Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and the third most common cause of cancer death, and accounts for 5.6% of all cancers. Nearly 82% of the approximately 550,000 liver cancer deaths each year occur in Asia. In some regions, cancer-related death from HCC is second only to lung cancer. The incidence and mortality of HCC are increasing in America countries as a result of an ageing cohort infected with chronic hepatitis C, and are expected to continue to rise as a consequence of the obesity epidemic. Clinical care and survival for patients with HCC has advanced considerably during the last two decades, thanks to improvements in patient stratification, an enhanced understanding of the pa-thophysiology of the disease, and because of developments in diagnostic procedures and the introduction of novel therapies and strategies in prevention. Nevertheless, HCC remains the third most common cause of cancer-related deaths worldwide. These LAASL recommendations on treatment of hepatocellular carcinoma are intended to assist physicians and other healthcare providers, as well as patients and other interested individuals, in the clinical decision-making process by describing the optimal management of patients with liver cancer.
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and the third most common cause of cancer death, and accounts for 5.6% of all cancers. Nearly 82% of the approximately 550,000 liver cancer deaths each year occur in Asia. In some regions, cancer-related death from HCC is second only to lung cancer. In Latin America it has been suggested that this neoplasia has increased. However, there is little information. Nevertheless, according to the prevalence of hepatitis C virus infection (and hepatitis B virus infection in some areas), obesity and alcohol intake in our region is expected that these speculations regarding its increase are true.
MethodologyA steering committee was invited among the members of the Latin American Association for the Study of the Liver (LAASL) to proposed the content of the Hepatocellular Cancer Consensus based on previous international consensuses as well as in topics of regional interest. A list of members from all participant countries in the LAASL was selected by the steering committee based on expertise and trajectory. The list of chapters for the Consensus was then matched to each expert so that one member would be responsible for drafting an initial version. Each author received an instruction manual prepared by the steering committee with specific instructions and a precise objective for each chapter. Authors were instructed to prepare all manuscripts following a concise and clear logical argument based on the best available evidence. Chapters were exhaustive to avoid duplicity of content. Recommendations had to be weighted according to another classification of other scientific societies of scientific evidence (Table 1). Once all initial drafts were written the steering committee selected another member of the ALEH to provide blinded comments. Reviewers were instructed to analyze each chapter carefully evaluating the relevance, completeness, and present importance of the content and references. Reviewers were specifically asked to analyze the recommendations and confirm the assessment of the strength of the evidence. An external scientific group double checked all references and provided editorial services including the translation of the chapters to Spanish or English. Final chapters of the consensus were made available to all members of LAASL for external evaluation and comments.
Strength of evidence classification.
| Class of evidence |
| • Class 1. Conditions for which there is evidence and/or general agreement that a given diagnostic evaluation procedure or treatment is beneficial, useful, and effective. |
| • Class 2. Conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a diagnostic evaluation, procedure, or treatment. |
| ° Class 2a. Weight of evidence/opinion is in favor of usefulness/efficacy. |
| ° Class 2b. Usefulness/efficacy is less well established by evidence/opinion. |
| • Class 3. Conditions for which there is evidence and/or general agreement that a diagnostic evaluation, procedure, or treatment is not useful/effective and in some cases may be harmful. |
| Level of Evidence |
| • Level A. Data derived from multiple randomized clinical trials or meta-analyses. |
| • Level B. Data derived from a single randomized trial or nonrandomized studies. |
| • Level C. Only consensus opinion of experts, case studies, or standard of care. |
HCC prevalence varies by geographic region. The incidence is highest (20 per 100,000 individuals) in areas with endemic hepatitis B virus (HBV) infection, such as sub-Saharan Africa and Eastern Asia.1 Mediterranean countries such as Italy, Spain, and Greece have intermediate incidence rates (10 to 20 per 100,000 individuals), whereas North and South America have a relatively low incidence (< 5 per 100,000 individuals). The age distribution of HCC largely depends on the dominant type of viral hepatitis and the age at which it was acquired. In regions with a high HBV incidence, infection occurs at birth and HCC diagnosis is established 10 years earlier than in regions where HBV is less prevalent, such as North America or Europe, where the most common etiology is hepatitis C virus (HCV) acquired later in life. HCC is more common in men than in women probably because infection by HBV or HCV and alcohol consumption are more prevalent and possibly more carcinogenic in men. In 80 to 90% of cases, HCC occurs with cirrhosis.2
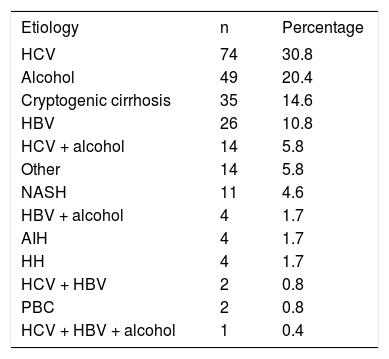
HCC in Latin AmericaInformation about the prevalence and incidence of, and risk factors for, HCC in Latin America is scarce. There are no reliable sources for the prevalence and incidence of HCC, but an approximation can be obtained from the cause-specific mortality rate, which was 4.1 per 100,000 in Mexico in 2000 and increased to 4.7 per 100,000 in 2006.3 A recent prospective study analyzed epidemiological aspects of HCC in Latin American countries.4 A total of 240 patients with HCC from nine countries were included in this study; the median age was 64 years, 72.5% of them were men, and 85.4% had underlying cirrhosis. The etiology of chronic liver disease (CLD) was HCV in 30.8% of patients, alcohol in 20.4%, cryptogenic cirrhosis in 14.6%, HBV in 10.8%, and HCV plus alcohol in 5.8% (Table 2). These results contrast with a retrospective study performed in Argentina,5 in which the main etiologies were chronic alcoholism in 41.6%, HCV in 40.5%, HBV in 13.4%, and cryptogenic cirrhosis in 9.2% of patients. In another recent study from Brazil,6 215 patients with diagnosis of HCC had a mean age of 57.3 (± 14.1) years, and 76.2% were men. The etiology of CLD was HCV and HBV infection in 43% and 23% of patients, respectively. Alcohol abuse alone or combined with other etiologies was identified in 32% of the patients. Schistosomiasis was found in 9% of the patients. Further studies are required to identify accurately the incidence, prevalence, mortality rate, and risk factors in Latin America.
Etiology of CLD in 240 patients with a diagnosis of HCC.
| Etiology | n | Percentage |
|---|---|---|
| HCV | 74 | 30.8 |
| Alcohol | 49 | 20.4 |
| Cryptogenic cirrhosis | 35 | 14.6 |
| HBV | 26 | 10.8 |
| HCV + alcohol | 14 | 5.8 |
| Other | 14 | 5.8 |
| NASH | 11 | 4.6 |
| HBV + alcohol | 4 | 1.7 |
| AIH | 4 | 1.7 |
| HH | 4 | 1.7 |
| HCV + HBV | 2 | 0.8 |
| PBC | 2 | 0.8 |
| HCV + HBV + alcohol | 1 | 0.4 |
HCC: hepatocellular carcinoma. HCV: hepatitis C virus. HBV: hepatitis B virus. NASH: nonalcoholic steatohepatitis. AIH: autoimmune hepatitis. PBC: primary biliary cirrhosis. Information from reference 4.
HCC etiology varies depending on the geographic location. In countries where HCC is endemic (subSaharan Africa, Asia, and Alaska), the most common cause is HBV infection, but in low-risk countries the most common HCC cause is cirrhosis, secondary to chronic viral infection or alcohol consumption.7
CirrhosisRegardless of its cause, cirrhosis is a major clinical and histopathological risk factor for HCC. One-third of cirrhotic patients will develop HCC during their lifetime.8 Long-term follow-up studies have reported that 1-8% of patients with cirrhosis develop HCC per year (e.g., 2% in HBV-infected cirrhotic patients and 3-8% in HCV-infected cirrhotic patients). In a Latin American study, 85% of HCC patients had underlying cirrhosis, whereas in a study from Argentina, cirrhosis was present in 93% of patients.4
The causes of cirrhosis include chronic viral hepatitis, alcohol abuse, inherited metabolic diseases such as hemochromatosis or alpha-1-antitrypsin deficiency, and nonalcoholic fatty liver disease (NAFLD). In a Mexican study of 1486 patients, the main risk factors for cirrhosis were alcohol consumption (39.5%), HCV (36.6%), cryptogenic (10.4%), primary biliary cirrhosis (5.7%), and HBV (5.0%).8
Hepatitis BChronic HBV infection is a well-established HCC risk factor. In the USA, up to 25% of HCC patients are HBV positive.9 Other HBV-related factors are high viral load10 and genotype C,11 which are independent predictors of HCC development. Sex is an important factor in these patients because there is an association between high testosterone level and tumor development in early stages of HCC.7
Hepatitis CHCV infection is recognized as a significant risk factor for HCC development, with 6-75% of HCC patients exhibiting antibodies to HCV.12,13 Some studies have identified genotype 1b as conferring a high risk for HCC development.14 A number of studies have demonstrated a direct relationship between HCC incidence and advanced stages of hepatic fibrosis in patients with chronic active hepatitis.15 Because of an HCV-related nonspecific inflammatory process that induces hepatocyte proliferation associated with an increase in alanine aminotransferase levels, patients with high inflammatory and proliferative activity are more prone to progress to HCC.16
AflatoxinAflatoxin is produced by Aspergillus flavus and A. parasiticus and is found in food such as peanuts and causes alterations in hepatocyte DNA.7 Aflatoxin is an important cofactor for HCC development in some parts of Africa and Asia. There is a strong correlation between the dietary intake of aflatoxin B1, TP53 mutations, and the incidence of HCC, specifically in HBV-infected individuals.12
Hereditary hemochromatosis (HH)HH is a significant risk factor for HCC development. Its presence is associated with a 200-fold increased risk for HCC.12 Iron toxicity in the liver is produced by free radical formation and lipid peroxidation within cells, and may eventually cause hepatocyte death, fibrosis, and cirrhosis.6
Wilson’s diseaseWilson’s disease is a heritable disease with mutations in the gene ATP7B and alterations in plasma copper circulation and its excretion in bile. Excessive free copper in the circulation can provoke cytoplasmic cell injury, cirrhosis, and sometimes HCC.12
Nonalcoholic fatty liver disease (NAFLD)NAFLD affects 10-24% of the population in various countries.17 The prevalence increases in high-risk groups, reaching 70-86% in obese and/or diabetic patients.18 Nonalcoholic steatohepatitis (NASH) is estimated to occur in 10% of NAFLD patients. NASH has been posited as a possible cause of cryptogenic cirrhosis.7 Patients with cryptogenic cirrhosis also develop HCC.
