Given the substantial burden of metabolic dysfunction-associated steatotic liver disease (MASLD), there is an urgent need to assess knowledge and awareness levels among physicians. We assessed MASLD knowledge among healthcare providers from Saudi Arabia, Egypt, and Türkiye.
Materials and MethodsTwo global surveys containing 54–59 items assessed awareness and knowledge of MASLD/NAFLD- one was for hepatologists and gastroenterologists, and the second was for non-specialists (e.g. endocrinologists, primary care providers [PCPs], and other healthcare professionals). Data were collected using an electronic data collection form. Knowledge scores and variables associated with higher knowledge scores were compared across all specialties.
ResultsA total of 584 physicians completed the survey (126 hepatologists, 178 gastroenterologists (GEs), 38 endocrinologists, 242 PCPs/others). Practice guidelines were the primary source for knowledge across all specialties (43–51%), then conferences (24–31%) except PCPs/others who selected the internet as the second common source (25%). Adherence to societal guidelines varied by specialty (81–84% of specialists vs 38–51% of non-specialists). Hepatologists and GEs showed similar mean knowledge scores (51–72% correct answers across three knowledge domains, p > 0.05); endocrinologists outperformed PCPs/others in knowledge scores in all knowledge domains, including Epidemiology/Pathogenesis (72% vs. 60%), Diagnostics (73% vs. 67%), and Treatment (78% vs. 67%) (all p < 0.01). Hospital-based practice and seeing a greater number of patients with MASLD/NAFLD were identified as independent predictors of higher knowledge scores among specialists (both p < 0.05).
ConclusionsA knowledge gap in the identification, diagnosis, and management of MASLD/NAFLD was found despite the growing burden of MASLD/NAFLD in Saudi Arabia, Egypt, and Türkiye. Education to increase awareness is needed.
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD), is believed to affect approximately 38% of the world population [1-3]. The prevalence is even higher in the Middle East and North Africa (MENA) region, affecting an estimated 46% of the population in this region [3]. Roughly 5% of individuals with MASLD/NAFLD are likely to develop end-stage liver disease, positioning MASLD/NAFLD as the leading cause of cirrhosis, liver cancer, and liver transplantation [4-6]. Beyond clinical implications, MASLD/NAFLD negatively impacts patients’ health-related quality of life (HRQL) and imposes a significant economic burden [7].
Recent data from the Global Burden of Disease study indicate a concerning rise in the prevalence and mortality associated with complications from MASLD/NAFLD in recent decades [8]. Despite these disturbing trends, there is a notable lack of awareness about the disease among patients and healthcare providers [9,10]. Accordingly, we conducted a global survey of providers and revealed a significant gap in knowledge, particularly among primary care providers [9]. This is significant given that individuals at high risk for progressive MASLD/NAFLD typically consult their primary care providers for management of their cardiometabolic risks [11]. Consequently, enhancing understanding of this liver disease and the importance of employing published algorithms for risk stratification in a primary care setting could improve patient and public health outcomes.
The lack of awareness about MASLD/NAFLD may also stem from other factors. Notably, the stigma linked to MASLD/NAFLD has led to a concerted effort by multiple societies to rename NAFLD to MASLD [1]. In this context, a recent global survey indicated that the stigma surrounding this liver condition is predominantly associated with obesity [12]. Furthermore, there is a notable difference in perception between healthcare providers and patients regarding the stigma of MASLD/NAFLD, with 38% of providers versus 8% of patients viewing MASLD/NAFLD as stigmatizing [12]. Another contributing factor to the low awareness could be the absence of approved treatments for MASLD/NAFLD. However, with the potential introduction of new drugs on the horizon, raising awareness among all stakeholders becomes increasingly critical [13]. This is particularly urgent in the MENA region, where the burden of MASLD/NAFLD is exceptionally high [3]. In this study, we provide a sub-analysis of data extracted from our global survey to enhance the understanding of physician knowledge about MASLD/NAFLD in the three MENA countries with high prevalence: Saudi Arabia, Egypt, and Türkiye.
2Materials and MethodsTwo separate knowledge and awareness surveys were developed globally by the members of the Global NASH Council (www.globalnashcouncil.org). The 59-item specialist version of the survey was designed to be completed by gastroenterologists (GEs) and hepatologists (MASLD/NAFLD specialists). Conversely, a 54-item version was offered to endocrinologists and internal medicine/primary care providers (PCPs) (non-specialists). The versions of the survey tailored for specialists and non-specialists contained questions pertinent to their respective fields, with 21 questions common to both. The Global NASH Council facilitated the distribution of the survey link to physicians within their countries. The survey was available online through Survey Monkey and could be completed in English or various national languages from April 2019 to September 2020 [9].
Both the specialist and non-specialist versions of the survey included questions about physicians' practices and awareness of MASLD/NAFLD. The specialist version featured 32 multiple-choice questions, whereas the non-specialist version had 24, each with only one correct answer. Knowledge scores were calculated based on the proportion of correct answers, ranging from 0 to 100, across three domains: 1) Epidemiology and Pathogenesis, 2) Diagnostics, and 3) Treatment. These scores were computed separately for each version of the survey. The overall knowledge score was then determined by averaging the scores from the three domains.
The description of the surveys has been previously published [9]. For the purpose of this study, we selected only those survey respondents who indicated their primary practice location was in a MENA country, specifically Saudi Arabia, Egypt, and Türkiye.
2.1Statistical analysisThe survey responses were summarized as counts (percentages) or medians (interquartile ranges [IQRs]). The chi-square and Kruskal-Wallis tests were applied for intergroup comparisons of categorical and continuous parameters, respectively, across specialties. The knowledge scores were summarized as mean (standard deviation) and were compared between specialties using Mann-Whitney U test for the specialist and non-specialist versions of the survey separately.
