Background and study aims. Chronic liver disease (CLD) can cause hepatopulmonary syndrome (HPS), defined as triad of liver disease, hypoxemia, and intrapulmonary vascular dilation (IPVD). The aim of this study was to determine the evidence of IPVD in a cohort of pediatric patients with CLD pre- and post-liver transplantation (LT).
Material and methods. All pediatric patients with CLD listed for LT were studied. Pulse oxygen saturation (SpO2), technetium-99m-labeled macroaggregated albumin (99mTc- MAA) perfusion scan (positive test: uptake of the isotope ≥ 6% in the brain), and echocardiography with saline bubble test (SBT) were performed. SBT was re-evaluated at 3-6 months after LT. Grading of SBT included grade 0 (no bubble), I (1-9 bubbles), grade II (10-20 bubbles), and grade III (> 20 bubbles).
Results. Eighteen patients, median age 22.5 months (8-108), were enrolled. Most had biliary atresia (77.8%). Pre-LT, all patients had SpO2 of 100% and none had positive 99mTc- MAA perfusion scan. Two patients (11%) had negative SBT (grade 0), 1 (5.5%) had grade I, 3 (16.5%) had grade II, and 12 (67%) had grade III, respectively. Post-LT SBT became negative in all survivors (n = 16), (p = 0.0001).
Conclusions. Most cirrhotic children in this cohort study had evidence of IPVD by positive SBT. However, none of these met the criteria for diagnosis of HPS. This evidence of IPVD subsided after LT.
Portopulmonary hypertension and hepatopulmonary syndrome (HPS) are two common complications causing hypoxemia in patients with advanced chronic liver disease (CLD).1 Portopulmonary hypertension, defined as an increased pulmonary arterial pressure in patients with liver disease caused by pulmonary arterial constriction, is found less common than HPS.2 HPS is characterized by triad of liver disease, arterial hypoxemia, and intrapulmonary vascular dilation (IPVD).3 The prevalence of HPS in patients with liver disease has been reported to be between 4% and 47%.4,5 The data on prevalence of HPS in children with CLD have been limited, however, it is estimated to be less than that found in adult patients.6 IPVD plays an important role in the HPS.3 Liver transplantation (LT) is the only definitive treatment for HPS.7 The aim of this study was to determine the evidence of IPVD in a cohort of pediatric patients with CLD pre- and post-LT.
Material and MethodsPediatric patients with chronic liver disease listed for liver transplantation were included. Chronic liver diseases in this study meant cirrhosis which defined as progressive chronic liver diseases with poor synthetic functions and/ or an evidence of portal hypertension. The etiologies of cirrhosis were determined according to clinical findings. Standard investigations included liver function tests, hepatobiliary ultrasonography, hepatobiliary scintigraphy and percutaneous liver biopsy. Intraoperative cholangiography was done if biliary atresia was suspected. Investigations for metabolic liver diseases and genetic studies were performed in selected cases. If the causes could not be identified the diagnosis was categorized as unknown cause. The diagnosis of liver disease was based on the standard criteria for each disease.
The informed consent in writing was obtained from each patient’s parents. This study which the study protocol was conformed was approved by the Institute Research Board Committee. The study protocol conformed to the ethical guide lines of the Declaration of Helsinki (1975). Demographic data including laboratory findings and pediatric end-stage liver disease (PELD) score8 were recorded. Pre-LT studies included oxygen saturation measurement, contrast-enhanced transthoracic echocardiography (CEE) and Technetium-99m (99mTc)-labeled macroaggregated albumin (MAA) perfusion scan. CEE was re-evaluated in all survivors at 3-6 months after LT. MAA perfusion scan was be re-evaluated at 3-6 months after LT if pre-LT results were abnormal.
Measure of oxygen saturationA pulse oximetry was used to measure pulse oxygen saturation (SpO2) in all patients while breathing in room-air at rest.