Recommendations- 1.
Governmental health agencies must recommend policies to prevent HCV/HBV transmission, to encourage a healthy lifestyle to prevent obesity and alcohol abuse (Class 1, Level A), and to establish measures to control metabolic conditions such as diabetes and obesity (Class 3, Level B).
Prevention of HCC can be classified as: a) primary, implying prevention of the development and progression of liver diseases; b) secondary, implying prevention of premalignant conditions such as cirrhosis; and c) tertiary, implying prevention of HCC reappearance after curative treatment.
HCC prevention requires the identification of risk factors, the mechanisms involved in hepatocarcinogenesis, and the potential therapeutic agents (che-moprevention). As a general rule, patients with liver diseases progressing to cirrhosis are at increased risk of HCC; thus, it is paramount to prevent or treat liver diseases to modify the incidence of HCC. Effective treatment of CLDs may halt disease progression and prevent cirrhosis development. HCC prevention may be achieved through general preventive measures applicable to most CLDs or through specific treatment of primary liver disease.
Primary preventionThe single most important measure in the prevention of HCC is to prevent HBV or HCV infection.19 HBV infection can be prevented through HBV vaccination of infants and sexually active adults.20,21 Prevention of HBV and HCV transmission by blood contamination in medical settings can be achieved by testing blood products; using disposable needles, syringes, or other devices that can become contaminated by blood or serum; adequate cleansing and sterilization of endoscopic equipment; wearing gloves to handle wounds and blood products; avoiding multiple-use injectable vials; and following general recommendations to avoid transmission from viremic patients to health care workers.19,22 Educational and needle- and syringe-exchange programs for injecting drug users have also proven effective in reducing hepatitis infection.
Secondary preventionTreatment of chronic hepatitis BThe viral load of HBV is a powerful independent risk factor for HCC development.23 Interferon (IFN)-based therapy reduces HCC risk, particularly in the early stages of cirrhosis and by suppressing serum HBV DNA.24,25 Lamivudine reduces the risk of HCC in patients with HBV, particularly in the advanced fibrosis stages, including cirrhosis.23,26 However, it is not certain how long the risk of HCC remains elevated after therapy-induced suppression of HBV DNA in patients with cirrhosis; in a previous study, the difference in HCC incidence between lamivudine- and placebo-treated groups became evident after 18 months.19,26 Treatment with more effective antiviral therapies that include the newer, more potent antivirals that are effective against viral strains with greater drug resistance, such as entecavir and tenofovir, appears to also reduce HCC risk in patients with chronic hepatitis B.27,28 In addition, case-control and cohort studies of noncirrhotic chronic hepatitis B patients suggest that antiviral therapy can reduce HCC when treatment is delivered early, although the level of evidence remains low.29
Treatment of chronic hepatitis CMore than 90% of hepatitis C patients with cirrhosis develop HCC. Thus, prevention of cirrhosis by successful elimination of HCV infection is likely to be a highly effective strategy for preventing HCC. Almost all studies to date have shown that HCC rates are highest in patients with cirrhosis and lower in those who respond to antiviral therapy that includes IFN;30,31 the rates are lowest in those exhibiting a sustained virological response (SVR).32 The preventive effect of antiviral therapy in patients with chronic hepatitis C may be even greater using pegylated IFN plus ribavirin because it achieves higher SVR rates than standard IFN.33,34 Even though HCC risk is markedly reduced in cirrhotic patients who exhibit a SVR, a long-term risk for HCC development remains for more than 5 years.35 It is important to mention that continued low dose of pegylated IFN therapy for those without a SVR fails to reduce the HCC risk.36 However, it is unknown whether treatment with triple therapy: pegylated IFN, ribavirin and protease inhibitors (Boceprevir and Telaprevir) or with the new direct acting antiviral may have an anticarcinogenic effect.
Treatment of non-viral liver diseasesThe percentage of HCC cases unrelated to either HBV or HCV varies between regions but is usually 10-20%.37 Such occurrences of HCC are related to alcoholic cirrhosis, NASH, genetic hemochromatosis, autoimmune liver diseases, and other infrequent diseases.
Prevention of alcoholic liver disease by reducing excessive alcohol intake is a strategy to lower the incidence of HCC in countries where this is a preva- lent disorder. On the other hand, there is no evidence to date that the discontinuation of chronic excessive alcohol intake reverses the elevated HCC risk once cirrhosis is established, at least during the first 10 years (although some studies have suggested the opposite).19 Other lifestyle modifications, such as changes in diet, may prove beneficial in preventing HCC.38
Metabolic factors such as obesity and type 2 diabetes mellitus independently increase HCC risk in patients with hepatitis C or other causes of cirrhosis.39–41 Thus, prevention of obesity and its metabolic complications (insulin resistance, metabolic syndrome, type 2 diabetes mellitus) should reduce HCC incidence. There is evidence that treatment with metformin but not with other antidiabetic medications can reduce the risk of HCC in patients with type 2 diabetes mellitus.42–44 Statins may also reduce HCC incidence independently of the cause of primary liver disease.45–47
The treatment of hemochromatosis is particularly suitable for HCC prevention because early treatment of hepatic iron overload prevents fibrotic liver disease and its complications. However, phlebotomy therapy at the stage of advanced fibrosis is ineffective in preventing HCC.48,49
Recommendations- 1.
The most important preventive strategy against HBV-related HCC is adoption of universal hepatitis B vaccination (Class 1, Level B).
- 2.
All countries should prioritize efforts to adopt extended infant-immunization schedules that include hepatitis B vaccination and that ensure that coverage of these programs extends to all communities (Class 1, Level B).
- 3.
Testing blood products for HBV and HCV is an essential strategy for preventing HCC related to chronic viral hepatitis (Class 1, Level A).
- 4.
Adoption of universal precautions to avoid transmission of blood-borne viruses in health care settings is advocated as another effective measure to reduce HCV-related HCC (Class 2, Level C).
- 5.
Effective antiviral therapy for chronic hepatitis B is a very important measure for preventing HCC (Class 2, Level B).
- 6.
Effective antiviral therapy for chronic hepatitis C (SVR, normalization of alanine aminotransferase) is a very important measure for preventing HCC (Class 1, Level B); however, HCC risk is not removed entirely in patients with underlying cirrhosis (Class 2, Level C).
- 7.
Preventing alcoholic liver disease should prevent some cases of HCC; dietary modifications should also be considered (Class 2b, Level C).
- 8.
Adequate treatment of obesity and type 2 diabetes may also prevent some cases of HCC (Class 2, Level A).
- 9.
Early detection of hemochromatosis by genetic screening for affected family members and serum studies of iron stores is important because iron overload correction by venesection prevents cirrhosis and the development of HCC (Class 2, Level A).
HCC surveillance is a critical process for improving the survival of cirrhotic patients. Surveillance increases the survival of patients with Child-Pugh class A.50,51 In patients with Child-Pugh class B, survival was higher for patients under surveillance (17.1 months) compared with those whose HCC was detected incidentally (12.0 months).52 For patients with Child-Pugh class C, there was no significant difference in survival between surveillance and no surveillance.
Surveillance in decompensated cirrhosisPeriodic screening for HCC is recommended only in decompensated patients who are on a waiting list for liver transplantation.52 Cucchetti and colleagues performed a cost-effectiveness analysis of HCC surveillance in decompensated cirrhotic patients. After 10 years of follow-up, 6.6% were alive without HCC, 17.5% had been diagnosed with HCC, and 75.9% had died of cirrhosis-related causes without HCC. The short life expectancy in this specific group of patients made surveillance ineffective unless patients were on a waiting list for liver transplantation.53
Recommendation- 1.
HCC surveillance of patients with Child-Pugh class C or decompensated cirrhosis is recommended only for patients on a waiting list for liver transplantation (Class 2a, Level B).