Independent predictors of the total knowledge scores were assessed using generalized linear regression models. In these models, potential predictors of the scores included physician's country of practice, medical specialty, practice setting, the number of years in practice, self-reported number of patients with MASLD/NAFLD seen over a predetermined period, and their primary source of knowledge about MASLD/NAFLD. Predictors with two-sided p < 0.05 were considered statistically significant.
2.2Ethical statementAll analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA). The WIRB-Copernicus Group Institutional Review Board (WCG® IRB) exempted the study from requiring participant consent due to the survey's content and the anonymity of both responses and data analysis.
3ResultsA total of 584 physicians from MENA countries completed the survey, comprising 126 hepatologists, 178 GEs, 38 endocrinologists, and 242 internal medicine/PCPs. Among the physicians who participated, 59% were from Türkiye, 25% were from Egypt, and 16% were from Saudi Arabia. Hepatologists were predominantly from Egypt, accounting for 64% of the total, whereas GEs were significantly represented from Saudi Arabia at 30%. PCPs/others were mainly from Türkiye, making up 91% of respondents in that category.
Compared to other specialties, hepatologists reported the most extensive duration of practice, with a median of 14 years in contrast to 3–10 years for other groups (p < 0.0001) (Table 1). Over 80% of providers across all specialties were hospital-affiliated, although hepatologists also frequently practiced in clinics (16%, compared to 4–7% in other specialties) (Table 1). For all specialties, practice guidelines were the primary source for the latest knowledge about MASLD/NAFLD (43–51%), with national or international conferences being the next common source (24–31%). However, for PCPs/others, the internet was the second most common source of information (25%) (Table 1).
Demographics of participating physicians from Saudi Arabia, Egypt, and Türkiye.
| Characteristics | Hepatologists | Gastroenterologists | Endocrinologists | PCPs and others | p-value | All |
|---|---|---|---|---|---|---|
| N | 126 | 178 | 38 | 242 | 584 | |
| Age, years | ||||||
| < 25 | 0 (0.0%) | 2 (1.1%) | 0 (0.0%) | 12 (5.0%) | <0.0001 | 14 (2.4%) |
| 26–30 | 6 (4.8%) | 4 (2.2%) | 2 (5.3%) | 102 (42.1%) | 114 (19.5%) | |
| 31–35 | 22 (17.5%) | 33 (18.5%) | 7 (18.4%) | 54 (22.3%) | 116 (19.9%) | |
| 36–40 | 28 (22.2%) | 37 (20.8%) | 9 (23.7%) | 32 (13.2%) | 106 (18.2%) | |
| 41–45 | 19 (15.1%) | 41 (23.0%) | 13 (34.2%) | 19 (7.9%) | 92 (15.8%) | |
| 46–50 | 19 (15.1%) | 25 (14.0%) | 3 (7.9%) | 9 (3.7%) | 56 (9.6%) | |
| 51–55 | 16 (12.7%) | 19 (10.7%) | 3 (7.9%) | 10 (4.1%) | 48 (8.2%) | |
| 56–60 | 7 (5.6%) | 12 (6.7%) | 1 (2.6%) | 3 (1.2%) | 23 (3.9%) | |
| > 60 | 9 (7.1%) | 5 (2.8%) | 0 (0.0%) | 1 (0.4%) | 15 (2.6%) | |
| Gender | ||||||
| Female | 34 (27.0%) | 35 (19.9%) | 15 (40.5%) | 110 (46.2%) | <0.0001 | 194 (33.6%) |
| Male | 92 (73.0%) | 141 (80.1%) | 22 (59.5%) | 128 (53.8%) | 383 (66.4%) | |
| Practice setting: | ||||||
| Hospital-based | 101 (80.2%) | 153 (86.0%) | 36 (94.7%) | 203 (83.9%) | <0.0001 | 493 (84.4%) |
| Group practice | 0 (0.0%) | 3 (1.7%) | 0 (0.0%) | 3 (1.2%) | 6 (1.0%) | |
| Solo private practice | 1 (0.8%) | 5 (2.8%) | 0 (0.0%) | 1 (0.4%) | 7 (1.2%) | |
| Clinic-based | 20 (15.9%) | 12 (6.7%) | 2 (5.3%) | 9 (3.7%) | 43 (7.4%) | |
| Other | 4 (3.2%) | 5 (2.8%) | 0 (0.0%) | 26 (10.7%) | 35 (6.0%) | |
| Number of years in practice, median (IQR) | 14 (8–20) | 10 (6–17) | 8 (3–10) | 3 (2–7) | <0.0001 | 8 (3–15) |
| Number of NAFLD patients seen per year, median (IQR) | 130 (60–300) | 110 (50–250) | 200 (100–600) | 25 (8–100) | <0.0001 | 100 (20–200) |
| Primary source of knowledge about NAFLD: | ||||||
| Guidelines | 62 (49.2%) | 91 (51.1%) | 19 (50.0%) | 105 (43.4%) | <0.0001 | 277 (47.4%) |
| Local Conferences | 5 (4.0%) | 10 (5.6%) | 2 (5.3%) | 8 (3.3%) | 25 (4.3%) | |
| National or international conferences or meetings | 39 (31.0%) | 43 (24.2%) | 9 (23.7%) | 34 (14.0%) | 125 (21.4%) | |
| Medical Journals | 12 (9.5%) | 12 (6.7%) | 7 (18.4%) | 25 (10.3%) | 56 (9.6%) | |
| Internet | 6 (4.8%) | 15 (8.4%) | 1 (2.6%) | 60 (24.8%) | 82 (14.0%) | |
| Other | 2 (1.6%) | 7 (3.9%) | 0 (0.0%) | 10 (4.1%) | 19 (3.3%) |
N, number; IQR,interquartile range; NAFLD,non-alcoholic fatty liver disease; PCPs, Primary care providers.
