transforming growth factor-β, leading to lung tissue fibrosis.7
Although the tolerability of P-IFN α is similar to that of conventional IFN a, IFN toxicity is generally dose and duration dependent.3 For example, Glue et al. reported that P-IFN α-2b produced a dose-related reduction in white blood cells, neutrophils, and platelets, and a dose-related increase in oral temperature.8 This leads us to speculate that pulmonary toxicity may occur more frequently with long-acting P-IFN α therapy at inappropriately high doses. The present case was receiving 120, μg/week of P-IFN α-2b.
Our patient also presented with refractory thrombocytopenia despite multiple platelet transfusions. A bone marrow aspiration biopsy showed hyperplasia of the megakaryocytes and grade II fibrosis, which could also be explained by chronic hepatitis C therapy because this toxic effect has been described previously.9
Plasmapheresis therapy was an alternative and last resource, given to remove humoral factors and proinflammatory and fibrinogenic cytokines from the circulation, thereby decreasing the pulmonary activity and toxicity associated with the host IFN response.10
Acute cholestatic hepatitis is a disease associated with viral infections and with diverse drugs, but it has not been reported to be secondary to IFN α therapy. Fibrosing cholestatic hepatitis has been related to liver and kidney posttransplantation status,11 hepatitis B virus infection and postche mo therapy immunosuppressed conditions,12 renal and cardiac posttransplantation status in the context of HCV infection,13,14 and a special type of recurrent infection with hepatitis B virus after liver transplantation;15 it can be a complication of cyclophosphamide and corticosteroid treatment of active glomerulonephritis,16 but it has not been reported to be associated with IFN therapy. Our patient had an undetectable viral load 10 days before her death. Therefore, this could be the first reported case of acute fibrosing cholestatic hepatitis associated with IFN α therapy.
This is the third fatal case related to P-IFN α-2b. In a previous case, the patient developed acute respiratory distress syndrome and died from multiple organ failure.17 Another case report mentioned a patient who developed progressive dyspnea requiring steroid therapy, with relapse after steroid withdrawal, who became steroid dependent in order to control symptoms.18
The limitations of the present case are the lack of respiratory function tests, bronchoalveolar liquid specimen analysis, and lung biopsy, which were not possible because of thrombocytopenia and patient deterioration. However, the absence of mediastinal lymphadenopathy on a computed tomographic scan excluded sarcoidosis and there was no evidence of infection (negative expectoration and blood cultures, normal procalcitonin levels, etc.), and these exclusions support our diagnosis of fatal and complicated interstitial pneumonitis associated with P-INF α-2b therapy in a women with chronic hepatitis C.