Early HCC detection is paramount for providing effective treatment to compensated cirrhotic patients.54 In cirrhotic patients, early HCC detection has proven to increase the 2- and 5-year survival.55
SurveillanceThere are two classic screening tools for early detection: α-fetoprotein (AFP) level and ultrasound. Although a recommendation has been made to use both strategies at 6 months,54 a systematic review found that ultrasound as an isolated strategy is superior.56 It has now been established that measuring the AFP level provides no extra benefit for the early detection in patients with cirrhosis.57
It is important to assure the quality of both ultrasound equipment and radiologists to detect early lesions.58 When access to quality ultrasound is limited, AFP may be considered.59
New serological biomarkers might improve the early diagnosis of HCC, particularly Golgi protein 73, but further studies are required.60
Recommendations- 1.
In cirrhotic patients, ultrasound should be performed every 6 months (Class 1, Level A).
- 2.
If quality ultrasound is not available, AFP level may be considered as a biomarker (Class 2, Level B).
Cirrhosis is the main risk factor for HCC.61–64 Less common causes include HH, chronic hepatitis B without cirrhosis, alpha-1 antitrypsin deficiency, aflatoxin exposure, autoimmune hepatitis, some porphyrias, and Wilson’s disease.64 Family history of hepatic cancer is also an important risk factor; family clusters of HCC have been frequently reported in Asian countries and less often in Europe or America.65,66 In these high-risk groups ultrasound-based HCC screening has proven to improve mortality.67
Hereditary hemochromatosis (HH)Chronic hepatic deposition of iron in HH leads to fibrosis, cirrhosis, and finally HCC.68 The iron overload in the liver causes dysfunction of intracellular organelles such as the mitochondria, microsomes, lysosomes, peroxisomes, and endoplasmic reticulum, leading to cellular dysfunction and hepatocellular injury. Iron-induced DNA damage has been also demonstrated in both animal and hepatocyte culture models of iron overload.69 Cirrhosis is the most important adverse prognostic factor in HH, with a 5-year survival in untreated patients as low as 50%.70 Patients with cirrhosis have 100- to 200-fold increased risk of developing HCC. HCC accounts for about 30% of HH-related deaths. Adequate management of iron stores significantly decreases, without eliminating, the risk of HCC development. Therefore, patients with cirrhosis, independent of phlebotomy treatment, should continue to be screened for HCC following apheresis procedures.70,71
Non-cirrhotic chronic hepatitis B and hepatocellular carcinomaChronic hepatitis B patients are at risk of HCC even in the absence of cirrhosis. HCC incidence in noncirrhotic HBV carriers ranges from 0.25% to 0.5% per year in Asia and Africa, and from 0.1% to 0.4% per year in Europe and America.72,73 The mechanisms involved in the progression to HCC in chronic hepatitis B patients are not known. Host-virus interactions in infected hepatocytes and cells involved in the inflammatory response to HBV infection could explain the progression to HCC. An alternative mechanism considers the oncogenic potential of HBV through DNA integration into the genome of liver cells.74,75 Although the mechanisms are not clear, some characteristics of the infection are important predictors of HCC,76 such as hepatitis B e antigen seropositivity,77 genotype C,74 and high viral load.
Familial history of hepatocellular carcinomaFamilial aggregation of HCC has been reported frequently in China where HBV infection is common. Familial aggregations have also been reported in other populations, although less frequently.66,78 Family history of HCC has been found to increase HCC risk even in persons with no hepatitis B or C infection.65,79 People with a family history of liver cancer have a two- to threefold increase in HCC risk, independent of the presence of chronic hepatitis B or C. This risk increases further in the presence of hepatitis B surface antigen and/or anti-HCV positivity to a 70-fold increased HCC risk.65 Some studies have suggested that a recessive inheritance model may play a role in familial HCC.
Recommendations- 1.
HCC surveillance should be performed for patients with HH and Child-Pugh class A or B cirrhosis, as well as those awaiting liver transplantation by experienced personnel using ultrasonography at 6-month intervals (Class 2, Level B).
- 2.
Surveillance for HCC in noncirrhotic HBV carriers should be performed using ultrasonography at 6-month intervals (Class 1, Level B).
- 3.
Surveillance for HCC in people with familial history of HCC, even in the absence of viral hepatitis B or C infection, should be performed using ultrasonography at 6-month intervals (Class 1, Level B).
The decision to initiate a noncirrhotic patient into a surveillance program for early HCC detection is driven by the HCC risk level. The recommended cutoff of annual incidence above which surveillance should be initiated is based on expert opinion and cost-benefit models, and can thus vary according to the underlying condition.80
Hepatitis CPatients with chronic hepatitis C without cirrhosis are at risk of HCC development. Unfortunately, evidence about the incidence of HCC in this group is still too limited to recommend a surveillance program. Surveillance is deemed cost-effective if the expected HCC risk exceeds 1.5% per year in patients with hepatitis C.81 According to the HALT-C trial in noncirrhotic patients, the cumulative incidence rates of HCC at 3, 5, and 7 years were 1.4%, 2.9%, and 6.8%, respectively, with similar values for patients receiving pegylated IFN. These data confirmed that HCC can occur in noncirrhotic chronic HCV patients, although the incidence is lower than in cir- rhotic patients and may not reach the threshold required to initiate surveillance.82
Non-alcoholic fatty liver disease (NAFLD) and Non-alcoholic steatohepatitis (NASH)For surveillance to be cost-effective in noncirrhotic patients with NAFLD, the HCC incidence must exceed 1.5% per year.83 A significant number of noncirrhotic individuals with NAFLD or NASH develop HCC, raising the possibility that NAFLD/NASH constitutes a risk factor for HCC independent of cirrhosis.84,85 A recent systematic review performed to evaluate the association between NAFLD/NASH and HCC concluded that the increased HCC risk in this setting seems to be predominantly limited to patients with cirrhosis.86 Therefore, no surveillance program for NAFDL/NASH in noncirrhotic patients should be implemented under the current state of evidence.
Other chronic liver disease (CLDs)There is a paucity of data to support a surveillance program for noncirrhotic patients with CLDs such as autoimmune liver disease, alpha-1 antitrypsin deficiency, Wilson’s disease, or HH.87–89
Recommendations- 1.
In noncirrhotic patients with chronic hepatitis C, the benefits of surveillance are uncertain (Class 2b, Level B).
- 2.
HCC surveillance is not recommended for noncirrhotic patients with NAFLD/NASH or other CLDs (Class 3, Level A).
Over the past decade, HCC survival has improved by more than 20%, largely because of advances in early diagnosis.90 Screening and surveillance of patients at increased risk of HCC are cost-effective and have been proven to reduce mortality.91 However, adherence to surveillance is less than 60%,92 limiting the impact of this strategy.
Non-invasive diagnosisCurrently, HCC is diagnosed using contrast-enhanced dynamic imaging methods, either computed tomography (CT) or magnetic resonance imaging (MRI). Comparison of CT and MRI show the latter to be superior (sensitivity 78 vs. 84%; specificity 77 vs. 84%, respectively), although the accuracy of MRI is only 33% in small lesions.92 Nodular lesions detected by imaging methods are classified in accordance with the International Consensus Group for HCC as follows.93
- •
Regenerative macronodules: not premalignant.
- •
Low-grade dysplastic nodules (DNs): difficult to differentiate from regenerative macronodules.
- •
High-grade DNs: most frequent precursor of HCC.
- •
Small HCC: < 2 cm, can be of vaguely nodular appearance (early) or distinctively nodular pattern (progressive).
Vascularization is a critical aspect in the evaluation of a DN because HCC tends to develop arterial vascularization that is independent from the portal system. HCC diagnostic accuracy increases when CT or MRI examination shows arterial wash-in in the nodule followed by venous wash-out.94,95 The arterial supply pattern helps to differentiate a DN from HCC,96,97 except when the DN reaches an intermediate degree of capilarization.98 Despite this limitation, the vascular pattern is pathognomonic for HCC.99
The diagnosis of small nodules (< 2 cm) is challenging because it is difficult to differentiate a DN from a small HCC. This differentiation is crucial because one-third of DNs are malignant and a timely diagnosis is critical to being able to offer a curative treatment.100 The specificity of an isolated dynamic imaging method in the diagnosis of small liver nodules is excellent when the vascular pattern of the lesion is typical, making it unnecessary to submit patients to other imaging methods to other imaging methods or biopsy as biopsy.101,102
MRI diagnosis is expected to improve through second-generation cellular contrast agents such as gadoxetic acid. Gadoxetic acid increases the diagnostic accuracy for HCC, but evidence about its usefulness in small lesions (< 2 cm) is limited. Nevertheless, even when second-generation contrast agents are used, it is difficult to differentiate high-grade DNs from HCC because of their similarities.103–105 The use of a diffusion-weighted technique may increase further the diagnostic accuracy of MRI.106
Contrast-enhanced ultrasonography is better than conventional ultrasound for diagnosing HCC. Findings such as hyperenhancement during the arterial phase and hypoenhancement during the late phase have a sensitivity of 88.8% and specificity of 100%.103 However, external validation and specific training are important concerns when using this diagnostic technique.
The measurement of AFP as an HCC diagnostic test must be abandoned.94,95 The AFP level has no satisfactory cutoff point and low sensitivity and specificity.104,105 Only one-third of patients with liver nodules have AFP levels > 100 ng/mL.92,106
Invasive diagnosisDespite the use of imaging methods, nearly 30% of patients will need a biopsy to establish the final diagnosis.92 Nodule biopsy must be conducted only when dynamic imaging examination is inconclusive, particularly in small lesions measuring 1-2 cm.107 Biopsy can be conducted through fine needle aspiration or core-cutting needle biopsy and may be difficult to perform depending on the location of the lesion or the coagulation status of the patient.