P-value returned by chi-square or Mann-Whitney (for continuous parameters) tests.
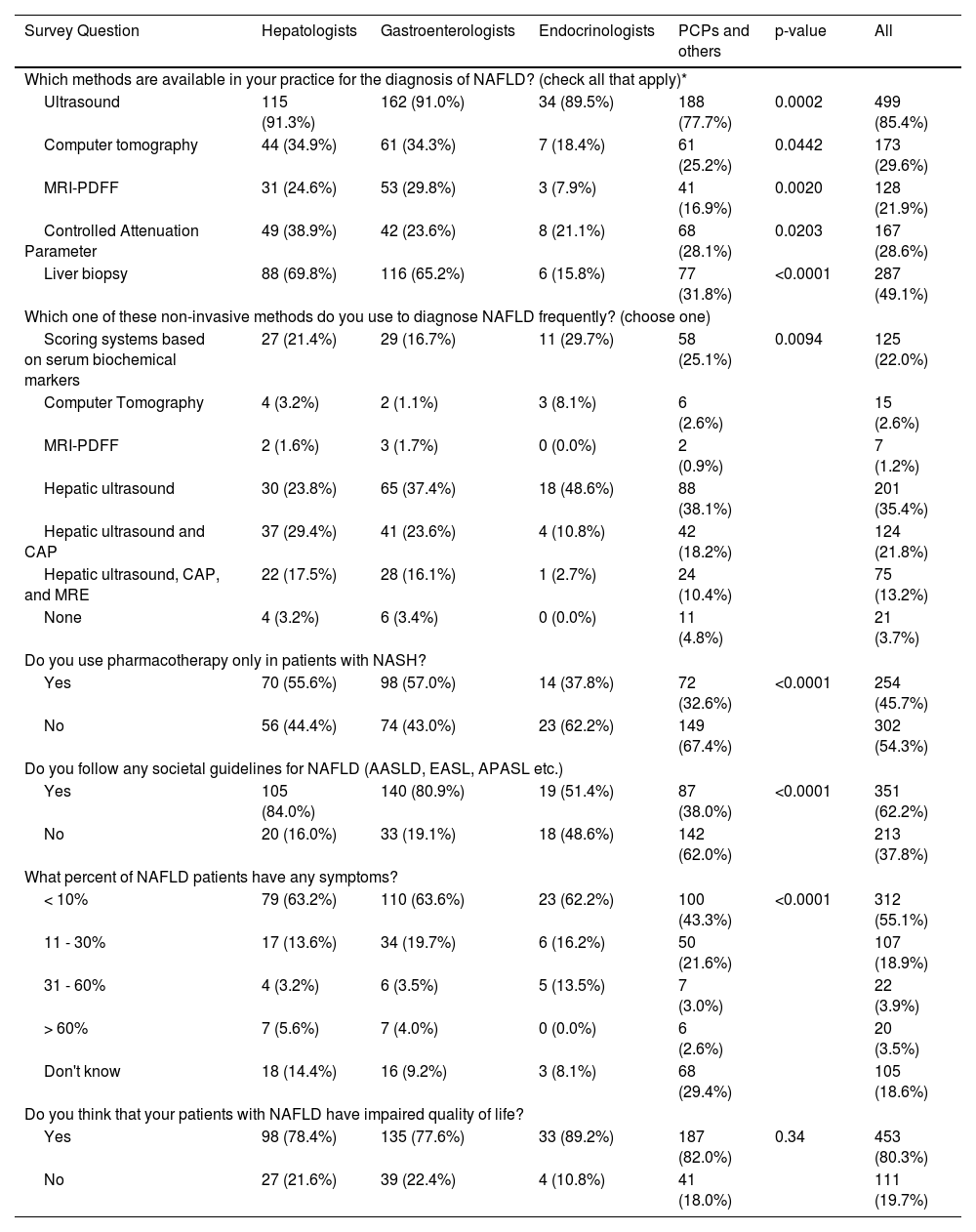
Hepatologists and GEs reported significantly greater access to diagnostic modalities for MASLD/NAFLD (ultrasound, computer tomography, magnetic resonance imaging derived proton density fat fraction [MRI-PDFF], and liver biopsy) compared to non-specialists (all p < 0.05) (Table 2). Ultrasound was the most widely available diagnostic tool, accessible to 85% of physicians across all specialties, including 78% of PCPs/others, whereas liver biopsy was available to 70% of hepatologists but only 16% of endocrinologists (p < 0.0001) (Table 2). MRI-PDFF was the least accessible MASLD/NAFLD diagnostic modality across all medical specialties (8–30%) (Table 2). Among hepatologists, hepatic ultrasound combined with controlled attenuation parameter (CAP) was the preferred diagnostic choice (29%), whereas hepatic ultrasound alone was favored by other specialties (37–49%) (Table 2). Additionally, serum biomarkers were the second most commonly utilized diagnostic tool among non-specialists (25–30%) (Table 2). In contrast, a very small percentage (0–8%) of physicians used CT scans or MRI-PDFF as their primary method for diagnosing MASLD/NAFLD (Table 2).
