Non-alcoholic fatty liver disease (NAFLD) is a relevant issue in public health owing to its epidemiological burden. It represents the most common chronic liver disease in the general population and is expected to increase in future as a result of an ageing population. The only currently recommended treatment for NAFLD is lifestyle modification. However, literature reports pre-clinical and clinical studies on the use of antioxidant supplementation in NAFLD. A new antioxidant complex, called Bilirel (BIL) (Pharmaluce, Republic of San Marino), have recently introduced in the Italian market. However no data are reported on his effects on liver steatosis. Here we report on a cases series of seven overweight patients with NAFLD, in which the association of an Italian Mediterranean diet, increased physical activity, and daily administration of two pills of BIL for 6 weeks, have induced the rapid improvement of fatty liver accumulation, glucose and lipid metabolism, and weight reduction.
Non-alcoholic fatty liver disease (NAFLD) is a relevant issue in public health owing to its epidemiological burden. It represents the most common chronic liver disease in the general population and is expected to increase in future as a result of an ageing population.1 It is estimated that the prevalence of NAFLD in the general adult population worldwide is approximately between 20 and 30%, with approximately 10% (2-3% overall) meet diagnostic criteria for non-alcoholic steatohepatitis (NASH).2 The prevalence increase between 70 and 90% in people with obesity or type 2 diabetes.3
NAFLD is currently recognized as a manifestation of metabolic syndrome. Central obesity, hyperglycemia, type 2 diabetes, arterial hypertension, and hypertriglyceridemia, which are elements of metabolic syndrome, are also known to be risk factors for NAFLD.4 It is a multifaceted metabolic disorder identified in the clinical practice by a variety of health care specialists, ranging from the primary care physicians and gastroenterologists, to cardiologists, radiologists, and gynaecologists. The biological mechanism of the underlying steatosis occurrence and the progression to the liver disease is not entirely understood and is probably due to a number of factors that are expressed in the context of genetic predisposition. In such a complex repertoire a “two-hits” hypothesis has been proposed.5,6 The “first hit” induces liver fat accumulation (steatosis), and the “second hit” prompts steatosis progression to the NASH. Insulin resistance, oxidative stress, cytokines, intestinal permeability and obesity, are identified as the major factors involved in NAFLD pathogenesis.7 These factors can promote intra-hepatic fat accumulation and lipotoxicity, and develop inflammation, oxidative stress, apoptosis and fibrogenesis that determines the progression of the disease.8 With regard to therapy, the approach to NAFLD is based on lifestyle intervention. Accordingly, regular physical activity and hypocaloric diets, with weight reduction, is the clinical approach well identified in international guidelines.9–13 The general recommendations for the diet are individualized and one should aim to achieve energy deficit of 500-1,000 kcal/day depending on the body mass index (BMI) of the patient. On the other hand, at least 150 min per week of moderate-intensity physical activity and at least 75 min per week of vigorous-intensity physical activity, further to muscle strengthening twice a week are been suggested by European guideline.10 However, there is no consensus concerning pharmacological treatment.
This report presents the data of seven overweight patients with NAFLD, in which the lifestyle changes, associated with the administration of a new antioxidant formulation, have induced the rapid improvement of fatty liver accumulation, glucose and lipid metabolism, and weight reduction.
Material and MethodsWe analyze retrospectively seven consecutively overweight patients with NAFLD, referred to our Department between March and May 2014 (Table 1). Patients included in the study were 4 men and 3 women, with a mean age of 32 (range 31-59) and 43 (range 32-52) years old, respectively. In all cases, other causes of liver disease were excluded, through viral markers (for hepatitis B and C), lack of alcohol intake in the anamnesis more than 20 g/daily, presence of autoimmune or metabolic disease, the use of drugs known to induce liver steatosis. For six weeks, all patients followed an Italian Mediterranean diet based on the body weight.14 The recommended composition of the dietary regimen was as follows: carbohydrates, 50 to 60%; proteins, 15 to 20% (of which about 50% was comprised of vegetable proteins); total fat, less than 30% (saturated fat, less than 10%; and cholesterol consumption, less than 300 mg per day), and 30 g of fibers. The composition of the diet in terms of foods and food combinations was planned to obtain an animal to vegetable protein ratio as close to 1:1 as possible. The Italian Recommended Dietary Allowances were incorporated to ensure proper vitamin and mineral intake.14 At the same time the subjects taken 2 pills/daily of a new antioxidant complex recently introduced in the Italian market called Bilirel (BIL) (Pharmaluce, Republic of San Marino). The composition of one pill of BIL was: silymarin 75 mg, chlorogenic acid 3.75 mg, protopine 0.02 mg, L-methionine 75 mg, and L-glutathione 75 mg. A daily physical activity was strongly recommended. The pills are taken far from the meals, to not influence its absorption.