Although a positive biopsy result settles the diagnosis, a negative one may not rule out a diagnosis of HCC. The sensitivity and specificity of liver biopsy are 100 and 75%, 100 and 100%, and 96 and 71% for nodules ≤ 2 cm, > 2 and ≤ 3 cm, and > 3 and ≤ 5 cm, respectively.108 Because nodules < 1 cm are difficult to characterize through biopsy and have a low probability of malignancy, a follow-up plan must be established that includes ultrasonography every 3 months. Biopsy complications are infrequent.109 The possibility of neoplastic cell seeding is close to 2.5% in large nodules110 and is expected to be lower in small nodules. The use of immunohistochemical panels does not increase the accuracy of the diagnosis.111
Recommendations- 1.
For liver nodules >1 cm, a single dynamic imaging examination with typical findings is sufficient for HCC diagnosis. If findings are not typical or if the vascular pattern is not characteristic, a second imaging technique should be used (Class 1, Level A).
- 2.
MRI that includes evaluation of the hepatic biliary phase using a second-generation contrast agent is useful in the diagnosis of HCC (Class 1, Level A).
- 3.
Contrast-enhanced ultrasonography and AFP level are not recommended as a primary tool for diagnosis (Class 1, Level A).
- 4.
Liver nodules >1 cm with inconclusive imaging results should be biopsied (Class 1, Level A).
- 5.
Surveillance of liver nodules < 1 cm should be conducted every 3 months with ultrasonography (Class 1, Level B).
Staging systems are designed to predict the overall prognosis of patients with HCC, to classify patients according to the prognostic variables, to provide a common system to compare results of various clinical trials, and to guide treatment choices. In the past decades, several staging systems have been proposed,112 leading to improvement in survival as treatments are tailored to the specific stage of HCC.
Various classifications have been adopted to stage HCC (Tables 3 and 4). Although some systems have been validated in specific settings and countries, no single staging system has been validated across the spectrum of HCC patients and treatment options. The difficulty in establishing a universal staging system resides in the confluence of HCC and cirrhosis because both the characteristics of the tumor and the degree of liver dysfunction contribute to the overall prognosis.113 Moreover, the heterogeneity of HCC around the world, which reflects underlying differences in epidemiological background, etiology, and risk factors, further increases the complexity in creating a common staging system.
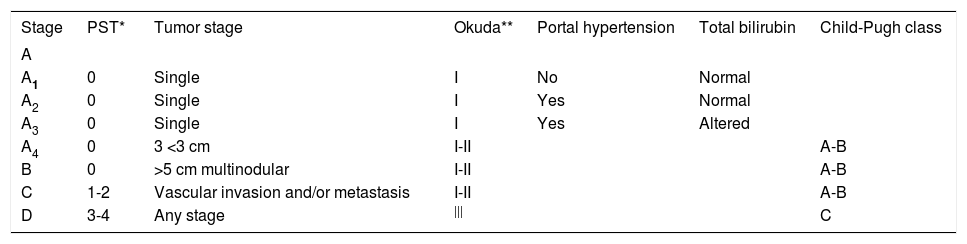
The Barcelona Clinic Liver Cancer (BCLC) Group classification of hepatocellular carcinoma.
| Stage | PST* | Tumor stage | Okuda** | Portal hypertension | Total bilirubin | Child-Pugh class |
|---|---|---|---|---|---|---|
| A | ||||||
| A1 | 0 | Single | I | No | Normal | |
| A2 | 0 | Single | I | Yes | Normal | |
| A3 | 0 | Single | I | Yes | Altered | |
| A4 | 0 | 3 <3 cm | I-II | A-B | ||
| B | 0 | >5 cm multinodular | I-II | A-B | ||
| C | 1-2 | Vascular invasion and/or metastasis | I-II | A-B | ||
| D | 3-4 | Any stage | ||| | C |
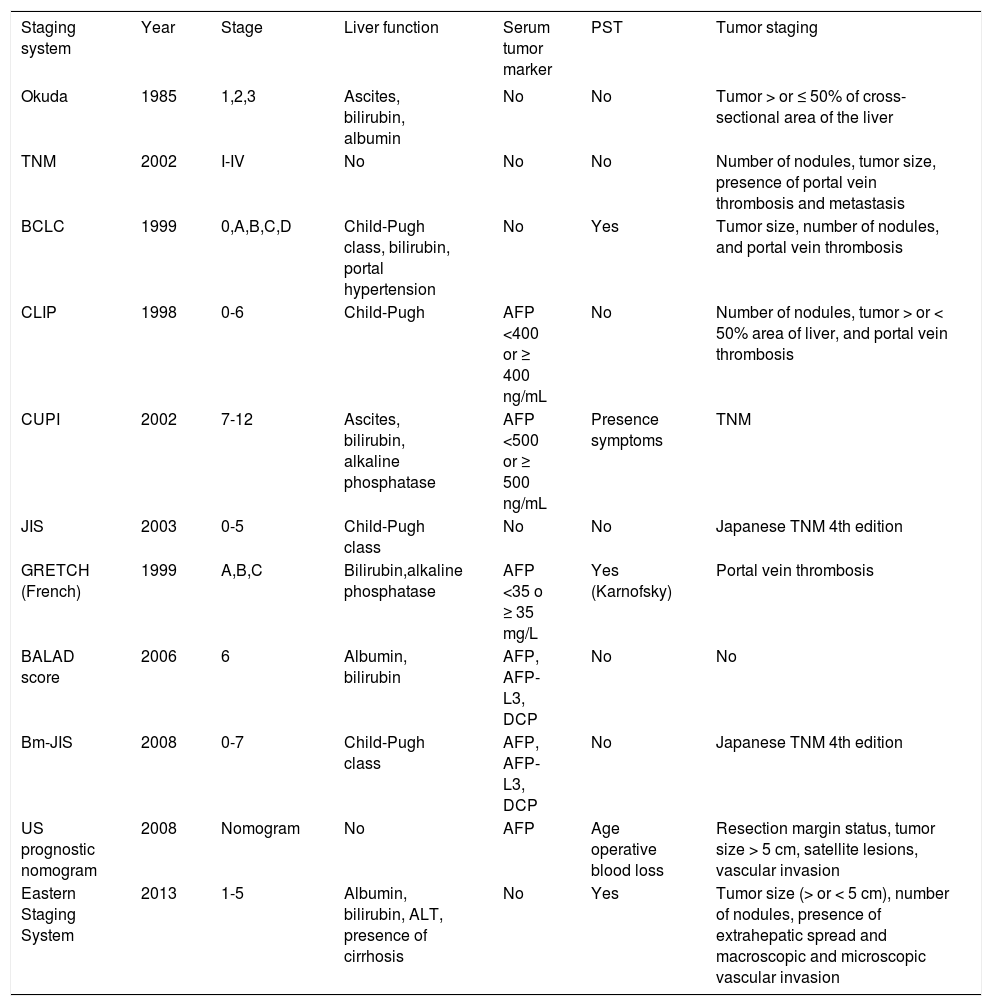
Main prognostic staging systems for hepatocellular carcinoma and included variables.
| Staging system | Year | Stage | Liver function | Serum tumor marker | PST | Tumor staging |
|---|---|---|---|---|---|---|
| Okuda | 1985 | 1,2,3 | Ascites, bilirubin, albumin | No | No | Tumor > or ≤ 50% of cross-sectional area of the liver |
| TNM | 2002 | I-IV | No | No | No | Number of nodules, tumor size, presence of portal vein thrombosis and metastasis |
| BCLC | 1999 | 0,A,B,C,D | Child-Pugh class, bilirubin, portal hypertension | No | Yes | Tumor size, number of nodules, and portal vein thrombosis |
| CLIP | 1998 | 0-6 | Child-Pugh | AFP <400 or ≥ 400 ng/mL | No | Number of nodules, tumor > or < 50% area of liver, and portal vein thrombosis |
| CUPI | 2002 | 7-12 | Ascites, bilirubin, alkaline phosphatase | AFP <500 or ≥ 500 ng/mL | Presence symptoms | TNM |
| JIS | 2003 | 0-5 | Child-Pugh class | No | No | Japanese TNM 4th edition |
| GRETCH (French) | 1999 | A,B,C | Bilirubin,alkaline phosphatase | AFP <35 o ≥ 35 mg/L | Yes (Karnofsky) | Portal vein thrombosis |
| BALAD score | 2006 | 6 | Albumin, bilirubin | AFP, AFP-L3, DCP | No | No |
| Bm-JIS | 2008 | 0-7 | Child-Pugh class | AFP, AFP-L3, DCP | No | Japanese TNM 4th edition |
| US prognostic nomogram | 2008 | Nomogram | No | AFP | Age operative blood loss | Resection margin status, tumor size > 5 cm, satellite lesions, vascular invasion |
| Eastern Staging System | 2013 | 1-5 | Albumin, bilirubin, ALT, presence of cirrhosis | No | Yes | Tumor size (> or < 5 cm), number of nodules, presence of extrahepatic spread and macroscopic and microscopic vascular invasion |
AFP: alpha-fetoprotein. PST: performance status test (based on the Eastern Cooperative Oncology Group performance scale: 0: asymptomatic. 1: symptomatic and fully ambulatory. 2: symptomatic and in bed ≤ 50% of the day. 3: symptomatic and in bed >50% of the day. 4: bedridden). DCP: des-gamma-carboxyprothrombin; AFP-L3: Lens culinaris agglutinin-reactive. AFP. ALT: alanine aminotransferase.