Availability of MASLD/NAFLD diagnostic methods and awareness among physicians of different specialties.
| Survey Question | Hepatologists | Gastroenterologists | Endocrinologists | PCPs and others | p-value | All |
|---|---|---|---|---|---|---|
| Which methods are available in your practice for the diagnosis of NAFLD? (check all that apply)* | ||||||
| Ultrasound | 115 (91.3%) | 162 (91.0%) | 34 (89.5%) | 188 (77.7%) | 0.0002 | 499 (85.4%) |
| Computer tomography | 44 (34.9%) | 61 (34.3%) | 7 (18.4%) | 61 (25.2%) | 0.0442 | 173 (29.6%) |
| MRI-PDFF | 31 (24.6%) | 53 (29.8%) | 3 (7.9%) | 41 (16.9%) | 0.0020 | 128 (21.9%) |
| Controlled Attenuation Parameter | 49 (38.9%) | 42 (23.6%) | 8 (21.1%) | 68 (28.1%) | 0.0203 | 167 (28.6%) |
| Liver biopsy | 88 (69.8%) | 116 (65.2%) | 6 (15.8%) | 77 (31.8%) | <0.0001 | 287 (49.1%) |
| Which one of these non-invasive methods do you use to diagnose NAFLD frequently? (choose one) | ||||||
| Scoring systems based on serum biochemical markers | 27 (21.4%) | 29 (16.7%) | 11 (29.7%) | 58 (25.1%) | 0.0094 | 125 (22.0%) |
| Computer Tomography | 4 (3.2%) | 2 (1.1%) | 3 (8.1%) | 6 (2.6%) | 15 (2.6%) | |
| MRI-PDFF | 2 (1.6%) | 3 (1.7%) | 0 (0.0%) | 2 (0.9%) | 7 (1.2%) | |
| Hepatic ultrasound | 30 (23.8%) | 65 (37.4%) | 18 (48.6%) | 88 (38.1%) | 201 (35.4%) | |
| Hepatic ultrasound and CAP | 37 (29.4%) | 41 (23.6%) | 4 (10.8%) | 42 (18.2%) | 124 (21.8%) | |
| Hepatic ultrasound, CAP, and MRE | 22 (17.5%) | 28 (16.1%) | 1 (2.7%) | 24 (10.4%) | 75 (13.2%) | |
| None | 4 (3.2%) | 6 (3.4%) | 0 (0.0%) | 11 (4.8%) | 21 (3.7%) | |
| Do you use pharmacotherapy only in patients with NASH? | ||||||
| Yes | 70 (55.6%) | 98 (57.0%) | 14 (37.8%) | 72 (32.6%) | <0.0001 | 254 (45.7%) |
| No | 56 (44.4%) | 74 (43.0%) | 23 (62.2%) | 149 (67.4%) | 302 (54.3%) | |
| Do you follow any societal guidelines for NAFLD (AASLD, EASL, APASL etc.) | ||||||
| Yes | 105 (84.0%) | 140 (80.9%) | 19 (51.4%) | 87 (38.0%) | <0.0001 | 351 (62.2%) |
| No | 20 (16.0%) | 33 (19.1%) | 18 (48.6%) | 142 (62.0%) | 213 (37.8%) | |
| What percent of NAFLD patients have any symptoms? | ||||||
| < 10% | 79 (63.2%) | 110 (63.6%) | 23 (62.2%) | 100 (43.3%) | <0.0001 | 312 (55.1%) |
| 11 - 30% | 17 (13.6%) | 34 (19.7%) | 6 (16.2%) | 50 (21.6%) | 107 (18.9%) | |
| 31 - 60% | 4 (3.2%) | 6 (3.5%) | 5 (13.5%) | 7 (3.0%) | 22 (3.9%) | |
| > 60% | 7 (5.6%) | 7 (4.0%) | 0 (0.0%) | 6 (2.6%) | 20 (3.5%) | |
| Don't know | 18 (14.4%) | 16 (9.2%) | 3 (8.1%) | 68 (29.4%) | 105 (18.6%) | |
| Do you think that your patients with NAFLD have impaired quality of life? | ||||||
| Yes | 98 (78.4%) | 135 (77.6%) | 33 (89.2%) | 187 (82.0%) | 0.34 | 453 (80.3%) |
| No | 27 (21.6%) | 39 (22.4%) | 4 (10.8%) | 41 (18.0%) | 111 (19.7%) | |
AASLD, American Association for the Study of Liver Disease; APASL, Asia Pacific Association for the Study of Liver; EASL, European Association for the Study of Liver; MRE, magnetic resonance enterography; MRI-PDFF, magnetic resonance imaging derived proton density fat fraction; NAFLD, non-alcoholic fatty liver disease; PCPs, primary care providers; P-value, returned by chi-square test for independence.
A marked disparity existed in adherence to societal guidelines for MASLD/NAFLD management, with 81–84% of specialists following them compared to only 38–51% of non-specialists (p < 0.0001) (Table 2). Additionally, 62–64% of hepatologists, GEs, and endocrinologists believed that fewer than 10% of patients with MASLD/NAFLD exhibit symptoms, in contrast to 43% of PCPs/others who shared this view. Notably, 29% of PCPs/others were uncertain about the proportion of patients with symptomatic MASLD/NAFLD (Table 2) (p < 0.0001). Although the perceived prevalence of symptomatic MASLD/NAFLD was generally low, the vast majority of physicians across all specialties (78–89%, p > 0.05) believed that patients with MASLD/NAFLD experience an impaired quality of life (Table 2).
