Background and aim. Receptor for advanced glycation end products (RAGE) blockade by a soluble form of RAGE (sRAGE) appears to be protective against hepatocellular death and necrosis after I/R injury. Little is known about the role of the hepatic RAGE, its ligands, and the plasma levels of sRAGE in liver transplantation (LT).
Material and methods. This was a prospective study on patients (n = 28) undergoing deceased donor LT. RAGE ligands [the N(epsilon)-carboxy-methyl-lysine (CML) adduct and the high-mobility group box 1 (HMGB1) protein] and sRAGE levels were measured in donors at the time of organ procurement, while in recipients they were tested before surgery (baseline), after graft reperfusion, and on day 1 and 7 posttransplantation. Donors and recipients liver biopsies were collected to assess the transcriptional expression of the full-length RAGE and of its truncated isoform, the endogenous secreted RAGE (esRAGE).
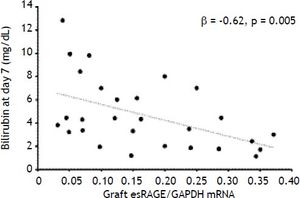
Results. At baseline, CML levels were higher in LT recipients than in donors (p = 0.02), decreased immediately after graft reperfusion (p < 0.0001) and returned to baseline values on day 7. Baseline HMGB1 levels (3.8 ± 2.3 ng/mL) increased after graft reperfusion (39.9±18 ng/mL, p < 0.0001), and returned to baseline values within day 1, while circulating sRAGE decreased significantly on day 7 (p < 0.0001). The graft esRAGE mRNA expression was inversely associated with bilirubin on day 7 (β = -0.62, p = 0.005).
Conclusions. Early on after LT, there is accumulation of CML and a rapid increase of HMGB1 concurrent with a remarkable decline in circulating sRAGE. The RAGE-ligand axis may also be involved in early graft dysfunction.
Early allograft dysfunction (EAD) may affect up to 25% of liver transplant (LT) recipients and entails a sevenfold and a tenfold higher risk for graft loss and patient death, respectively.1 Both during surgery and in the post-operative period, the liver graft may be injured by several toxic factors, such as hypotension, hypoxia, ischemia/reperfusion (I/R) injury, and use of hepatotoxic drugs. Although the mechanisms leading to EAD have not yet been entirely elucidated, its development is believed to be largely due to I/R injury, which is associated with cell death, oxidative damage and a severe inflammatory response within the liver.2–4 In turn, the extent of I/R injury may be related to the cold ischemia time, graft quality, donor age, the recipient’s clinical status at transplantation, and surgical challenges. From a bio-molecular perspective, liver graft injury and EAD may result in altered expression of serum proteins associated with an inflammatory response.5 Recently, several chemokines and cytokines –such as the monocyte chemotactic protein-1, interleukin-8, RANTES, interferon-γ inducible protein 10 and the interleukin-2 receptor- appear to increase in the early post-operative period of EAD recipients.6
There is experimental evidence that interaction between the receptor for advanced glycation end products (RAGE) and its ligands plays a pivotal role in liver inflammation and tissue regeneration after liver injury.7,8 RAGE interacts with pro-inflammatory ligands, such as the carboxy-methyllysine ad-ducts (CML), the high-mobility group box 1 (HMGB1) protein and the S100/calgranulins among others, thus resulting in amplification of inflammation and tissue injury.9,11 On the other hand, RAGE blockade by a soluble form of RAGE significantly increases animal survival after extended liver resection and appears to be protective against hepatocellular death and necrosis after I/R injury.7,8,12 RAGE is detectable in blood as a soluble isoform that consists of the extracellular ligandbinding domain only. The total circulating isoforms of soluble RAGE (sRAGE) include endogenous secreted isoforms (esRAGE) generated via alternative splicing, and a truncated form generated through proteolytic cleavage of the full-length cell-surface receptor.13 Both isoforms can bind RAGE ligands and contribute to their removal by competing with the membrane-bound RAGE, thus resulting in a cytoprotective effect.14,15
Over the last decade, clinical studies performed on patients undergoing lung, kidney, or LT have documented the involvement of the RAGE-ligand axis in the post-transplantation inflammation and regeneration processes.16–19 Cirrhotic patients undergoing LT show high levels of plasma CML before surgery which decrease by 50% at 3 months post-transplantation, thus confirming that the liver acts as a clearing organ for these adducts.18 The circulating and pro-inflammatory RAGE ligand HMGB1 -which is present in the cell nucleus and can be released by necrotic cells or in response to hypoxia−20,21 peaks in blood after graft reperfusion concurrent with hepatic HMGB1 expression up-regulation.19
The interaction between RAGE and its ligands can thus impair liver regeneration, while sRAGE can have a protective role. Hence, we set up a prospective trial to evaluate the plasma levels of circulating RAGE ligands and/or sRAGE peri-operatively and in the early post-operative period, concurrent with assessment of the hepatic sRAGE/RAGE transcriptional expression in both graft recipients and their donors.
Material and MethodsPatient selectionThis was a 12-month, prospective, single-centre study on the behaviour of the RAGE-ligand axis in 28 patients submitted to primary, whole-size, deceased donor LT performed at our institution from November 2010 to April 2012. The current report focuses on the first 7 day post-transplantation.
Patients were enrolled if adults (≥ 18 years) and affected with clinically documented chronic liver disease of viral, metabolic, autoimmune, toxic and/or innate aetiology with or without hepatocellular carcinoma (HCC), and if they provided informed consent. Exclusion criteria called for: acute liver failure; a model for end-stage liver disease (MELD) score ≥ 30 at transplantation; HCC beyond Milan criteria as per the pre-transplant clinical investigations; non-HCC liver neoplasms (i.e. cholangiocarcinoma, adenomatosis, hemangioendothelioma); acute and/or chronic kidney disease (defined as serum creatinine ≥ 1.5 mg/dL at transplantation); a body mass index (BMI) > 27 kg/m2; combined organ transplantation; abstinence from alcohol use < 6 months; autoimmune liver disease on active steroid treatment; hepatitis C virus (HCV)-related disease on treatment with interferon at the time of transplantation; history of major cardiovascular event, such as acute myocardial infarction, unstable angina, previous coronary revascularization, stroke and peripheral vascular disease grade ≥ 2; hepato-pulmonary syndrome with arterial hypoxemia (PaO2) < 70 mmHg or an alveolar-arterial gradient > 20 mmHg; porto-pulmonary hypertension with a mean pulmonary arterial pressure > 25 mmHg; and mental impairment. At the time of transplantation, patients were re-screened for compliance with the eligibility criteria and underwent laboratory tests. The MELD score at transplantation was derived as indicated elsewhere.22 Patients were re-evaluated for shortterm complications for 7 days following LT by collecting clinical data, and routine blood parameters. Patients did not develop any complications within this observation period.
Approval was obtained from the institutional review board. The study was carried out in compliance with the principles set forth in the 2008 Seoul revision of the declaration of Helsinki.
Liver biopsiesTwo biopsies were obtained from the donor liver graft during procurement and from the recipient’s liver explant, respectively, and were stored at −20°C in RNAlater (Sigma-Aldrich, St. Louis, MO, USA) for tissue stabilization.
Blood sample collectionBlood samples were obtained preoperatively from donors and recipients. In the recipient, further samples were collected within 30 min after graft reperfusion, on days 1 and 7. Blood samples were centrifuged at 4 °C and plasma was immediately stored at −80 °C until analysis. All laboratory tests were performed in blinded fashion with respect to the identity of the samples. Routine biochemical analyses were determined by standard laboratory methods.
Determination of plasma sRAGE levelsPlasma sRAGE levels were determined using a double-sandwich ELISA kit (DuoSet ELISA development kit; R&D Systems, Minneapolis, MN, USA) as previously described.23 Intra-assay and inter-assay coefficients of variation was 5.9% and 8.2%, respectively. The lower limit of detection of sRAGE was 21.5 pg/mL.
Determination of plasma CML levelsPlasma CML levels were measured by an inhouse competitive ELISA using the mouse F(ab’)2 fragment anti-AGE monoclonal antibody (clone 6D12) (ICN Biochemical Division, Aurora, OH,USA), as previously described.24 The lower limit of detection of CML was 0.5 μg/mL.
Determination of plasma HMGB1 levelsPlasma HMGB1 levels were determined using the double-sandwich ELISA Kit II (IBL International, Hamburg, Germany) according to the manufacturers’ description. Intra-assay and inter-assay coefficients of variation were <8% and 10% respectively. Plasma samples from 30 healthy voluntaries were assayed as control (49.3 ± 12.3 years). The sensitivity of the assay was 0.1 ng/mL.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)Total RNA was extracted from liver specimens by using the RNeasy Midi kit (Qiagen S.p.A, Milan, Italy) as previously described.25 The RNA concentration was determined spectrophotometrically at 260 nm (BioPhotometer Eppendorf Italia, Milan, Italy). Integrity and purity of total RNA were checked by electrophoresis of samples on ethidium bromide 2% agarose gel and visualized at 302 nm (2UVTM Transilluminator, UVP, Upland, CA, USA).
Total RNA was reverse-transcribed using the iS-cript cDNA synthesis kit (Bio-Rad Laboratories, Inc,Hercules, CA, USA). The cDNA was amplified using the Taq PCR Core Kit (Qiagen, Germany) with specific primers and conditions as shown below, in a GeneAmp PCR System 9700 thermal cycler (Perkin Elmer/Applied Biosystems, Foster City, CA, USA).
All PCR reactions were performed in a 50 µL total volume, containing Taq polimerase (Qiagen, Germany) 2.5 U, deoxyribonucleotide triphosphate (dNTP) 0.2 mmol/L, MgCl2 1.5 mmol/L and 1 µmol/ L of both forward and reverse primers. The Primers, annealing temperature and cycles used were respectively:
- •
For RAGE gene, F: 5’-CAGGAATGGAAAGGAGACCA-3’, R: 5’-CCCTTCTCATTAGGCACCAG3’, 62°C (30 sec.) and 40 cycles.
- •
For esRAGE gene, F: 5’-GGGGATGGTCAACAAGAAAGG-3’, R: 5’- AGGTTCCTCCGACTGATTCAGTTC -3’, 55°C (15 sec) and 40 cycles.
- •
For GAPDH gene, F: 5’-GGTCTCCTCTGACTTCAACAGCG-3’, R: 5’-GGTACTTTATTGATGGTACATGAC-3’, 55° (1 min) and 30 cycles.
The amplified products were electrophoresed on ethidium bromide 2% agarose gel, in parallel with a DNA Ladder 100 bp (Qiagen, Germany). After gel image acquisition by a digital camera (Kodak DC290 Digital camera System™; Eastman Kodak, Rochester, NY, USA), the band intensity of PCR DNA was quantified using densitometric analysis by Scion image™. PCR products of esRAGE and full-length RAGE were normalized to transcript levels of the control gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical analysisThe sample size was calculated with the Stata software (version 9.2; Stata Corp. College Station, TX, USA) by the estimated power for two-sample comparison of means of CML plasma values. A sample size of 56 patients (28 donors and 28 recipients) would provide 85% power to detect differences of 30% in CML value between two groups with a two-sided Student t-test at an αlevel of 0.05.
Data were analysed with the use of statistical software SPSS 13.0 (SPSS Inc, Chicago, IL, USA). The Kolmogorov-Smirnov test of normality was used to verify whether the distribution of variables followed a Gaussian pattern. According to the level of distribution, data are presented as mean ± standard deviations (SD), medians and interquartile ranges. Variables with a non-normal distribution were logarithmically transformed before each analysis. The unpaired Student’s t-test was used to compare normally distributed variables between donors and recipients. Multiple differences were evaluated by one-way ANOVA followed by the Bonferroni’s post-hoc test. Simple correlations were evaluated by linear regression analysis. A two-tailed p-value < 0.05 was considered statistically significant.
ResultsDonor age, cold and warm ischemia, recipient clinical and biochemical characteristics, and post-transplant markers of liver injury are illustrated in table 1. Recipients were mainly males (82%), and the most frequent indication for LT was HCC (64%). Mean recipients’ age was lower than their donors’ (53 ± 8.7 vs. 62.1 ± 17.3 years, respectively; p = 0.017).
Donor and recipient clinical and biochemical characteristics.
| • Donors (N = 28) | ||
| Age (years) | 62.1 ± 17.3 | |
| • Recipients (N = 28) | ||
| Age (years) | 53 ± 8.7* | |
| Male (%) | 23 (82) | |
| Primary diagnosis, n (%) | ||
| HCC | 7 (25) | |
| HCC + HCV + cirrhosis | 7 (25) | |
| HCC + HBV + cirrhosis | 2 (7.1) | |
| HCV + cirrhosis | 5 (17.9) | |
| HCC + HBV | 1 (3.6) | |
| HCC + HCV | 1 (3.6) | |
| Caroli’s syndrome | 2 (7.1) | |
| Primary sclerosing cholangitis | 2 (7.1) | |
| Alcoholic cirrhosis | 1 (3.6) | |
| Ascites, n (%) | 10 (36) | |
| MELD score | 9.48 ± 3.79 | |
| Graft cold ischemia time (min) | 451.4 ± 91 | |
| Graft warm ischemia time (min) | 87 ± 15 | |
| Prior LT | On day 7 | |
| ALT (U/L) | 69 (42-90.5) | 89 (76.2-119.8) † |
| AST (U/L) | 72.5 (59-134.5) | 40 (31.2-56.8)‡ |
| GGT (U/L) | 141.5 (104-197.5) | 159 (106-220) |
| LDH (U/L) | 232.7 ± 59.1 | 234.8 ± 39.4 |
| Bilirubin (mg/dL) | 1.51 (1.1-2.5) | 3.28 (1.8-4.4)§ |
| INR | 1.27 ± 0.19 | 1.17 ± 0.13 |
| Creatinine (mg/dL) | 0.826 ± 0.202 | 0.937 ± 0.495 |
| Antithrombin III (%) | 50 (38-73) | |
| Cholinesterase (U/L) | 2663 (2,049-5,335) |
Data are presented as mean ± SD, median and interquartile range or percentage. HCC: hepato-cellular carcinoma. ALT: alanine aminotransferase. AST: aspartate aminotransferase. GGT: gamma-glutamyl transpeptidase. LDH: lactate dehydrogenase. MELD: model for end-stage liver disease.
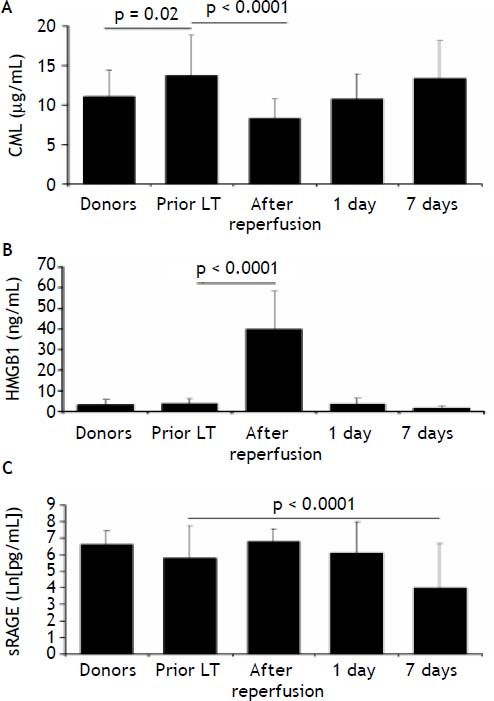
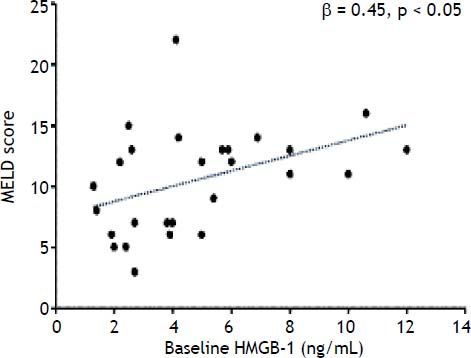
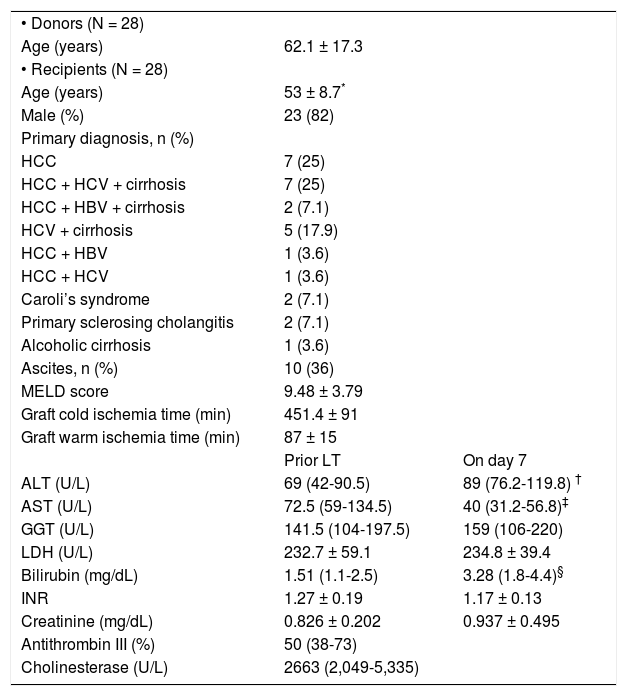
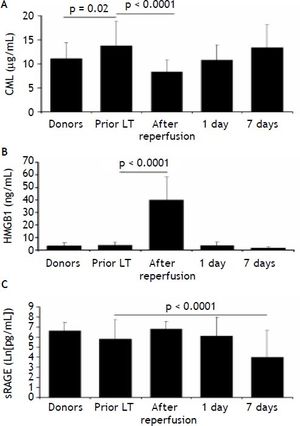
At baseline, among the RAGE-ligands CML plasma levels were higher in LT recipients than donors (p = 0.02) (Figure 1A) and decreased immediately after graft reperfusion (p < 0.0001) to return to baseline values on day 1 (Figure 1A). The baseline HMGB1 levels did not differ between LT recipients and donors (3.8 ± 2.3 ng/mL vs. 3.3 ± 2.4 ng/mL, respectively) (Figure 1B). However, in both groups they were significantly (p < 0.0001) higher than in a group (30 subjects) of normal controls (0.45 ± 0.3 ng/mL). The baseline HMGB1 levels in LT recipients correlated with the preoperative MELD score (ß = 0.45, p < 0.05) (Figure 2). The baseline HMGB1 levels increased dramatically after graft reperfusion (39.9 ± 18 ng/mL, p < 0.0001) returning rapidly to baseline values one day after LT (3.6 ± 2.9 ng/mL) (Figure 1B). The peak values of HMGB1 after reperfusion tended to correlate directly with the MELD score on day 7 (β = 0.42, p = 0.07).
Comparison between donors and recipients (n = 28) prior to LT in plasma levels of CML (A), HMGB1 (B) and sRAGE (C) and kinetics of the same parameters in LT recipients at 7 days after LT. Data are presented as mean ± SD. Comparison between donors and LT recipients was evaluated by t-test while kinetics in LT recipient was evaluated by ANOVA followed by Bonferroni test.
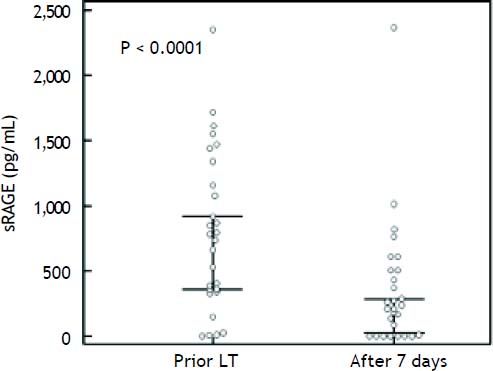
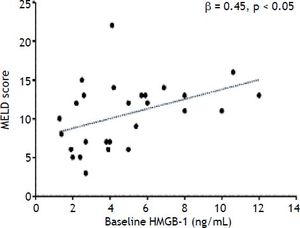
The preoperative plasma levels of sRAGE did not differ between LT recipients and donors (Figure 1C). Plasma sRAGE levels did not change early after LT, but decreased significantly on day 7 (p < 0.0001) (Figure 1C). The decrease of sRAGE levels observed in the current patients’ population is illustrated in figure 3.
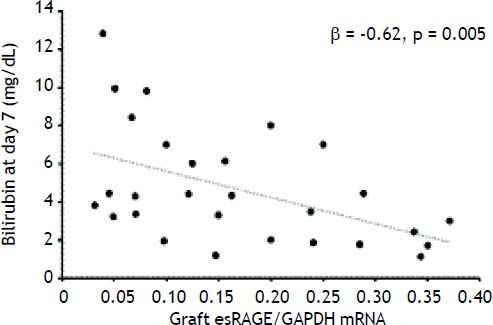
Semi-quantitative RT-PCR analysis revealed that hepatic full-length RAGE mRNA expression differed between donors and LT recipients (p = 0.005) while esRAGE mRNA did not differ between two groups (Table 2). The graft esRAGE mRNA expression was significantly and inversely associated with bilirubin levels on day 7 (β = - 0.62, p = 0.005) (Figure 4).
This study provides evidence supporting the involvement of RAGE and RAGE-ligands in the cascade of biological events following liver graft procurement and transplantation.
We found that, after transplantation, the circulating levels of sRAGE -which are the result of its expression in tissues and release into the bloodstream- decrease by day 7. Since sRAGE might exert inhibitory effects on RAGE, acting as a decoy, but also binding to cell surface RAGE to block the formation of homodimers,26 decreased levels of sRAGE may contribute to enhanced RAGE-mediated pro-inflammatory signalling after transplantation and I/R injury. These changes in circulating sRAGE levels after LT may not only be due to decreased expression/release -and possibly affected by immunosuppression-27 but also to enhanced clearance. However, in the current series baseline, serum creatinine did not change significantly vs. day 7, and the first hypothesis seems much more plausible and warrants further investigation. In agreement with our observation, previous research in kidney transplantation has demonstrated that circulating sRAGE levels decline after surgery and this is associated with a two- to threefold higher risk for mortality in kidney recipients.17
Among RAGE ligands, the most remarkable indicator of liver impairment is the HMGB1 protein, which is present into the cell nucleus and can be released by cells undergoing necrosis or in response to hypoxia.20,21 In agreement with Ilmakunnas, et al.,19 our data confirm that HMGB1 plasma levels increase early on after reperfusion, probably as a result of I/R injury and tended to correlate to MELD score on day 7. That is compatible with the hypothesis that, following ischemia-reperfusion, the early release of HMGB1 activates the RAGE and as a result of this interaction the effects of the injury are amplified and revealed later.
Furthermore, we also found higher values of HMGB1 in LT recipients than in normal subjects, and a positive relationship between baseline HMGB1 protein and pre-operative MELD score. These results underline the role of this marker in chronic liver disease and suggest that HMGB1 may in turn have a harmful impact on graft function. The LT recipients had higher hepatic expression of RAGE than donors. Nevertheless, we do not have a plausible explanation for this and we believe that other investigations are need to know the reason of this difference. We also found higher CML levels in LT recipients than in donors, possibly as consequence of reduced AGE clearance due to liver impairment. However, after a rapid drop ensuing graft reperfusion, CML levels returned to pre-transplant values on day 7, underscoring a poorly clarified reduced detoxification capacity of the liver graft early on after transplantation. An interesting relationship was the inverse association between esRAGE liver expression in donor grafts and recipients’ serum bilirubin on day 7 post-transplantation because low levels of the protective esRAGE could negatively influence the transplant in early follow-up, although the assessment of esRAGE in post-transplant biopsy (unfeasible for ethical concerns) would be of greater utility. Considering all of these results as a whole -i.e. the association between graft esRAGE and bilirubin levels on day 7, the rapid increase of HMGB1 after graft reperfusion, and the CML accumulation in the bloodstream followed by a dramatic decline of the protective sRAGE in the early postoperative period- we believe that these biological markers point out to metabolic events with the potential to affect graft survival and patient outcomes. The RAGE-ligand axis is a promising target of further investigation, as a biological marker of liver injury prior to and after LT. Identification of patients at risk of complications is crucial for improving post-operative care and transplant outcomes. The RAGE-ligand axis may also be used as a therapeutic target in order to enhance early graft function and improve survival.
Our study has several inherent limitations. This work is exploratory in nature and is a descriptive study, and therefore we cannot assign causality to our findings, nor can we recommend specific clinical strategies based on our conclusions. The sample size is the major limiting factor, and a prospective, larger validation set is needed for conclusions that are more robust. Despite these limitations, this study provides evidence for several associations between the cascade of biological events ensuing graft procurement, I/R, and reperfusion and the RAGEligands axis, and this can guide future investigation.
Abbreviations- •
CML: N(epsilon)-Carboxy-Methyl-Lysine.
- •
EAD: early allograft dysfunction.
- •
esRAGE: endogenous secreted RAGE.
- •
HCC: hepatocellular carcinoma.
- •
HMGB1: high-mobility group box 1.
- •
I/R: ischemia/reperfusion.
- •
LT: liver transplantation.
- •
MELD: Model for End-Stage Liver Disease.
- •
RAGE: receptor for advanced glycation end products.
- •
sRAGE: soluble RAGE.
This work was funded by institutional sources.
Conflict of InterestThe authors have no conflicts of interest to disclose.
AcknowledgmentsThe authors wish to thank Alison Frank for her editorial assistance and owe a deep debt of gratitude to the nurse staff of the Hepatobiliary surgery and liver transplant Unit, University of Pisa Medical School Hospital, Pisa, Italy.