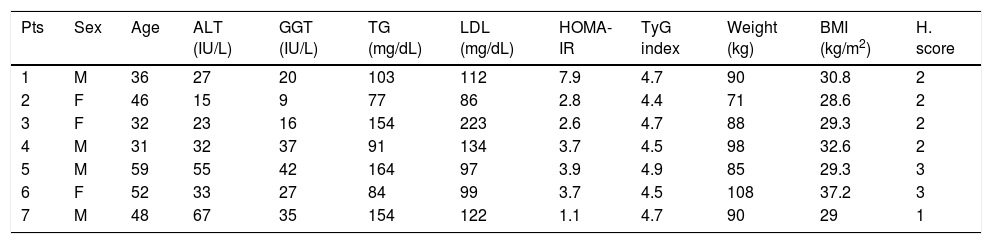
Characteristics of the patients at the baseline.
| Pts | Sex | Age | ALT (IU/L) | GGT (IU/L) | TG (mg/dL) | LDL (mg/dL) | HOMA-IR | TyG index | Weight (kg) | BMI (kg/m2) | H. score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 36 | 27 | 20 | 103 | 112 | 7.9 | 4.7 | 90 | 30.8 | 2 |
| 2 | F | 46 | 15 | 9 | 77 | 86 | 2.8 | 4.4 | 71 | 28.6 | 2 |
| 3 | F | 32 | 23 | 16 | 154 | 223 | 2.6 | 4.7 | 88 | 29.3 | 2 |
| 4 | M | 31 | 32 | 37 | 91 | 134 | 3.7 | 4.5 | 98 | 32.6 | 2 |
| 5 | M | 59 | 55 | 42 | 164 | 97 | 3.9 | 4.9 | 85 | 29.3 | 3 |
| 6 | F | 52 | 33 | 27 | 84 | 99 | 3.7 | 4.5 | 108 | 37.2 | 3 |
| 7 | M | 48 | 67 | 35 | 154 | 122 | 1.1 | 4.7 | 90 | 29 | 1 |
Normal value: ALT < 40 IU/L. GGT < 50 IU/L. TG < 150 mg/dL. LDL < 130 mg/dL. HOMA-IR ≤ 2.8. TyG index ≤ 4.5. BMI < 25.
The serum levels of total cholesterol, lipoproteins (i.e., high density HDL and low density LDL), triglycerides (TG) as well as the standard liver tests, including total bilirubin, alanine aminotransferase (ALT) and gamma glutamyltranspeptidase (GGT), were performed at the start of the therapy and after 6 weeks. Also fasting glucose and insulin levels were measured. To evaluate insulin resistance, we calculated two surrogate index: the homeostasis model assessment technique (HOMA-IR), and the product of plasma triglyceride and glucose concentrations (TyG) index.15,16 BMI was calculated as body weight divided by height squared (kg/m2). Finally, abdominal ultrasound were performed by one of the investigators with previous experience in performing and interpreting echography, to describe the grade of steatosis according to Hamaguchi score (H. score), a semiquantitative classification, that ranged from 0 (no steatosis) to 3 (severe steatosis).17 For ethical reasons and in according with international recommendations, no liver biopsies were performed.
ResultsAll patients have strictly follow the alimentary regimen prescribed and completed a six weeks treatment with BIL. All patients have reported an increase in physical activity. During the period of observation, none of other treatment for NAFLD was started. Considering the whole group of the patients (n: 7), we describe in all patients the decrease of the weight and subsequently of BMI (Table 2). In particular, the median weight reduction registered was 6.1 kg (range 5-8), with a median of 1 kg/week (range 0.8-1.3). On insulin resistance, we report that fasting glucose, insulin and consequently HOMA-IR were reduced from week 0 with a median value of 3.7 (range 1.1-7.9), to week 6 with a median value of 2.2 (range 0.7-2.8). In particular, the five patients with identified insulin resistance by HOMA-IR (patients No. 1, 2, 4, 5, and 6) showed normalization in four cases and a reduction in one case. The improvement of insulin resistance in our case series is confirmed by the analysis of TyG index. The four patients with initial high TyG index (patients No. 1, 3, 5, and 7), showed the reduction of the value after 6 weeks, with a normal value in three cases and in one the upper limit.