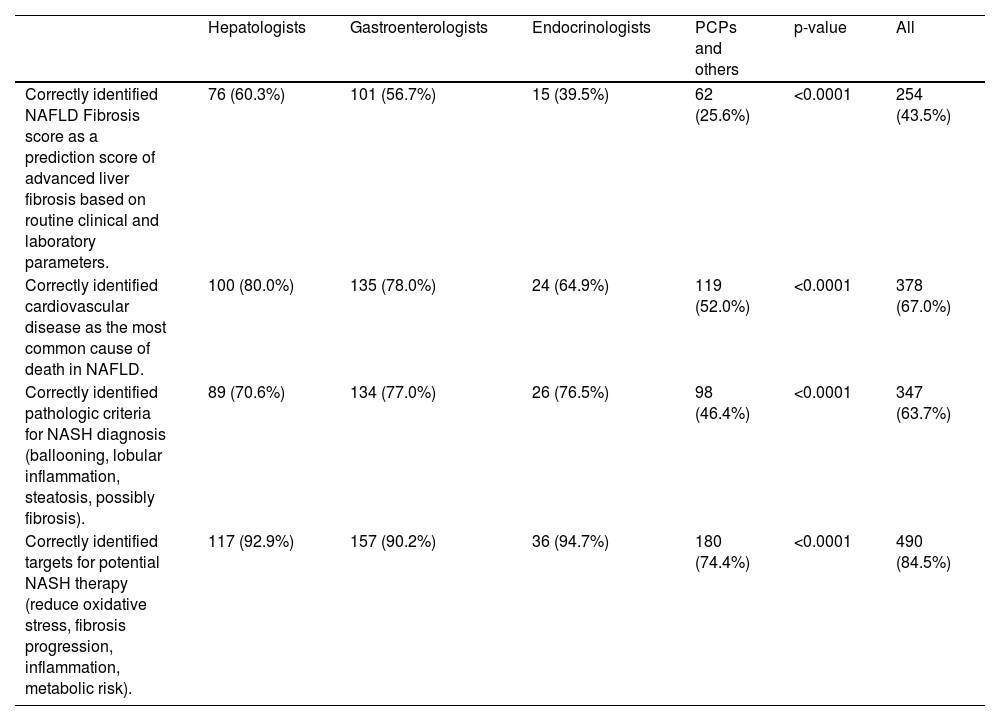
Specialists demonstrated the highest accuracy in answering questions about MASLD/NAFLD, whereas PCPs/others showed the lowest accuracy rates. The question regarding the definition of the MASLD/NAFLD Fibrosis Score saw the lowest correct response rates, with 60% among hepatologists and only 26% among PCPs/others. The correct understanding was that the NAFLD Fibrosis Score is based on laboratory parameters, not histology or imaging (Table 3). In the specialist version of the survey, hepatologists and GEs showed similar mean knowledge scores (all p > 0.05) (Table 4). Conversely, in the non-specialist version, endocrinologists outperformed PCPs/others in knowledge scores, particularly in the domains of Epidemiology/Pathogenesis (72% vs. 60%), Diagnostics (73% vs. 67%), and Treatment (78% vs. 67%) (all p < 0.01) (Table 4).
Knowledge about MASLD/NAFLD across medical specialties.
| Hepatologists | Gastroenterologists | Endocrinologists | PCPs and others | p-value | All | |
| Correctly identified NAFLD Fibrosis score as a prediction score of advanced liver fibrosis based on routine clinical and laboratory parameters. | 76 (60.3%) | 101 (56.7%) | 15 (39.5%) | 62 (25.6%) | <0.0001 | 254 (43.5%) |
| Correctly identified cardiovascular disease as the most common cause of death in NAFLD. | 100 (80.0%) | 135 (78.0%) | 24 (64.9%) | 119 (52.0%) | <0.0001 | 378 (67.0%) |
| Correctly identified pathologic criteria for NASH diagnosis (ballooning, lobular inflammation, steatosis, possibly fibrosis). | 89 (70.6%) | 134 (77.0%) | 26 (76.5%) | 98 (46.4%) | <0.0001 | 347 (63.7%) |
| Correctly identified targets for potential NASH therapy (reduce oxidative stress, fibrosis progression, inflammation, metabolic risk). | 117 (92.9%) | 157 (90.2%) | 36 (94.7%) | 180 (74.4%) | <0.0001 | 490 (84.5%) |
P-value returned by chi-square test for independence.
MASLD/NAFLD knowledge scores* across medical specialties (mean ± SD).
| Specialist version | Non-specialist version | |||||||
| Knowledge domain | Hepatologists | Gastroenterologists | p | All specialists | Endocrinologists | PCPs and others | p-value | All non-specialists |
| Epidemiology & pathogenesis | 53.6 ± 17.5 | 51.2 ± 15.6 | 0.23 | 53.6 ± 17.5 | 72.2 ± 17.2 | 59.9 ± 21.4 | 0.0010 | 61.6 ± 21.3 |
| Diagnostics | 69.3 ± 14.9 | 71.7 ± 14.9 | 0.13 | 69.3 ± 14.9 | 72.7 ± 10.6 | 67.0 ± 12.9 | 0.0083 | 67.8 ± 12.7 |
| Treatment | 58.1 ± 11.4 | 55.7 ± 12.2 | 0.13 | 58.1 ± 11.4 | 78.3 ± 13.9 | 67.1 ± 19.4 | 0.0004 | 68.6 ± 19.1 |
| Total | 60.3 ± 10.8 | 59.5 ± 10.0 | 0.45 | 60.3 ± 10.8 | 74.4 ± 9.5 | 64.7 ± 13.7 | <0.0001 | 66.0 ± 13.6 |
* The scores (proportions of correct responses, range 0–100) were calculated separately for the specialist and non-specialist versions of the survey using different subsets of questions and are not directly comparable between the two versions besides questions included in Table 3.
In multivariable analysis, adjusted for the participants’ country, independent predictors of higher total MASLD/NAFLD knowledge scores for specialists included having a practice affiliated with a hospital and seeing a greater number of patients with MASLD/NAFLD per month (p < 0.05) (Supplementary Table 1). In contrast, the knowledge scores were not significantly associated with the medical specialty (hepatologists vs. GEs), years of practice, or the source of knowledge about MASLD/NAFLD (p > 0.05) (Supplementary Table 1). Among non-specialists, being an endocrinologist (vs. PCP/other) and managing a higher number of patients with MASLD/NAFLD (p < 0.01), but not the type of practice setting or the length (p > 0.05), were associated with higher knowledge scores (Supplementary Table 1). Additionally, non-specialists who primarily relied on the internet for MASLD/NAFLD information had significantly lower knowledge scores (p = 0.01) (Supplementary Table 1).