Methods for the identification of intrapulmonary vascular dilation (IVPD)- •
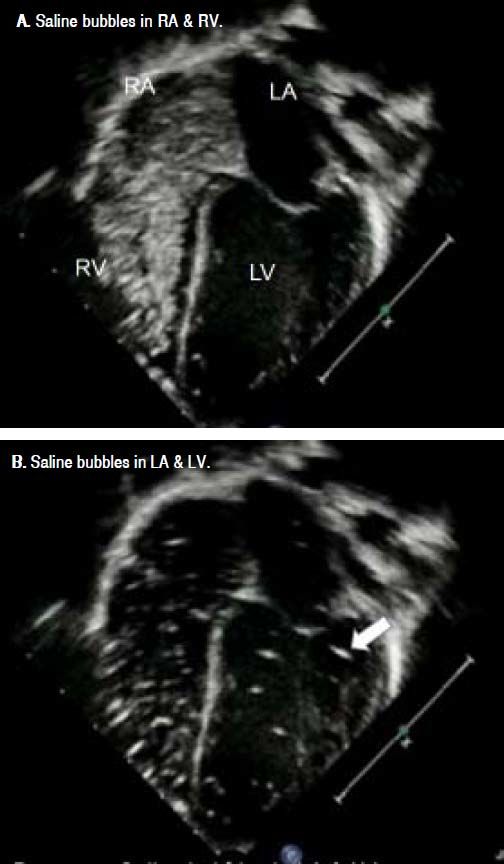
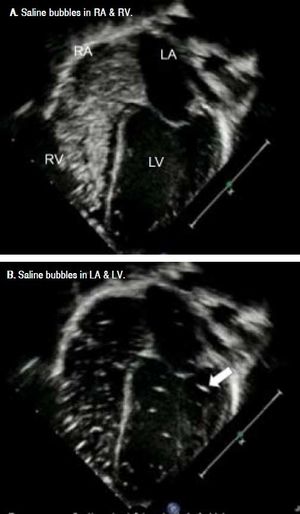
Contrast-enhanced transthoracic echocardiography (CEE), so called saline bubble test (SBT) was performed as previously described.9 A standard real-time two-dimensional (2-D) transthoracic echocardiography was performed in an apical four-chamber view. A 10 mL NSS in a luerlock syringe was injected into another syringe connecting by 3-way stopcock back and forth to create agitated (shaken) NSS. Then, this agitated NSS was injected via a peripheral vein into superior/inferior vena cava, right atrium (RA), and right ventricle (RV). The agitated NSS, acting like a contrast medium, created a stream of micro-bubbles (diameter >10 μm)9,10 seen as increased echogenicity in RA and RV on 2-D echocardiography.9,11
A positive SBT is defined as micro-bubble appearance in the LA after its appearance in RA and RV at the time of more than three cardiac cycles. The immediate appearance of micro-bubble in LA following its appearance in RA within 3 cardiac cycles suggested that the right to left shunt occurred across the patent foramen ovale or the atrial septal defect. SBT was grading as:
- °
Grade 0 (no micro-bubble in the left atrium).
- °
Grade I (1-9 bubbles).
- °
Grade II (10-20 bubbles).
- °
Grade III (> 20 bubbles).12
- °
- •
Technetium-99m (99mTc)-labeled macroaggregated albumin (MAA) perfusion scan was performed to detect uptake over the kidneys and brain, indicating that the MAA particles which were larger than 20 microns passed through abnormally dilated pulmonary vascular channels in case of normal cardiac chambers. Patient was in the standing position for 10 min and room air breathing, 2 mCi of 99mTc-MAA (Dupont Pulmolite; Billerica, MA; 90% of the MAA particle size between 10 and 90 mm) was injected via a peripheral IV site. At 20 min after injection, quantitative brain imaging was conducted in the supine position, and a brain uptake percent (assuming a constant 13% blood flow to the brain)13 was obtained via the following calculation, geometric mean of technetium (GMT) counts around the brain and lung: (GMTbrain)/GMTbrain + GMTlung).9 In normal healthy children, less than 5% of isotope can be quantified in the brain. IPVD was defined as either positive SBT or abnormal uptake in the brain (≥ 6%) with 99mTc-MAA perfusion scanning.