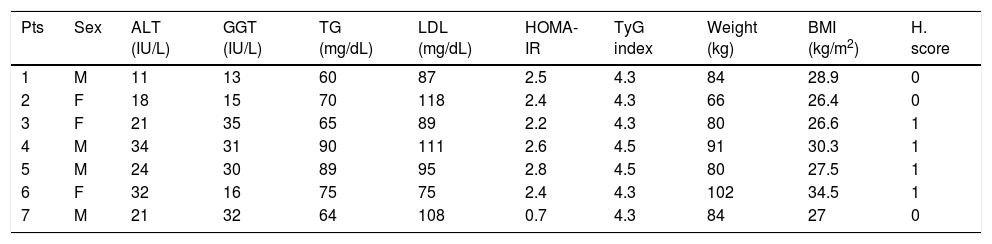
Characteristics of the patients after six weeks of lifestyle changes and treatment with BIL.
| Pts | Sex | ALT (IU/L) | GGT (IU/L) | TG (mg/dL) | LDL (mg/dL) | HOMA-IR | TyG index | Weight (kg) | BMI (kg/m2) | H. score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 11 | 13 | 60 | 87 | 2.5 | 4.3 | 84 | 28.9 | 0 |
| 2 | F | 18 | 15 | 70 | 118 | 2.4 | 4.3 | 66 | 26.4 | 0 |
| 3 | F | 21 | 35 | 65 | 89 | 2.2 | 4.3 | 80 | 26.6 | 1 |
| 4 | M | 34 | 31 | 90 | 111 | 2.6 | 4.5 | 91 | 30.3 | 1 |
| 5 | M | 24 | 30 | 89 | 95 | 2.8 | 4.5 | 80 | 27.5 | 1 |
| 6 | F | 32 | 16 | 75 | 75 | 2.4 | 4.3 | 102 | 34.5 | 1 |
| 7 | M | 21 | 32 | 64 | 108 | 0.7 | 4.3 | 84 | 27 | 0 |
Abdominal ultrasound, showed in all patients a reduction in the H. score for liver steatosis, that ranged from 1-3 points at the baseline to 0-1 at the 6-weeks evaluation. In particular, the hyperechogenity of the liver parenchyma, an echographic parameter directly linked with fat accumulation, was improved.
Considered lipid metabolism, TG value decreased in the three cases with initial hypertriglyceridemia (patients No. 3, 5 and 7), and in the two patients with higher LDL (patients No. 3 and 4). Finally, the two patients with initial hypertransaminasemia, showed the normalization of the ALT value at the end of the treatment (patients No. 5 and 7).
DiscussionDespite the rapidly growing recognition of NAFLD over the past decade, therapy directed at treatment or prevention of this condition remains to be define. Given the high prevalence of NAFLD in overweight patients, prevention of hepatic fat accumulation and weight reduction remains the cornerstone of the treatment of liver steatosis, and the management must focus on the treatment of metabolic syndrome, and, therefore, NAFLD is often considered as an individual entity in the clinical practice. Essential in the management of NAFLD, is to implies a dietary modification to decrease the body weight and to increase the physical exercise, in order to reduce insulin resistance and to normalize the transaminases levels.2
The occurrence of liver steatosis and its progression to fibrosis and cirrhosis, is a long process that includes consecutive steps. Liver steatosis is a consequence of an altered balance between fat accumulation and oxidation, both of which are regulated by insulin. Fatty liver is frequently associated with insulin resistance, and type 2 diabetes is associated with a two- to fivefold increased risk to develop NAFLD.16,17 The ideal treatment for NAFLD would reduce the liver damage and its progression. Therefore, drugs for the treatment of NAFLD should reduce body weight, improve insulin resistance and metabolic alterations, reduce the linkage between adipose tissue and liver function by acting as antiinflammatory and immune-modulatory agents by blocking oxidative stress. NAFLD patients are generally overweight or obese. A well balanced dietetic regimen and the increase in physical activity, are associated with an improvement in liver enzymes, lipid and glucose metabolism, in about 40% of overweight patients with NAFLD.14,18,19 Our results are in agreement with those studies, and we confirmed a direct correlation between lifestyle changes and BMI, insulin resistance, and hepatic fat accumulation.