3.3Assessment and management of MASLD/NAFLD among specialistsAmong GEs and hepatologists, 40–43% reported that the majority (>50%) of their patients with MASLD/NAFLD exhibited normal liver enzymes (Table 5). A significant majority (70–82%) seldom recommended liver biopsies for patients with MASLD/NAFLD, typically in fewer than 10% of cases (Table 5). While more than half of GEs/hepatologists (56–60%) would consider a biopsy if other liver conditions potentially coexist with steatohepatitis, less than half (35–38%) would do so based solely on elevated non-invasive fibrosis markers unless these markers persist for over 6 months (57–60%). Only a small fraction (14–20%) would recommend a biopsy for the presence of metabolic syndrome alone (Table 5). Notably, both GEs and hepatologists reported that most of their patients with metabolic dysfunction-associated steatohepatitis/non-alcoholic steatohepatitis (40–43%) had stage 2 fibrosis, with a small percentage (5–6%) diagnosed with cirrhosis at the time of liver biopsy (Table 5).
Survey of MASLD/NAFLD practice among completers of the specialist version of the survey (hepatologists, gastroenterologists).
| Hepatologists | Gastroenterologists | p-value | All specialists | |
| In your practice, what percentage of patients with NAFLD has normal aminotransferase levels? | ||||
| < 20% | 15 (11.9%) | 16 (9.1%) | 0.70 | 31 (10.3%) |
| 20 – 30% | 12 (9.5%) | 25 (14.3%) | 37 (12.3%) | |
| 31 – 40% | 13 (10.3%) | 24 (13.7%) | 37 (12.3%) | |
| 41 – 50% | 21 (16.7%) | 25 (14.3%) | 46 (15.3%) | |
| > 50% | 54 (42.9%) | 70 (40.0%) | 124 (41.2%) | |
| Don't know | 11 (8.7%) | 15 (8.6%) | 26 (8.6%) | |
| In your expert opinion, what are the indications for liver biopsy (check all that apply)?* | ||||
| Other liver diseases that can co-exist with steatohepatitis | 76 (60.3%) | 99 (55.6%) | 0.41 | 175 (57.6%) |
| Presence of metabolic syndrome that places the patient at risk for steatohepatitis | 25 (19.8%) | 25 (14.0%) | 0.18 | 50 (16.4%) |
| High NAFLD fibrosis score or other non-invasive markers of fibrosis with presence of fat by imaging | 48 (38.1%) | 63 (35.4%) | 0.63 | 111 (36.5%) |
| Chronically elevated liver enzymes for more than 6 months | 72 (57.1%) | 106 (59.6%) | 0.67 | 178 (58.6%) |
| None | 5 (4.0%) | 1 (0.6%) | 0.0354 | 6 (2.0%) |
| What proportion of your patients with NAFLD do you send for liver biopsy? | ||||
| < 10% | 103 (81.7%) | 122 (70.1%) | 0.12 | 225 (75.0%) |
| 10 to < 30% | 17 (13.5%) | 40 (23.0%) | 57 (19.0%) | |
| 30 to < 50% | 3 (2.4%) | 8 (4.6%) | 11 (3.7%) | |
| ≥ 50% | 3 (2.4%) | 4 (2.3%) | 7 (2.3%) | |
| In your practice, at what stage will NASH usually be diagnosed? | ||||
| F1 | 20 (15.9%) | 37 (21.3%) | 0.67 | 57 (19.0%) |
| F2 | 54 (42.9%) | 70 (40.2%) | 124 (41.3%) | |
| F3 | 25 (19.8%) | 34 (19.5%) | 59 (19.7%) | |
| F4 | 6 (4.8%) | 11 (6.3%) | 17 (5.7%) | |
| Don't know | 21 (16.7%) | 22 (12.6%) | 43 (14.3%) | |
| Do you use pharmacotherapy only in patients with NASH? | ||||
| Yes | 70 (55.6%) | 98 (57.0%) | 0.81 | 168 (56.4%) |
| No | 56 (44.4%) | 74 (43.0%) | 130 (43.6%) | |
P-value returned by chi-square test for independence.
Non-specialists were asked about their approach to the identification and early-stage management of patients with MASLD/NAFLD. They were found to prioritize screening for MASLD/NAFLD among patients with diabetes, unexplained elevated alanine transaminase (ALT) levels, and dyslipidemia, with screening rates of 84% and 74% for diabetes, 70–74% for elevated ALT, and 66–71% for dyslipidemia, respectively (Table 6). A minority would screen all patients for MASLD/NAFLD (18% of endocrinologists and 10% of PCPs/others), and a few PCPs/others (9%) did not screen for MASLD/NAFLD at all (Table 6). Upon incidental discovery of fatty liver on imaging, 3% of PCPs/others would take no action (Table 6).
Survey of MASLD/NAFLD practice among completers of the non-specialist version of the survey.