Attempts to improve the classification and prognostic capabilities for HCC are still evolving. The conventional staging systems for HCC, such as the Okuda stage114 or the TNM stage115 have been shown to have important limitations. New systems have been proposed, but only some have been validated in different settings. Based on common characteristics shared by several staging systems, the key factors that influence HCC prognosis and treatment options are solitary versus multifocal tumors, the presence of macrovascular invasion, ext- rahepatic disease, high serum AFP level, patient performance status, and the degree of hepatic impairment.112
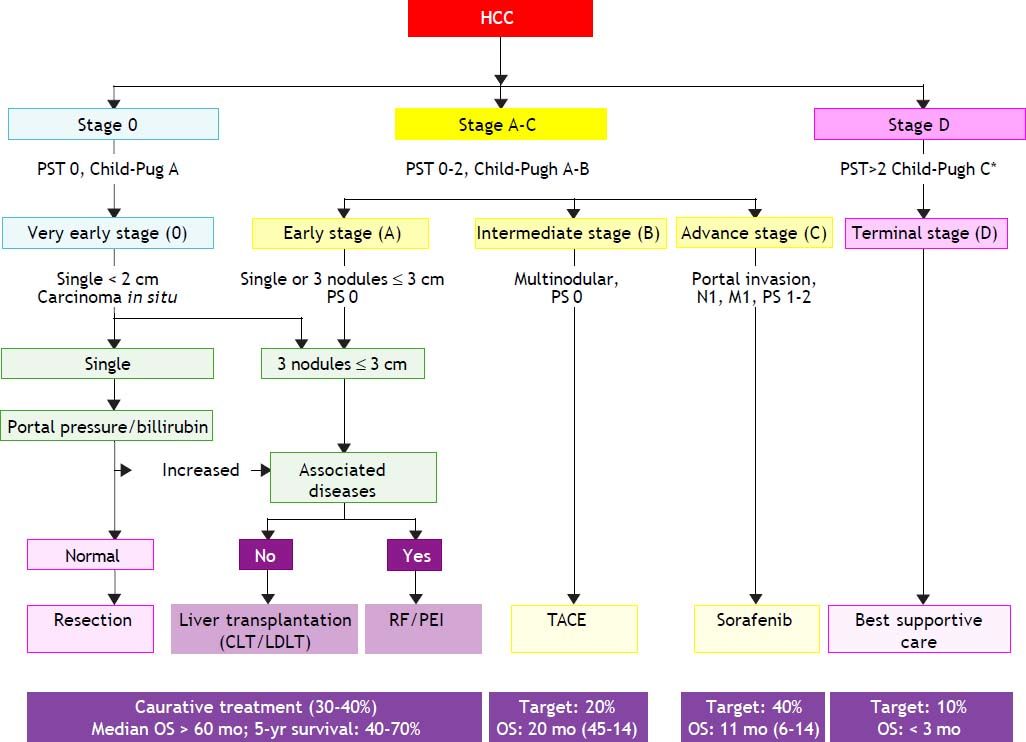
The Barcelona Clinic Liver Cancer Group (BCLC) staging classification was developed by Llovet and colleagues in 1999.116 When following patients with unresectable and nontransplantable HCC randomized to placebo, they observed that vascular invasion and extrahepatic spread were independent predictors of mortality. This allowed HCC patients to be classified into different categories based in variables related to hepatic function, portal hypertension, bilirubin level, cancer-related symptoms, physical status, and tumor stage (size, number, presence of distant metastases, and vascular invasion). Most therapeutic clinical trials have used the BCLC system as the reference staging system.112
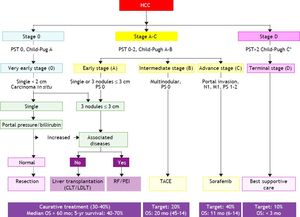
The BCLC classification links the stage of the disease to a specific treatment algorithm that correlate with life expectancy. Following the BCLC staging system (Table 3), patients may be classified according to the following staging.
- •
Stage 0 or very early. Asymptomatic HCC patients with a single nodule < 2 cm without portal hypertension, well-preserved liver function (Child-Pugh class A), and good performance status, which may benefit from curative therapies, with > 80% survival at 5 years. Currently 5-10% of Western patients are diagnosed at this stage compared with 30% in Japan diagnosed through intensive surveillance programs.
- •
Stage A or early. Asymptomatic patients with single nodule (2-5 cm) or three nodules ≤ 3 cm and Child-Pugh class A or B. These patients may also benefit from curative therapies (resection, liver transplantation, or local ablation), with 50-75% survival at 5 years.
- •
Stage B or intermediate. Large multinodular HCC with Child-Pugh class A or B, with adequate performance status. These patients may benefit from chemoembolization, with a median survival of 20 months.
- •
Stage C or advanced. Multinodular with portal invasion or extrahepatic spread. These patients may benefit from palliative treatments with new agents such as sorafenib, with a median survival of 11 months.
- •
Stage D or terminal. Child-Pugh class C patients, with very poor life expectancy. Symptomatic palliative treatment is proposed, with a median survival of 3-4 months.
The BCLC staging classification has been validated in the USA, Europe, and Taiwan and has been shown to have superior prognosis capabilities over a range of other classifications. The American Association for the Study of Liver Diseases and the European Association for the Study of the Liver have endorsed the BCLC staging system because it can be used to guide the choice of treatment and estimate life expectancy, whereas other staging systems focus exclusively on predicting survival (Figure 1).117,118 Thus, the BCLC system is now emerging as the standard staging system in Western populations.
The Barcelona Clinic Liver Cancer (BCLC) staging system and treatment allocation. HCC: hepatocellular carcinoma. M: metastasis classification. N: node classification. TACE: transcatheter arterial chemoembolization. Adapted from European Association for the Study of the Liver: EASL-EORTC Clinical Practice Guideline: Management of hepatocellular carcinoma. J Hepatol 2012; 56: 908-46.
Other staging or scoring systems for HCC have been proposed, such as the GRETCH (Groupe d’Etude et de Traitement du Carcinome Hepatocellu- laire) scoring system,119 the CUPI (Chinese University Prognostic Index) staging system,120 the Simplified Staging System,121 the CLIP (Cancer of the Liver Italian program) scoring system, the JIS (Japan Integrated Staging) staging system,122 and the Tokyo scoring system (Table 4).123 Most clinical studies from Japan have concluded that the JIS or the modified JIS staging system are the best syste- ms to stage their HCC patients. On the other hand, studies from China, Korea, and Taiwan have favored either the TNM or CLIP as the best staging systems. Most studies from Western countries have favored either the BCLC or CLIP system as the best staging system for their patients.124–127
Recommendations- 1.
An adequate assessment of the prognosis of HCC should consider the tumor stage, liver function, and physical status of the patient. The impact of therapy should also be considered when estimating life expectancy (Class 1, Level B).
- 2.
The BCLC staging system is recommended for prognostic prediction and treatment allocation (Class 1, Level B). This system can be applied to most HCC patients provided that specific considerations for special subpopulations (e.g., liver transplantation) are incorporated.
Resection and liver transplantation are curative surgical treatments for HCC. Liver transplantation removes both the tumor and the underlying cirrhosis, and represents the first-line treatment for cirrhotic patients. Unfortunately, there are no randomized clinical trials comparing surgical resection and liver transplantation for HCC treatment.128
For liver transplantation, it is paramount to carefully select the best candidates by taking into account tumor stage, liver function, functional status of the patient, availability of a liver graft, technical experience, and probably some biological characteristics.129 The BCLC staging system is the most widely accepted system for guiding treatment recommendations and is the preferred system for assessing the prognosis of these patients.130
Compliance with the Milan criteria is the main factor for determining the prognosis for liver transplantation in patients with HCC and cirrhosis,131–133 and has been integrated into the BCLC staging system,134,135 and the United Networks for Organ Sharing pretransplant staging for organ allocation in the USA.136 To be eligible, candidates should have an expected survival of at least 70% over 5 years with a recurrence rate < 15%.131 Both survival and recurrence are heavily dependent on tumor size as established by the Milan criteria (solitary tumor ≤ 5 cm in diameter, or ≥ 3 tumors, each ≤ 3 cm in diameter and no macrovascular invasion).132 According to a meta-analysis,133 the 5-year survival rate of patients with HCC meeting the Milan criteria (65-78%) is similar to the rate observed in patients with no tumor evidence (68-87%). Patients meeting the Milan criteria are also at lower risk for microvascular invasion and poorly differentiated tumor. When exceeding the Milan criteria, the 5-year survival rate could be as low as 46-60%.
An expanded version of the Milan criteria was tested at the University of California, San Francisco, with patients with a solitary tumor ≤ 6.5 cm or ≤ 3 nodules with the largest lesion ≤ 4.5 cm and total tumor diameter ≤ 8 cm, experienced survival rates of 90% and 75.2%, at 1 and 5 years, respectively, versus a 50% 1-year survival for patients with tumors exceeding these limits.137 However, other studies have failed to validate these expanded criteria.
Downstaging for transplantationPatients exceeding the Milan criteria might benefit from reducing pretransplantation tumor stage (downstaging) by locoregional treatment.137,138 According to a recent meta-analysis, downstaging HCC before liver transplantation in patients outside the Milan criteria improved the survival rates at 1, 3, and 5 years from 82 to 100%, 79 to 100%, and 55 to 94%, respectively.139 These survival rates are similar to those of patients within the Milan criteria.130 Although promising, the clinical application of downstaging should be considered carefully because there were important methodological limitations present in all studies included in this meta-analysis.
Incorporation of serum alpha-fetoprotein as patient selection criteria for liver transplantationPrevious studies have demonstrated that an elevated pretransplantation serum AFP level is an independent risk factor for HCC recurrence after liver transplantation, suggesting that AFP should be incorporated in the patient selection criteria.140–143 Several cutoff values (210, 400, and 1000 ng/mL) have been proposed, but to date none has been validated.144–147
Recommendations- 1.
Liver transplantation should be the first-line treatment for patients within the Milan criteria (single tumor ≤ 5 cm or ≥ 3 nodules ≤3 cm) and not suitable for resection (Class 1, Level A).
- 2.
Liver transplantation is not currently recommended for patients not meeting the Milan criteria because further prospective evidence of its benefit is required (Class 2a, Level B).
- 3.
Liver transplantation may be considered after successful downstaging to meet the Milan criteria (Class 2a, Level B).
- 4.
Further studies are required to validate the pretransplantation cutoff for serum AFP level as a criterion for liver transplantation (Class 2a, Level B).
Resection is the best therapeutic option for HCC in cirrhotic and noncirrhotic patients with a solitary nodule tumor, preserved hepatic function, and no portal hypertension (< 10% of cases).148–152 Resection has shown good results with low perioperative mortality (0.8-3%) and up to 60% survival at 5 years.150,153–162
Selection of the ideal candidate for resection depends on the careful CT or MRI evaluation of tumor size, presence of satellite lesions, and vascular involvement. The evaluation of preoperative liver function can be assessed using the indocyanine green retention rate at 15 min, directly by evaluation of portal hypertension by measuring the hepatic venous pressure gradient (desirable, < 10 mmHg), or indirectly by the platelet count (desirable, ≥ 100,000/ μL). Patients without direct hepatic venous pressure gradient measurement but with confirmed esophageal varices, diuretic therapy to control ascites, and high bilirubin level should not be considered for resection.156–163
The surgical procedure must aim to obtain at least 2 cm margins through anatomic resection, except when this procedure compromises the healthy residual liver volume of a cirrhotic patient; in this case, a minimum surgical margin is sufficient. Although anatomic resection remains controversial, the general trend is to perform it whenever possible, provided that the volume of the remaining parenchyma is not affected.163–168
Recurrence after resectionRecurrence after surgical resection can be 70% within 5 years and is more likely to occur within the first 3 years. The main mechanisms of recurrence are primary tumor dissemination, intrahepatic metastasis, and development of new tumors (de novo HCC).148,169–176 Factors contributing to recurrence are vascular invasion, presence of satellite lesions, histological differentiation grade, and size of the primary node resected.150,155,170,175 Many attempts have been made to find an effective adjuvant or neoadjuvant therapy to reduce the risk of recurrence. Several studies have been performed in Eastern countries, especially with IFN in the postoperative period. However, despite a recent meta-analysis showing a decrease in HCC recurrence in patients with viral hepatitis, data are lacking to safely recommend this alternative.157,177–181 In cases of recurrence, the patient must be reassessed by BCLC staging.177,178,182–190
Recommendations- 1.
Resection should be considered for patients with a solitary nodule tumor and preserved liver function; the tumor size, presence of satellite lesions, and vascular involvement should be considered (Class 2a, Level B).
- 2.
Patients with esophageal varices, diuretic therapy to control ascites, and high bilirubin level should not be considered for resection (Class 2a, Level B).
- 3.
Resection margins should aim for > 2 cm margins, except in patients with reduced parenchymal reserve (Class 3, Level B).
When resection or transplantation is not an option, locoregional therapy represents a viable alternative for patients with HCC confined to the liver. Tumor cell destruction can be achieved through chemical substance injection (e.g., ethanol, acetic acid, or boiling saline) or by modifying the temperature (e.g., radiofrequency, microwave, laser, or cryotherapy). Currently, radiofrequency ablation (RFA) is the first choice for local ablation, but ethanol injection remains an important tool. Although nonresectional locoregional therapies are not curative, they destroy some tumors while preserving nontumorous liver parenchyma and may thus serve as a bridge toward a more definitive therapy such as liver transplantation or as salvage treatment for postresection recurrence.
Percutaneous ethanol injection (PEI)PEI is a well-established technique for nodulartype HCC. PEI achieves complete necrosis in 90% of tumors < 2 cm, 70% of tumors 2-3 cm, and 50% of tumors 3-5 cm.191,192 In patients with Child-Pugh class A, cirrhosis, and early stage tumors, PEI has a 5-year survival rate of 47-53%.193,194 The major limitation of PEI is the high local recurrence rate, which may reach 43% in lesions > 3 m.195 It has been speculated that ethanol diffusion can be blocked by the intratumoral fibrotic septa or the tumor capsule, undermining its curative capacity (particularly in tumors > 2 cm). Another chemical ablation techni- que, percutaneous acetic acid injection, does not offer substantial advantages to PEI.196 The efficacy of percutaneous ablation is assessed by dynamic CT 1 month after therapy.191
Radiofrequency ablation (RFA)RFA is superior to PEI in patients with early stage HCC, particularly those with compensated liver disease (Child-Pugh class A).197 RFA has 5-year survival rates up to 76% when used as frontline therapy in patients with resectable HCC assessed by the BCLC criteria; this survival rate is similar to that after surgical resection.192,198 In two randomized trials, RFA was as effective as surgical resection in terms of overall survival and recurrence-free survival but was less invasive and had fewer complications.199,200 Therefore, RFA may represent a viable option to surgical resection in very early stage patients; however, more evidence is required before RFA can be recommended as a competitive alternative to resection.
RFA has several limitations and produces suboptimal results in patients with tumors > 3 cm or with a perivascular location.201 Complete tumor necrosis has been observed in < 50% of tumors > 3 cm because of heat loss caused by perfusion-mediated tissue cooling within the area ablated.201 To overcome these limitations, numerous refinements of ablation methods are under clinical testing, including laser ablation, microwave ablation, cryoablation, light-activated therapy, and irreversible electroporation.
Recommendations- 1.
The standard of care for patients with BCLC stage 0-A tumors not suitable for surgery is local ablation with RFA or PEI. Other ablative therapies, such as microwave or cryoablation, are still under investigation (Class 2a, Level B).
- 2.
RFA is recommended in tumors < 5 cm, and PEI is recommended in cases where RFA is not technically feasible (around 10-15% of patients) (Class 1, Level A).
- 3.
In tumors < 2 cm, BCLC 0, both techniques achieve complete responses in > 90% of patients and produce a good long-term outcome. Whether they can be considered as competitive alternatives to resection is uncertain (Class 1, Level C).
Curative therapies for HCC are available for about 30% of patients. Chemoembolization is an alternative for most patients, particularly those who are not candidates for resection, liver transplantation, or percutaneous ablation.
Chemoembolization is the direct delivery of a chemotherapeutic agent into the tumor followed by an embolizing agent. Overall, arterial embolization increases the 2-year survival rate to 41% compared with 27% in control patients. However, embolization alone may not increase survival, and the treatment may require the addition of a chemotherapeutic agent. Patients receiving cisplatin or doxorubicin along with arterial embolization had a 58% better survival rate at 2 years compared with conservative management (odds ratio (OR) = 0.42; 95% confidence interval (CI) 0.20-0.88), a benefit not observed with embolization alone (OR = 0.59; 95% CI 0.29-1.20).202 There are some important predictors for improved overall survival after chemoembolization such as BCLC stage (A and B vs. C, hazard ratio (HR) = 3.58), Child-Pugh classification (A vs. B, HR = 2.34), tumor size (< 4 cm vs. ≥ 4 cm, HR = 2.58), and distribution (unilobar vs. bilobar HR = 2.11).203 Further research is needed particularly regarding the benefits of embolization in patients with portal vein invasion.204
Microsphere and bead embolizationMicrosphere embolization represents a promising alternative to standard chemoembolization. Patients treated with microspheres exhibited an improved overall survival (HR 0.73; 95% CI 0.60-0.88) and longer time to progression (HR 0.61; 95% CI 0.41-0.89) compared with patients receiving stan- dard chemoembolization. Limited data suggest more benefits in patients treated with 32P glass microspheres than with yttrium 90 microspheres.205 In a similar manner, doxorubicin-eluting bead transarterial chemoembolization induced better 2- and 3-year survival rates compared with standard chemoembolization (2-year survival OR = 0.64, 95% CI 0.46-0.89; 3-year survival OR = 0.61, 95% CI 0.47-0.80).206
Preoperative transcatheter arterial chemoembolizationPreoperative transcatheter arterial chemoembolization is considered an alternative treatment for preventing recurrence after hepatectomy. However, a large cohort study207 and a meta-analysis of nonrandomized studies have shown no benefit of this strategy to the 5-year overall survival (OR = 0.85; 95% CI 0.59-1.22) or the 5-year disease-free survival (OR = 1.19; 95% CI 0.93-1.53).208
Combination therapyCombination therapy seeks to provide further benefits by combining an ablative therapy, such as RFA, with standard chemoembolization. A recent meta-analysis showed that combination therapy had better 1- to 5-year and overall survival compared with monotherapy. However, when compared with standard chemoembolization, the combination therapy improved the 1-, 3-, and 5-year survival rates but failed to significantly improve the 2-year and overall survival rates.209 More randomized controlled trials are required to evaluate further the potential advantages of combination therapy over standard chemoembolization.
Recommendations- 1.
Chemoembolization should be considered for patients with BCLC stage B without portal invasion (Class 1, Level A).
- 2.
The use of doxorubicin-eluting beads or yttrium 90 microspheres shows benefits over standard chemoembolization. However, the cost of these alternatives requires more research (Class 1, Level A).
- 3.
Preoperative transcatheter arterial chemoembolization should not be considered as the standard of care (Class 1, Level A).
HCC diagnosed at an advanced stage or with progression after locoregional therapy has a poor prognosis because of the tumor itself and the underlying liver disease.210,211 Systemic therapeutic agents are an option, although to date, only sorafenib has shown positive results under randomized controlled conditions.212,213
SorafenibSorafenib is a small molecule that inhibits tumor cell proliferation and angiogenesis while increasing apoptosis. It acts by inhibiting the serine/threonine kinases Raf-1 and B-Raf, and by blocking the vascular endothelial growth factor receptors 1, 2, and 3 as well as the platelet-derived growth factor β receptors.
Data on the efficacy of sorafenib come from the SHARP (Sorafenib HCC Assessment Randomized Protocol) and the Asia-Pacific randomized controlled trials, which involved patients with wellpreserved liver function (Child-Pugh class A) and HCC BCLC-C.212,213 In the SHARP trial, patients received sorafenib 400 mg b.i.d. or placebo; the median overall survival was 10.7 months in the sorafenib group and 7.9 months in the placebo group. Sorafenib was also superior to placebo for the time to radiological progression (5.5 vs. 2.8 months).212 In the Asia-Pacific trial, hepatitis B was the main cause of HCC, and patients had a more advanced disease (ECOG 1-2 or metastatic cancer); the median overall survival was 6.5 months in the sorafenib group versus 4.2 months in the placebo group.213 The most common grade 3 drug-related adverse events reported were diarrhea (8-9%) and hand-foot skin reaction (8-16%); drug discontinuation because of adverse events occurred in 15% of patients under sorafenib and in 7% of patients under placebo. Sorafenib must be maintained until clinical progression is observed. Sorafenib was discontinued upon decreased performance status, liver dysfunction progression, or other evidence of clinical progression.212
The evidence for sorafenib use in Child-Pugh class B patients is scarce because >95% of the patients with the SHARP and Asia-Pacific trials were Child-Pugh class A.212,213 Cohort studies analyzing Child-Pugh class B patients reported a similar frequency and profile of adverse events as in Child-Pugh class A patients; however, the Child-Pugh class B patients experienced a higher frequency of both drug discontinuation (38% vs. 24%) and se- vere adverse events (15% vs. 8%).214–217 Overall, the evidence suggests that sorafenib may be a safe option for Child-Pugh class B patients;214–217 however, at present, there are insufficient data to recommend its use.
Resistance of HCC to sorafenib is a major concern. The exact mechanisms by which sorafenib acts upon HCC and the potential pathways to resistance are largely unknown. No other agent has proven to be efficacious in improving survival in a phase III trial, and no alternative treatment exists for patients with acquired resistance or intolerance to sorafenib.212,218 Several agents are currently in phase II and III development including tivantinib,219 brivanib,220 erlotinib and bevacizumab,221 and everolimus.222 In addition, the role of molecularly targeted therapy in combination with transcatheter arterial chemoembolization in earlier stages of the disease or as adjuvants after potentially curative approaches is under investigation.223–227 The ongoing international STORM (Sorafenib as Adjuvant Treatment in the Prevention of Recurrence of Hepatocellular Carcinoma) trial aims to investigate the role of sorafenib in reducing the chance of tumor recurrence following radical therapy; no results have been published yet.
Other systemic therapiesDoxorubicin, either in combination with other agents or as a single treatment, is the most commonly studied form of HCC chemotherapy. Doxorubicin has failed to improve survival in patients with advanced HCC. A large multicenter phase III trial of 445 patients with HCC investigated the use of doxorubicin or nolatrexed to improve survival, but the results were disappointing to the extent that further exploration of the use of nolatrexed in HCC treatment was no longer recommended.228
The use of doxorubicin versus the combination of cisplatin, IFNα-2b, doxorubicin, and fluorouracil was investigated in a randomized controlled trial involving 188 patients.229 No statistically significant difference in overall survival was observed, despite better response rates in the combination group compared with the doxorubicin group (20.9% and 10.5%, respectively). However, the combination of cisplatin, IFNα-2b, doxorubicin, and fluorouracil was associated with a higher rate of myelotoxicity.
IFNs have immunomodulatory and antiproliferative effects on tumor cells and have been investigated in HCC. In one randomized study, IFNs were reported to be superior to doxorubicin in terms of survival, tumor response, and toxicity in patients with HCC.230 However, IFN treatment for HCC requires further investigation. Estrogen receptors have been found in HCC tumors, triggering the investigation of tamoxifen as a possible systemic treatment for HCC. However, the results from the randomized controlled trials involving tamoxifen were discouraging.231–234
Recommendations- 1.
Sorafenib is the standard systemic therapy for HCC in patients with Child-Pugh class A underlying cirrhosis and advanced tumor (BCLC stage C) or tumor progressing after locoregional therapy (Class 1, Level A).
- 2.
There is no alternative treatment for patients with intolerance or failure to respond to sorafenib (Class 2, Level B).
- 3.
Other systemic therapies are not recommended (Class 2a, Level A).
Palliative care is the comprehensive care for a patient with a terminal illness. It has three main objectives: control symptoms related to the disease or the treatment, improve quality of life, and provide psychosocial and spiritual support to both the patient and family.235 Most patients with HCC are diagnosed in an advanced stage requiring a multi-disciplinary approach to palliative care.236 However, scientific evidence regarding the best approaches to palliative care in HCC is lacking.
Abdominal painAbdominal pain is the most common symptom in patients with HCC and may be related to the size of the tumor or to metastatic lesions. According to the World Health Organization guidelines, pain management with opioids is recommended, although this recommendation is not based on clinical trial evidence.237 Preliminary information suggests that opioids have greater bioavailability in patients with HCC (64.8%) or liver metastases (62.1%) than in controls (16.8%).238
FatiguePatients with HCC frequently report fatigue. Fatigue is a multifactorial symptom that involves pain, emotional stress, sleep disturbance, anemia, nutritional deficiency, deconditioning, and comorbidities.239 Pharmacological interventions are directed toward altering the factors associated with fatigue such as the use of erythropoietin in patients with chemotherapy-induced anemia, antidepressants if depression is suspected to be the cause of fatigue, or psychostimulants to increase the level of energy.
Weight lossWeight loss affects 54-80% of HCC patients in the terminal stage and occurs mainly because of the anorexia-cachexia syndrome. Although the diagnosis of anorexia-cachexia syndrome is based mainly on weight loss and anorexia, other parameters such as hypoalbuminemia, fatigue, chronic nausea, decreased caloric intake, or decreased muscle mass and body fat are also strong indicators of the syndrome. Megestrol (320 mg/day) has been shown to reduce the loss of appetite, nausea, and vomiting while improving quality of life in patients with the anorexiacachexia syndrome.240–242
JaundiceJaundice is an important sign in patients with HCC with or without biliary obstruction. In HCC patients with jaundice and no biliary obstruction, it is important to identify the treatable and reversible causes of jaundice such as reactivation of viral hepatitis or drug-induced or alcoholic hepatitis. In HCC patients with obstructive jaundice, it is important to first stabilize the patient, drain the bile duct obstruction, and control tumor bleeding and then to evaluate tumor resectability. Depending on the degree of biliary obstruction and general condition of the patient, these two steps may be performed in one or two phases.242 In patients with severe jaundice, biliary obstruction must be released by endoscopic retrograde cholangiopancreatography with biliary stent placement or through percutaneous transhepatic drainage. Percutaneous drainage is the best method to resolve the biliary obstruction because the tumor may be friable and may disseminate small fragments into the bile duct and clog the drains. In the presence of hemobilia, it is important not to confuse intrabiliary tumors with extensive intrabiliary blood clots. After treatment of the hemobilia, the endoscopic retrograde cholangiopancreatography should be repeated to delineation of the extent of the tumor. In the presence of profuse hemobilia, embolization through selective hepatic angiography is recommended. In HCC patients with jaundice, palliative drainage should be performed to improve quality of life.243
ItchingItching secondary to cholestasis can be treated using cholestyramine to decrease the enterohepatic circulation of bile acids. Other general care measures include emollients to keep the skin hydrated and reduce itching, and use of unscented soap to prevent skin irritation and relieve symptoms.244
Variceal bleedingPatients with acute variceal bleeding and unresectable HCC experience high rates of recurrent bleeding and mortality.245,246 Endoscopic variceal ligation is highly effective in controlling bleeding and has proven to be superior to sclerotherapy.247,248 Portal vein thrombosis and tumors in both lobes are related to the recurrence of bleeding.249 The use of a transjugular intrahepatic portosystemic shunt is a palliative measure for the control of variceal bleeding and ascites.250
Radiation therapyPalliative radiation therapy for liver tumors is indicated in patients experiencing abdominal pain and to reduce the symptoms caused by the mass effect or bone pain caused by metastasis to bone metastasis, the adrenal glands, lymph nodes, central nervous system, or soft tissues.251 Surgical resection is an effective treatment for patients with a small or single metastatic lesion. Other options are RFA, highintensity ultrasound, cryoablation, and tumor ablation with ethanol; all previous approaches have shown limitations in treating multiple metastatic lesions. Stereotactic radiation is a new method for direct high-dose radiation aimed at a target volume. After stereotactic radiation, quality of life improves moderately and remains relatively stable, although depression, and the severity of symptoms such as fatigue, decreased appetite, nausea, and pain may remain.252
Psychosocial and spiritual supportPsychosocial and spiritual support of the patient must be provided by a multidisciplinary team of physicians, nurses, pharmacists, social workers, and religious advisors to help patients and families.253
Recommendations- 1.
Palliative care should be provided to all patients with advanced HCC with no other therapeutic alternative (Class 1, Level C).
- 2.
Primary symptoms should be treated with the less invasive alternatives. However, endoscopic procedures and radiotherapy may be used on a case-by-case basis (Class 1, Level C).
- 3.
More research is needed in this understudied group of patients (Class 1, Level C).
In the general population, 15-20% of HCCs occur in the noncirrhotic liver,254 but these figures vary from 7% to 54% between geographic areas and according to the liver disease etiology.255–260 Noncirrhotic HCC affects patients with no evidence of liver disease or with inflammatory, fibrotic, or degenerative liver diseases (e.g., chronic viral hepatitis, HH, and NASH). Fewer than 10% of noncirrhotic HCC cases occur with no evidence of liver disease; these cases are frequently associated with genotoxic agents such as aflatoxins, although an uncertain proportion arise through transformation of hepatic adenomas.255,261 Noncirrhotic HCCs follow a bimodal distribution with respect to age, with the first peak of incidence in the second decade of life (when there is no difference in gender distribution and the fibrolamellar variant is the main form of presentation), and the second peak around the sixth decade of life.255,262
SurveillanceIndividuals with a family history of HCC are at two- to threefold increased risk of developing HCC and should be included in a surveillance protocol independent of their hepatic health status.263 Patients with HBV infection and a family history of HCC are at a particularly high risk for HCC because of the synergistic effect of these two factors.264 HCC related to hepatitis C can be found in the noncirrhotic liver.265 In the HALT-C trial, the cumulative 5-year HCC incidence among patients with cirrhosis was 7.0% compared with 4.1% in those with bridging fibrosis.266 Among 720 HCC cases involving a noncirrhotic liver, almost 30% were related to HCV infection.255 Considering that the transition from advanced fibrosis to cirrhosis cannot be defined accurately, the EASL-EORT guidelines recommend surveillance for patients with bridging fibrosis, although its cost-effectiveness is yet to be established.267
Evidence about the relationships between noncirrhotic HCC, NASH, autoimmune liver disease, HH, and alpha-1 antitrypsin deficiency is still limited.267 An increasing number of reports of HCC in patients with a noncirrhotic liver and NASH have been published in recent years.268,269 Obesity and diabetes prevention represents the best longterm strategy for avoiding NASH-related HCC,270 although strong evidence for the efficacy of such interventions in preventing HCC has not been reported.
TreatmentThere are two therapeutic lines to follow in patients with noncirrhotic HCC. The first line is liver resection, which has an overall survival rate of 25-81% and tumor recurrence rate of 30-73%.271 Another approach is liver transplantation, either as primary or as a rescue treatment following recurrence after resection.272 Only patients free of pathological macrovascular invasion and lymph node involvement should be considered for liver transplantation. The 1- and 5-year overall and tumor-free survival rates were 84% and 49% for primary transplantation and 76% and 43% for rescue transplantation, respectively.273 A 1999 systematic review suggested that fibrolamellar HCC is a more favorable indication for orthotopic liver transplantation (40% 5-year survival rate) than is nonfibrolamellar HCC (11.2% 5-year survival rate) in patients with no underlying liver disease.274
Recommendations- 1.
HCC surveillance in noncirrhotic patients is indicated every 6 to 12 months using ultrasonography in:
- •
Patients with a family history of HCC (Class 2, Level B),
- •
Patients with HBV and active hepatitis (Class 2, Level B),
- •
Patients with HCV with bridging fibrosis in the liver biopsy (Class 2b, Level B).
- •
- 2.
Surgery with lobular resection is the first-line treatment for HCC in the noncirrhotic, nonfibrotic liver (Class 1, Level B).
- 3.
In patients for whom resection is not indicated, liver transplantation can be offered to those affected by fibrolamellar HCC (Class 1, Level B). Transplantation in nonfibrolamellar patients is indicated only if there is no macrovascular invasion or lymph node involvement (Class 2b, Level B).
Primary childhood liver tumors are rare, affecting 5 of 10,000,000 children younger than 19 years of age. HCC is typically diagnosed in children aged 10 years and older (75%). Fibrolamellar HCC is diagnosed at older ages and receives surgical treatment more frequently than does nonfibrolamellar HCC.275 Despite important advances in surgical treatment, fewer than 30% of affected children are cured. Results obtained through resection with partial hepatectomy remain dismal because of the high recurrence rate. Pediatric patients with unresected HCC remain largely unresponsive to chemotherapy and continue to have a very poor prognosis.
TreatmentResection and liver transplantComplete tumor resection is the cornerstone of HCC treatment; however, complete resection is achieved in only 25% of children. Pediatric patients undergoing tumor resection experience a significant increase in 5-, 10-, and 20-year survival compared with those who do not undergo resection,275 but the results are not as positive as those observed after orthotopic transplantation (53.4% 5-year survival for resection vs. 85.3% for orthotopic transplantation).276 The role of lymphadenectomy is not clear, but it may improve the prognosis of surgically treated patients.277
There is no consensus about which liver transplantation criteria should be used in children with HCC. The Milan criteria are used widely despite having been designed for adults. Limited nonrandomized evidence suggests that children not meeting the Milan criteria can be transplanted with no detectable difference in survival.278,279
ChemotherapyChemotherapy has proven to be only partially useful in treating HCC. In the Société Internatio- nale d’Oncologie Pédiatrique – Epithelial Liver Tumor Study Group trial 1 (SIOPEL-1) using preoperative chemotherapy with a combination of doxorubicin and cisplatin (PLADO), the overall survival at 5 years was 28% and event-free survival was 17%. In SIOPEL-2, 21 patients were treated with alternating cycles of cisplatin and carboplatin/doxorubicin; 18% had metastasis, 35% had extrahepatic extension/vascular invasion, and 53% had multifocal HCC. The 3-year overall survival was 28%.280,281 A North American trial that compared PLADO against vincristine/cisplatin/5-fluorouracil found no statically significant differences in survival.282,283 New drugs such as aflibercept (VEGF-Trap) are in phase I trials and have potential as new treatments.284
SorafenibSorafenib is a promising option for HCC treatment in children. The impact of sorafenib on survival of adult patients with advanced HCC has been tested in clinical trials and analyzed in a metaanalysis, leading to its approval as first-line systemic therapy.282,283,285–290 In children, the evidence is still scarce. In a retrospective analysis, 12 patients with HCC received chemotherapy treatment (PLADO) and sorafenib; six were in complete remission after 20 months, four of them were maintained on a PLADO/sorafenib/resection treatment and two required transplantation after local recurrence. Four of seven patients with an unresectable tumor had a partial response to PLADO/sorafenib, two were stabilized, and one progressed. Although promising, the use of sorafenib alone versus sorafenib/chemo-therapy should be evaluated further.289
Arterial chemoembolizationIndications for arterial chemoembolization in children are limited. The most significant indication should be the presence of tumors that remain unresectable after systemic chemotherapy to try to make the tumors resectable without the need for transplantation.291–293
Recommendations- 1.
Surgical resection is the best treatment option for HCC in children (Class 2, Level B).
- 2.
Liver transplantation is an alternative. However, reliance on the Milan criteria to indicate eligibility for transplantation is unclear, and children not meeting the criteria may still benefit from transplantation (Class 2, Level B).
- 3.
Chemotherapy may be considered in pediatric patients for whom surgical treatment is not viable. Further research is needed to evaluate this possibility (Class 2, Level B).
- •
AFP: alpha-fetoprotein.
- •
BCLC: Barcelona Clinic Liver Cancer Group.
- •
CI: confidence interval.
- •
CLD: chronic liver disease.
- •
CT: computed tomography.
- •
CUPI: Chinese University Prognostic Index.
- •
DN: dysplastic nodule.
- •
EASL: European Association for the Study of the Liver.
- •
GRETCH: Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
HH: hereditary hemochromatosis.
- •
HR: hazard ratio.
- •
IFN: interferon.
- •
JIS: Japan Integrated Staging.
- •
MRI: magnetic resonance imaging.
- •
NAFLD: nonalcoholic fatty liver disease.
- •
NASH: nonalcoholic steatohepatitis.
- •
OR: odds ratio.
- •
PEI: percutaneous ethanol injection.
- •
PLADO: cisplatin and doxorubicin.
- •
RFA: radiofrequency ablation.
- •
SHARP: Sorafenib hepatocellular carcinoma Assessment Randomized Protocol.
- •
SVR: sustained virological response.