Silybum marianum, commonly known as Milk Thistle (MT), is a plant native of the Mediterranean and North African regions and was used in the treatment of liver diseases for millennia.20 The active complex of MT is a lipophilic extract from plant seeds and it is composed of isomer flavonolignans (i.e. silibinin, isosilibin, silidianin, and silichristine), collectively known as silymarin. Silymarin comprises at least 70% of the standardized MT. Silibinin is the major isomer and the most active component and represents about 60-70%, followed by silichristin (20%), and silidianin (10%). Modern Western medicine has confirmed the hepato-protective properties of silymarin by conducting animal studies and clinical trials in NAFLD.20,21 Silymarin presents anti-oxidant, anti-inflammatory, anti-fibrotic, detoxifying and regenerative properties. In particular, silymarin contrasts lipid peroxidation by scavenging of free radicals and increases the levels of reduced glutathione. Silymarin treatment was associated with a reduction of insulin resistance and a significant decrease in fasting insulin levels, suggesting an improvement of the activity of endogenous and exogenous insulin.21–23 Is it also been reported that silymarin extract caused an improvement in ALT levels in human. Recently, Loguercio, et al. showed that a silybin vitamin E complex improves insulin resistance and liver damage in patients with NAFLD.24 Considered these properties, in our patients we administered the BIL complex with a daily dose of 120 mg of silymarin, with subsequently improvement of HOMA-IR, TyG index and ALT in the patients with hypertransaminasemia.
The changes on glucose and lipid metabolism, can also be explained by the action of chlorogenic acid, other component of the BIL complex. In fact, chlorogenic acid, is one of the most abundant polyphenol compounds in the human diet, and an important component of coffee.25 Chlorogenic acid exerts many biological properties and in particular stimulates glucose uptake in both insulin-sensitive and insulin-resistant adipocyte, with reduced early fasting glucose and insulin responses.25,26 In our cases the metabolic changes reported, can be explained by the synergic action of the hypocaloric diet in association with silymarin and chlorogenic acid.
Another component of the BIL complex is the protopine, an isoquinoline alkaloid contained in the Fumaria officinalis, that present choleretic and hepato-protective action.27 In particular, protopine selectively inhibits pro-inflammatory mediators (i.e. nitric oxide, cyclooxygenase-2, and prostaglandin E2) and the production of pro-inflammatory cytokines (i.e. tumor necrotic factor-α, interleukin-1 and -6).28 These data suggests that protopine could be a potential candidate for the treatment of inflammatory diseases, and in particular of NAFLD, in which hepatic lipid accumulation could induce an inflammatory status, with progression of the disease. Our data, showed the reduction of the H. score that ranged from 1-3 points at the initial time, to 0-1 at the 6-weeks evaluations.
The increase of the oxidative stress recording in NAFLD, causes not only consumption of the glutathione, the major intra-cellular anti-oxidant, with decrease of its levels, but also attenuates activity of s-adenosyl-L-methionine the principal biological methyl donor and a precursor of glutathione, essential for the anti-oxidant pathways.29 Is hypothesizable that as a result of oxidant insult, reduction of glutathione levels in combination with lower ATP availability due to mitochondrial dysfunction, results in unbalanced reactive oxygen species production with progression of the hepatic injury.30 The administration of glutathione and methionine, help to restore the oxidative balance.
Finally, the influence of the dietary nutrients in the pathogenesis of NAFLD has been reported. A diet regimen rich in monounsaturated fatty acids and omega-3, fruit, vegetables, fibre and reduced intake of saturated fats, simple carbohydrates, sweetened drinks and moderate alcohol intake should be recommended in the NAFLD patients.31 Considered these basis, we have prescribed to our patients, a personalized Mediterranean diet that has extensively been associated with a favourable health outcome, with beneficial effects both on the prevention and the treatment of the metabolic syndrome.
With the limits posed by the small number of subjects described, we indicate that BIL complex associated with a dietetic regimen and an increase in physical activity, improve after a short time, liver damage, BMI and hepatic fat accumulation, and we suggest the potential role of BIL complex to treat overweight patients with NAFLD. Whether the results are related to a direct effect of the complex on the activation of hepatic stellate cells or via its anti-oxidant or metabolic activity, is still to clarify. Future studies with larger patient samples and longer periods of observation are surely warranted, to define the exact mechanisms of action of the complex.
Conflict of InterestThere are no conflicts of interest associated with this work.
Abbreviations- •
ALT: alanine aminotransferase.
- •
BIL: Bilirel.
- •
BMI: body mass index.
- •
GGT: gamma glutamyltranspeptidase.
- •
H. score: Hamaguchi score.
- •
HDL: high density lipoprotein.
- •
HOMA-IR: homeostasis model assessment technique.
- •
LDL: low density lipoprotein.
- •
MT: Milk Thistle.
- •
NAFLD: non-alcoholic fatty liver disease.
- •
NASH: non-alcoholic steatohepatitis.
- •
TG: triglycerides.
- •
TyG: triglyceride x glucose.