| Endocrinologists | PCPs and others | p-value | All | |
| Which type of individual do you screen for NAFLD? (check all that apply)* | ||||
| Everyone | 7 (18.4%) | 25 (10.3%) | 0.15 | 32 (11.4%) |
| Diabetes | 32 (84.2%) | 179 (74.0%) | 0.17 | 211 (75.4%) |
| Hypertension | 16 (42.1%) | 70 (28.9%) | 0.10 | 86 (30.7%) |
| Dyslipidemia | 27 (71.1%) | 160 (66.1%) | 0.55 | 187 (66.8%) |
| Sleep apnea | 17 (44.7%) | 69 (28.5%) | 0.0438 | 86 (30.7%) |
| Hypothyroidism | 17 (44.7%) | 73 (30.2%) | 0.07 | 90 (32.1%) |
| Cardio-vascular disease | 21 (55.3%) | 108 (44.6%) | 0.22 | 129 (46.1%) |
| Polycystic ovary syndrome | 18 (47.4%) | 67 (27.7%) | 0.0142 | 85 (30.4%) |
| Unexplained elevated ALT | 28 (73.7%) | 169 (69.8%) | 0.63 | 197 (70.4%) |
| Cryptogenic liver disease | 21 (55.3%) | 124 (51.2%) | 0.64 | 145 (51.8%) |
| I do not screen for NAFLD | 0 (0.0%) | 23 (9.5%) | 0.0473 | 23 (8.2%) |
| What do you do if you find NAFLD incidentally in an imaging in your patient? (check all that apply)* | ||||
| Investigate presence of metabolic alterations and the absence of others liver diseases | 31 (81.6%) | 166 (68.6%) | 0.10 | 197 (70.4%) |
| Asses the cardiovascular risk and investigate if the patients have a liver disease | 27 (71.1%) | 113 (46.7%) | 0.0052 | 140 (50.0%) |
| Assess the risk of metabolic alterations and liver function with non-invasive scoring system in absence of other a chronic liver disease | 22 (57.9%) | 127 (52.5%) | 0.53 | 149 (53.2%) |
| Investigate presence of alcohol consumption. | 23 (60.5%) | 116 (47.9%) | 0.15 | 139 (49.6%) |
| Do nothing | 0 (0.0%) | 7 (2.9%) | 0.29 | 7 (2.5%) |
| In your expert opinion, what is the initial assessment of patients with suspected NAFLD? (check all that apply)* | ||||
| Elevated liver enzymes | 25 (65.8%) | 156 (64.5%) | 0.87 | 181 (64.6%) |
| Exclusion of other liver diseases | 20 (52.6%) | 131 (54.1%) | 0.86 | 151 (53.9%) |
| Controlled Attenuation Parameter | 4 (10.5%) | 31 (12.8%) | 0.69 | 35 (12.5%) |
| Liver biopsy | 6 (15.8%) | 16 (6.6%) | 0.05 | 22 (7.9%) |
| A liver imaging such as ultrasound, MRI or CT scan | 7 (18.4%) | 55 (22.7%) | 0.55 | 62 (22.1%) |
| None | 0 (0.0%) | 8 (3.3%) | 0.26 | 8 (2.9%) |
| In your opinion, what are the indications for liver biopsy? (check all that apply)* | ||||
| At risk for steatohepatitis or cirrhosis | 20 (52.6%) | 124 (51.2%) | 0.87 | 144 (51.4%) |
| Other conditions that cause steatohepatitis can't be excluded | 21 (55.3%) | 130 (53.7%) | 0.86 | 151 (53.9%) |
| Other conditions that cause steatohepatitis coexist | 10 (26.3%) | 56 (23.1%) | 0.67 | 66 (23.6%) |
| Presence of metabolic syndrome | 6 (15.8%) | 17 (7.0%) | 0.07 | 23 (8.2%) |
| High NAFLD fibrosis score | 20 (52.6%) | 114 (47.1%) | 0.53 | 134 (47.9%) |
| Do you refer cases of NAFLD to a gastroenterologist or hepatologist? | ||||
| Yes | 31 (83.8%) | 172 (75.8%) | 0.28 | 203 (76.9%) |
| No | 6 (16.2%) | 55 (24.2%) | 61 (23.1%) | |
| When do you send a patient with NAFLD to a specialist? (check all that apply)* | ||||
| At risk for steatohepatitis or cirrhosis | 26 (68.4%) | 167 (69.0%) | 0.94 | 193 (68.9%) |
| Other conditions that cause steatohepatitis can't be excluded | 24 (63.2%) | 126 (52.1%) | 0.20 | 150 (53.6%) |
| Other conditions that cause steatohepatitis coexist | 12 (31.6%) | 90 (37.2%) | 0.50 | 102 (36.4%) |
| Presence of metabolic syndrome | 4 (10.5%) | 49 (20.2%) | 0.15 | 53 (18.9%) |
| High NAFLD fibrosis score | 18 (47.4%) | 129 (53.3%) | 0.50 | 147 (52.5%) |
| Do you use pharmacotherapy only in patients with NASH? | ||||
| Yes | 14 (37.8%) | 72 (32.6%) | 0.53 | 86 (33.3%) |
| No | 23 (62.2%) | 149 (67.4%) | 172 (66.7%) | |
| What are the barriers for NAFLD management in your practice? (select one most important) | ||||
| Lack of confidence in managing it | 3 (8.1%) | 13 (5.7%) | 0.07 | 16 (6.0%) |
| Time constrains | 11 (29.7%) | 30 (13.0%) | 41 (15.4%) | |
| Cost of evaluation and treatment | 2 (5.4%) | 12 (5.2%) | 14 (5.2%) | |
| Failure of patients to adhere to the lifestyle modifications | 18 (48.6%) | 124 (53.9%) | 142 (53.2%) | |
| Lack of availability of effective drugs | 1 (2.7%) | 37 (16.1%) | 38 (14.2%) | |
| No barriers | 2 (5.4%) | 14 (6.1%) | 16 (6.0%) | |
ALT, alanine transaminase; CT, computed tomography; MRI, magnetic resonance imaging; PCPs, primary care providers; P-value, returned by chi-square test for independence.
On the initial assessment of patients with suspected MASLD/NAFLD, 65–66% would assess liver enzymes, and 53–54% would try to exclude other liver diseases (Table 6). Over half of the physicians (51–55%) would only recommend a biopsy for patients at risk of steatohepatitis and cirrhosis or when other liver diseases could not be excluded. In addition, nearly half of the survey completers (47–53%) would order a liver biopsy for patients with a high MASLD/NAFLD Fibrosis Score (Table 6).