Data were expressed in median and range for continuous variables, number, percentage for categorical variables. Data of the numbers of patients with positive and negative saline bubble test pre- and post-liver transplantation were compared using McNemar’ s test. A p value of < 0.05 was considered statistical significant. Data were analyzed using the Stata 13 software (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
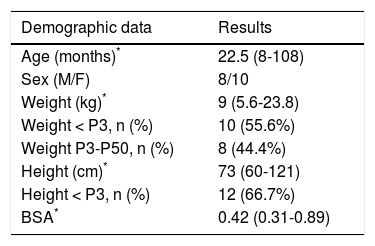
ResultsAll cirrhotic children listed for liver transplantation and undergoing liver transplantation at our center were consecutively enrolled in 18-month period resulting in 18 patients. Median age of the study children was 22.5 months (range 8-108) and median weight was 9 kg (range 5.6-23.8). Most patients were less than 24 months of age (55.6%) and had body weight less than the 3rd percentile (66.7%) (Table 1). The most common etiology was biliary atresia (77.8%). The median PELD score was 19 (range 12-25) (Table 2). All patients in this study had at least one of the clinical evidences of portal hypertension including variceal bleeding, ascites and splenomegaly. All patients underwent LT after performing all tests with median duration of 37 days (range 2-104).
Demographic data of the study children (N = 18).
| Demographic data | Results |
|---|---|
| Age (months)* | 22.5 (8-108) |
| Sex (M/F) | 8/10 |
| Weight (kg)* | 9 (5.6-23.8) |
| Weight < P3, n (%) | 10 (55.6%) |
| Weight P3-P50, n (%) | 8 (44.4%) |
| Height (cm)* | 73 (60-121) |
| Height < P3, n (%) | 12 (66.7%) |
| BSA* | 0.42 (0.31-0.89) |
BSA: body surface area. F: female. M: male. P: percentile.
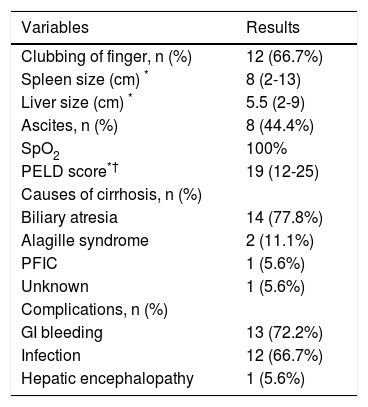
Characteristics of variables in the study patients
| Variables | Results |
|---|---|
| Clubbing of finger, n (%) | 12 (66.7%) |
| Spleen size (cm) * | 8 (2-13) |
| Liver size (cm) * | 5.5 (2-9) |
| Ascites, n (%) | 8 (44.4%) |
| SpO2 | 100% |
| PELD score*† | 19 (12-25) |
| Causes of cirrhosis, n (%) | |
| Biliary atresia | 14 (77.8%) |
| Alagille syndrome | 2 (11.1%) |
| PFIC | 1 (5.6%) |
| Unknown | 1 (5.6%) |
| Complications, n (%) | |
| GI bleeding | 13 (72.2%) |
| Infection | 12 (66.7%) |
| Hepatic encephalopathy | 1 (5.6%) |
PELD: pediatric end-stage liver disease. PFIC: progressive familial intrahepatic cholestasis. SpO2. pulse oxygen saturation.
PELD = [0.480] x Loge [bilirubin (mg/dL)] + 1.857 x loge [INR] -[0.687] x Loge [albumin (g/dL)] + [0.436 (if less than one year)] + [0.667 (if growth failure, < 2 SD)8]. Multiply the score by 10 and round to the nearest whole number.
Although 12 patients (66.7%) had clubbing of fingers, none of them had cyanosis. All children had pulse oxygen saturation (SpO2) of 100% at rest and breathing in room air.
Pre-LT, there was no saline bubble detected in the left sided heart within the first three cardiac cycles, but saline bubbles were detected in the left sided heart after 3 cardiac cycles in 16 of 18 children (89%) (Figure 1). Severity of positive SBT was as following; grade I (n = 1, 5.5%), grade II (n = 3, 16.5%), and grade III 12 (67%). However, none of them had positive 99mTc- MAA perfusion scan. There was no correlation between PELD score and SBT grade since most patients had high PELD score. Two children died after LT, resulting in 16 children with complete studies. Importantly, post-LT, SBT became negative in all 16 survived children including 14 children with positive pre-LT SBT (p = 0.0001).
DiscussionNormally, the aorta and its branches (arteries) bring the oxygenated blood thru the tissues, their lumens being smaller until they connect to the capillary plexuses. After delivery of oxygen, the deoxygenated blood circulates into veins and back to the right-sided heart. The pulmonary arteries bring deoxygenated blood thru the lungs, their lumens being smaller until they connect to pulmonary capillary plexuses, receiving oxygen into the circulation of the pulmonary veins and back to the left-sided heart. The pulmonary capillary plexuses act as filters for small particles such as small blood clots coming back from the body to prevent them from circulating to the left sided heart. The intrapulmonary vascular dilation is the main pathological feature of HPS in which many potential mechanisms explained this vascular dilation including endothelins, nitric oxide, and tumor necrotic factor-alpha.14–16 The more degree of this intrapulmonary vascular dilation, the more arteriovenous shunting which leading to HPS.14,17 The capillaries (parts of intrapulmonary vascular beds) where gas exchange occurs, dilates to 15–50 mm in HPS.18 Intrapulmonary right to left shunt can be caused by pulmonary arteriovenous malformation, pulmonary arteriovenous anastomoses (fistulas), or intrapulmonary vascular dilation. Consequently, some of the deoxygenated blood could circulate into pulmonary veins, and when the degree of intrapulmonary shunt is much enough, hypoxemia and cyanosis can occur.
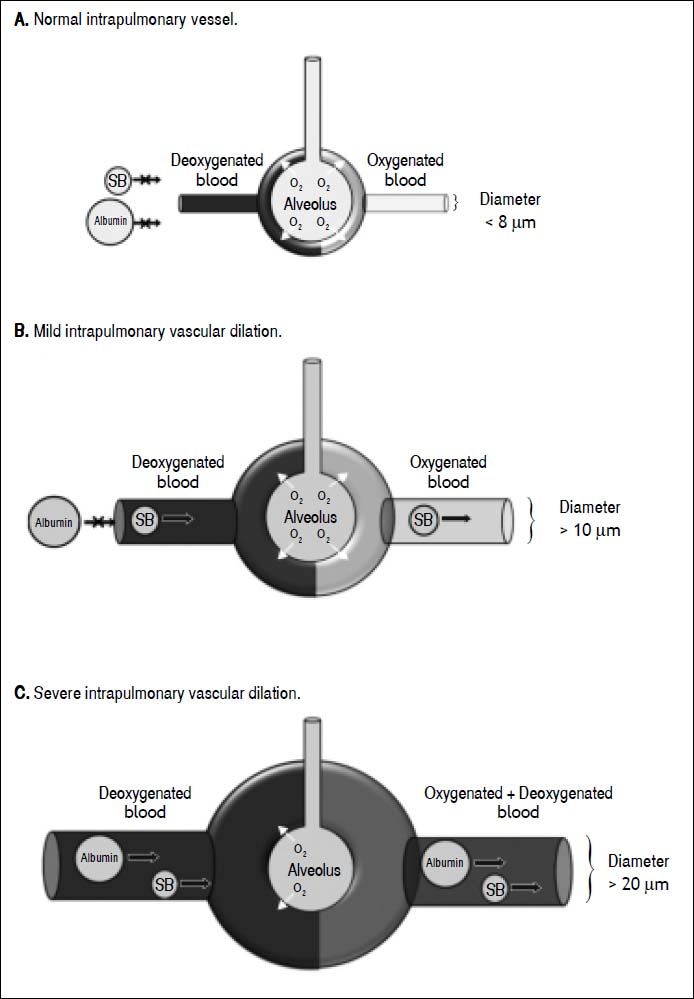
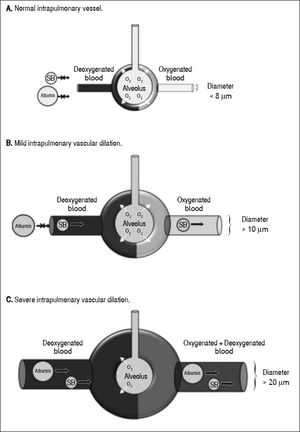
In this cohort study, among 18 children with cirrhosis, 16 children (89%) had evidence of intrapulmonary shunting according to positive saline bubble test. Most (67%) had positive SBT grade III (> 20 bubbles). Normally, these microbubbles which larger than 10 μm9,10 are trapped and absorbed in the normal pulmonary capillary bed during the first pass, but they could pass through if the pulmonary vessels are dilated. When the agitated saline are seen in the left heart after 3 cardiac cycles, the intracardiac right to left shunt could be excluded,19 however, we could not differentiate pulmonary arteriovenous malformation, pulmonary arteriovenous fistula, and intrapulmonary vascular dilation from each other. Nevertheless, in our study, all positive saline bubble tests disappeared post LT. This indirectly indicated that the positive SBT in all patients was due to intrapulmonary vascular dilation rather than the two formers. Since all pre-LT children did not have desaturation at room air, the definite diagnosis of HPS could not be made, although mild or early HPS was not excluded. In addition, the 99mTc- MAA perfusion scan was negative in all pre-LT children. This indicated that the degree of IPVD was not much enough to let the molecule of MAA which had diameter larger than the intrapulmonary vessel to pass through the intrapulmonary vascular beds. Although the Tc-99m MAA perfusion scan is the standard method for the diagnosis of HPS, the major disadvantage of Tc-99m MAA perfusion scan is, first, it could not differentiate intracardiac shunt from intrapulmonary shunt.20 Secondly, the MAA perfusion scan is less sensitive for the detection mild IPVD when compared to saline bubble as shown in this study. This could be explained by the size of Tc-99m-albumin (approximately more than 20 μm)21,22 will be trapped in pulmonary vascular bed whereas the size of saline bubble (approximately more than 10 μm)9,10 could pass thru the pulmonary vascular bed if there is mild IPVD (Figure 2). Previous studies demonstrated that saline bubble test or contrast enhanced echocardiography was more sensitive than MAA perfusion scan in detection of intrapulmonary vascular dilation.9,23 European Respiratory Society (ERS) Task Force on Pulmonary-Hepatic Vascular Disorders (PVD) has recommended contrast-enhanced echocardiography as the first line screening test for HPS.24 From our study, we agree with this recommendation in performing echocardiography with saline bubble test as part of investigations in all children with chronic liver disease. Studies demonstrated that most patients (> 85%) with HPS had improvement or resolution in hypoxemia after LT.7,25 Moreover, the mortality after LT was significantly increased in patients who developed HPS before performing liver transplantation.26 Our findings indicate that IPVD or probable early stage of HPS can be identified in most children with CLD, since 89% of these children had evidence of IPVD by SBT. HPS has also been reported in patients with non-hepatic portal hypertension.27 In our study, all cirrhotic patients had clinical evidences of portal hypertension, we speculated that portal hypertension may play role in the findings of IPVD. Early LT in patients with evidence of IPVD might prevent the progression to definite HPS and thereby could improve the survival rate after LT.
Drawing demonstrates normal intrapulmonary vessel (A) which saline bubble and technetium-99m-labeled macroaggregated albumin could not pass thru, mild intrapulmonary vascular dilation (B) which only saline bubble could pass thru but technetium-99m-labeled macroaggregated albumin could not pass thru, and severe intrapulmonary vascular dilation (C) which boths could pass thru. Albumin: technetium-99m-labeled macroaggregated albumin. O2: Oxygen. SB: saine bubble.
Most cirrhotic children in this cohort study had evidence of IPVD by positive SBT. However, none of these met the criteria for diagnosis of HPS. This might indicate mild degree of IPVD and might represent early stage of HPS. IPVD resolved after LT in all patients.
Abbreviations- •
2-D: 2 dimension.
- •
99mTc: Technetium-99m.
- •
CEE: contrast-enhanced transthoracic echocardiography.
- •
CLD: chronic liver disease.
- •
GMT: geometric mean of technetium.
- •
HPS: hepatopulmonary syndrome.
- •
IPVD: intrapulmonary vascular dilation.
- •
LA: left atrium.
- •
LT: liver transplantation.
- •
LV: left ventricle.
- •
MAA: macroaggregated albumin.
- •
NSS: normal saline solution.
- •
PELD: pediatric end-stage liver disease.
- •
RA: right atrium.
- •
RV: right ventricle.
- •
SBT: saline bubble test.
This study was financially supported by Research Grant of Faculty of Medicine Ramathibodi Hospital, Mahidol University.
AcknowledgementsWe acknowledge Mr. Dittapol Muntham for his statistical analysis, liver transplantation team at Faculty of Medicine Ramathibodi Hospital, Mr. Uthen Pandee for performing echocardiography, and all patients participating in this study.