Regarding treatment, 33–38% of non-specialists would prescribe pharmacotherapy for patients with MASLD/NAFLD (p > 0.05) (Table 6). Moreover, 76%−84% would refer their patients with MASLD/NAFLD to a specialist (GE/hepatologist), especially if they identify them to be at risk for steatohepatitis or cirrhosis (68–69%), if other conditions that cause steatohepatitis cannot be excluded (52–63%), or if they have a high MASLD/NAFLD Fibrosis Score (47–53%) (all p > 0.05) (Table 6). The main barrier to MASLD/NAFLD management cited among non-specialists was patient non-adherence to lifestyle modifications (49–54%) (Table 6), with endocrinologists more frequently mentioning time constraints (30% vs. 13%) and PCPs/others pointing to the lack of effective drugs (16% vs. 3%) (p = 0.07) (Table 6).
4DiscussionThis study suggests a substantial knowledge gap about MASLD/NAFLD among healthcare providers in the three MENA countries: Saudi Arabia, Egypt, and Türkiye. This sub-analysis of our global physician survey primarily focuses on the MENA region [9]. Given the enormous burden of MASLD/NAFLD in the MENA region, assessment of physician knowledge in this region is of great importance. Our data show that the knowledge gap about MASLD/NAFLD in the MENA region is most pronounced among PCPs/others and less so among GEs and hepatologists. However, even these specialists, who should be experts in liver diseases, did not consistently recognize the prognostic significance of hepatic fibrosis stages and cardiovascular disease in patients with MASLD/NAFLD, with only 60–80% identifying these correctly. Furthermore, there was a paradox in providers’ perceptions of MASLD/NAFLD symptomatology. While many acknowledged that patients with these conditions experience reduced HRQL, they also predominantly considered these patients to be “asymptomatic”. This contradiction highlights the need to emphasize that MASLD/NAFLD is not truly asymptomatic, as a considerable number of patients suffer from significant fatigue, which greatly affects their HRQL [13,14].
Another interesting observation from this study is that most providers, particularly PCPs/others, relied on elevated ALT levels to screen for MASLD/NAFLD. However, this method only partially captures those at risk for progressive liver disease and may overlook many patients with cardiometabolic risks who do not exhibit elevated liver enzymes [15]. To enhance the detection of high-risk patients with MASLD/NAFLD, it is crucial to increase adherence to the latest guidelines from professional societies. These recommendations urge for a comprehensive approach that integrates clinical assessments, physical examinations, laboratory data, and non-invasive tests [16-19].
Expectedly, both specialist and non-specialist providers from the three MENA countries who treated more patients with MASLD/NAFLD demonstrated higher knowledge and awareness scores. This correlation was particularly strong among specialists such as GEs and hepatologists, especially those affiliated with hospitals. These findings imply that such affiliations may provide better access to current knowledge and resources, and the demands of a busy practice necessitate a deeper understanding of conditions like MASLD/NAFLD. This inference can also be seen with the number of hepatologists who participated in this study from Egypt. Indeed, Egypt has had a long history of high rates of liver disease (e.g., schistosomiasis and hepatitis C virus). As a result of concerted efforts of hepatologists and the local governments, the prevalence of these liver diseases are decreasing significantly [20-21]. As such, hepatologists from Egypt appear in a prime position to lead efforts to implement strategies effectively to decrease the burden of MASLD/NALFD in the MENA region.
While specialists often rely on medical journals, society guidelines, and conferences for information on MASLD/NAFLD, a significant number of PCPs/others turned to the internet. However, internet reliance as a primary information source was linked to lower knowledge scores among non-specialists. Therefore, as efforts are made to enhance knowledge and awareness of liver diseases, it is crucial to employ varied strategies tailored to different healthcare provider groups.
The study limitations include a potential bias, favoring responses from specialists and PCPs/others with a particular interest or expertise in MASLD/NAFLD, as well as those affiliated with hospitals. The extent of this bias is difficult to quantify due to the unrecorded number of survey invitations distributed. Consequently, the survey may have overlooked a significant segment of the medical community, including community-based providers, those practicing in rural areas, and non-physician healthcare professionals. This limitation could affect the generalizability of the study findings and suggests the need for a more comprehensive approach to future research in this area. The sample of survey completers used in this study has an overrepresentation of hepatologists from Egypt and PCPs from Türkiye, which may have caused a bias of an unknown direction. Inclusion of cardiology providers in the survey could have been informative given that cardiovascular events are the most common cause of mortality in NASH; that group of providers was not reached by the survey.
5ConclusionsIn conclusion, we have demonstrated a non-negligible knowledge gap about MASLD/NAFLD among providers in the three MENA countries, especially among PCPs/others. Given that these providers are often the first point of contact for affected patients, targeted educational programs are warranted. Such initiatives should prioritize those most susceptible to MASLD/NAFLD and focus on the disease pathophysiology, patient-reported outcomes, and diagnostic techniques, including their limitations. This educational effort is crucial in the MENA area, where the prevalence of the disease is notably high.
FundingThis research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statementSaleh A. Alqahtani: Conceptualization, Project administration, Resources, Supervision, Validation, Writing – review & editing. Yusuf Yilmaz: Data curation, Validation, Writing – review & editing. Mohamed El-Kassas: Data curation, Validation, Writing – review & editing. Khalid Alswat: Data curation, Validation, Writing – review & editing. Mohamed Mawardi: Data curation, Validation, Writing – review & editing. Faisal M. Sanai: Data curation, Validation, Writing – review & editing. Faisal Abaakhail: Data curation, Validation, Writing – review & editing. Saad Alghamdi: Data curation, Validation, Writing – review & editing. Waleed K. Al-Hamoudi: Data curation, Validation, Writing – review & editing. Fatema Nader: Project administration, Resources, Supervision. Maria Stepanova: Data curation, Formal analysis, Methodology, Writing – original draft. Zobair M. Younossi: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft.