Bile is primarily secreted in hepatocytes (i.e. the canalicular bile) and subsequently delivered to the intrahepatic bile ducts, where is modified by cholangiocytes (i.e. the ductal bile). Bile formation is the result of the coordinated interactions of membrane-transport systems that generate the vectorial movement of solutes and osmotically driven water molecules. Hepatocytes and cholangiocytes express aquaporins, specialized membrane channel proteins that facilitate the osmotic transport of water. In this review, we provide a summary of what is known on liver AQPs and their significance in canalicular and ductal bile formation under normal and pathological conditions.
Abbreviations:
AQP, aquaporin
Bsep, bile salt export pump
Mrp2, multidrug resistance-related protein 2
CFTR, Cystic Fibrosis Transmembrane Regulator
AE2, Cl-/HC03- anion exchanger 2
This work was supported by Grant PICT 05-10590 (to R.A. Marinelli) from Agencia Nacional de Promoción Científica y Tecnológica, and by Grant PIP 03020 (to R.A. Marinelli) from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Primary bile is secreted by hepatocytes at the bile canaliculus and is subsequently modified in composition and volume by cholangiocytes the cells that line the intrahepatic bile ducts.1 Bile consists of about 95% water and water transport by liver cells is thought to occur passively in response to local, transient, osmotic gradients generated by the active transport of certain solutes. Both hepatocytes and cholangiocytes express aquaporins (AQPs),2,3 a family of membrane channel proteins that facilitate the osmotically driven movement of water molecules.4 There is compelling experimental evidence suggesting that AQPs are key players in bile formation.
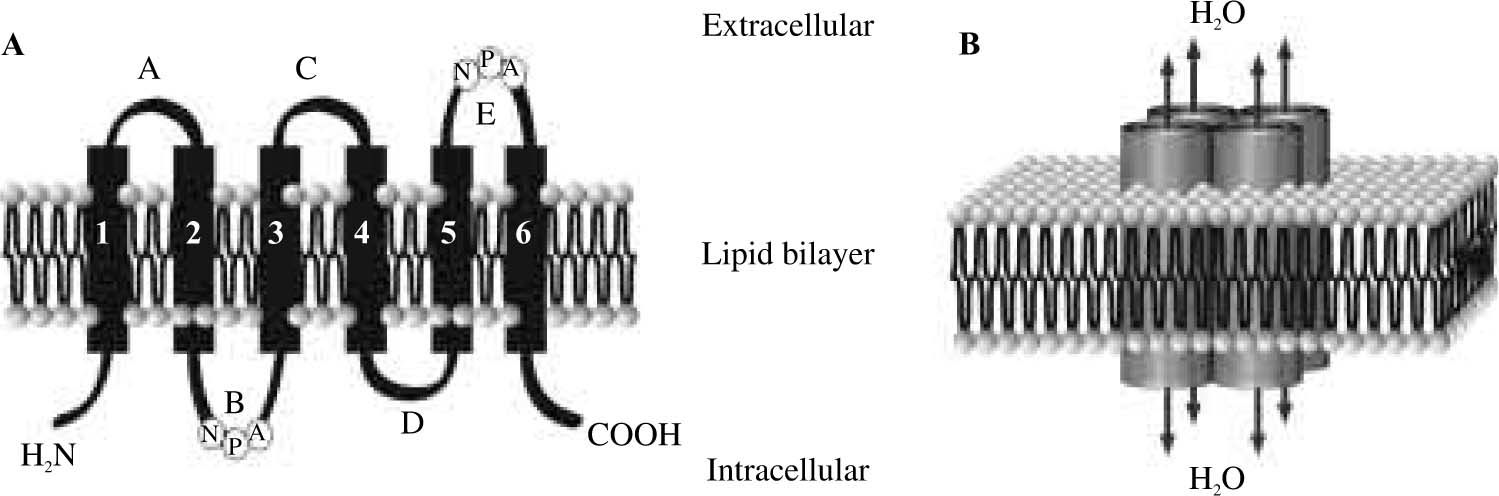
Aquaporin water channelsThe first AQP was identified in erythrocytes by Dr. Peter Agre from Johns Hopkins University, U.S.A.5,6 who was awarded with the Nobel Prize in Chemistry 2003. AQPs are a family of integral homotetrameric membrane proteins widely distributed in mammals, plants, and lower organisms. Eleven mammalian aquaporin proteins, numbered 0 to 10, have been identified thus far.7 Each aquaporin consists of four independent channels assembled into a functional unit. Primary sequence of AQPs revealed that each subunit is composed of approximately 270 amino acids with cytoplasmic carboxy-and amino-terminal ends. They consist of six bilayer-spanning domains connected by three extracellular (A, C, and E) and two intracellular loops (B and D). Two of these loops enclose the conserved motif Asn-Pro-Ala (NPA) which is part of the aqueous pore4(Figure 1). Several recent studies have revealed the high-resolution structures of the water channel protein family that explain their selectivity. The three key features for channel selectivity are:8 (i) size restriction. The pore narrows to a diameter of 2.8 Å (approximately the diameter of a water molecule); (ii) electrostatic repulsion. The residue Arg-195 imposes a barrier to cations, including protonated water (H3O+); (iii) water dipole reorientation. Positively charged dipoles reorient water molecules and prevent the formation of a proton conductance.
Membrane topology and organization of the aquaporin water channel molecule. A: A single AQP monomer is composed of six transmembrane domains connected by loops A to E, with two NPA boxes and with the amino and carboxy termini oriented intracellularly; B: The AQP subunits are assembled into a tetramer with the four sets of B and E loops constituting four central water pores.
Aquaporins are characteristically inhibited by mercurials. The inhibitory site corresponds at the Cys-189, proximal to the NPA motif in loop E.9 Certain AQPs (e.g., AQP4) lack the cysteine at this site leading to the lack of inhibition by mercurials.10,11 AQPs work primarily as water-transporting channels, although some of them (e.g., AQP9) are also permeable to certain small solutes such as glycerol and urea.12
AQPs have been shown to be expressed in many epithelial cells; typically, one or more specific AQPs exist in a particular water-transporting epithelial cell. They are either constitutively expressed or regulated, often by agonist-induced trafficking from an intracellular vesicular compartment to the plasma membrane allowing for rapid changes in membrane permeability, depending upon physiological needs. An example of this is the translocation of AQP2 in response to vasopressin in the tubule epithelia of the collecting duct of the kidney.13
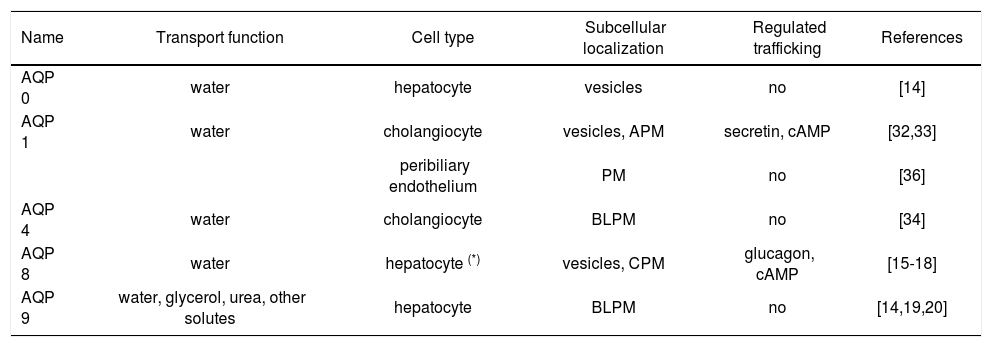
Five members of the mammalian aquaporin family were found to be expressed in liver cells both at mRNA and protein level (Table I).
Liver aquaporin proteins.
| Name | Transport function | Cell type | Subcellular localization | Regulated trafficking | References |
|---|---|---|---|---|---|
| AQP 0 | water | hepatocyte | vesicles | no | [14] |
| AQP 1 | water | cholangiocyte | vesicles, APM | secretin, cAMP | [32,33] |
| peribiliary endothelium | PM | no | [36] | ||
| AQP 4 | water | cholangiocyte | BLPM | no | [34] |
| AQP 8 | water | hepatocyte (*) | vesicles, CPM | glucagon, cAMP | [15-18] |
| AQP 9 | water, glycerol, urea, other solutes | hepatocyte | BLPM | no | [14,19,20] |
CPM, canalicular plasma membranes; APM, apical plasma membranes; BLPM, basolateral plasma membranes; PM plasma membranes.
It has been recently shown that rat hepatocytes express mRNA and protein for AQP0,14 AQP8,15-18 and AQP9.14,19,20
Aquaporin-8 (AQP8)Hepatocyte AQP8 is an N-glycosylated protein of about 34 kDa. Initial biochemical, immunohistochemical, and confocal immunofluorescence studies15,16 showed that AQP8 is largely intracellular in hepatocytes. Immunoelectron microscopy studies localized AQP8 to pericanalicular vesicles as well as to the canalicular plasma membrane.15,21,22
It was observed that AQP8 can redistribute from the intracellular vesicular compartment to plasma membrane in hepatocytes stimulated by the cell permeable cAMP analog, dibutyryl cAMP.15 The cAMP-induced increased levels of AQP8 on hepatocyte surface are accompanied by an increase in membrane water permeability.15 The microtubule blocker colchicine specifically inhibits the dibutyryl cAMP effect on both AQP8 redistribution to cell surface and hepatocyte membrane water permeability,15 providing evidence for the involvement of microtubules for AQP8 trafficking. More recent studies in isolated rat hepatocytes show that the cAMP-mediated hormone glucagon, is also able to induce the translocation of AQP8 to canalicular membrane.23 Glucagon effect requires activation of the cAMP-dependent protein kinase A and an intact microtubular network. Since the AQP8 molecules lack consensus protein kinase A phosphorylation sites24 and therefore may not be phosphorilated, the effect of glucagon is believed not to be directed towards AQP8 protein itself but rather to microtubule-associated proteins involved in vesicle trafficking. Confocal immunofluorescence microscopy and functional studies in polarized isolated rat hepatocyte couplets14 demonstrated that AQP8 is specifically targeted to the canalicular plasma membrane domain, which promotes osmotically-driven water secretion.14
Recent detailed immunoelectron microscopy studies revealed that AQP8 in hepatocytes is also present on smooth endoplasmic reticulum adjacent to glycogen granules as well as in some mitochondria.22 These novel findings suggest the involvement of AQP8 in preserving cytoplasmic osmolarity during glycogen metabolism and in mediating mitochondrial volume changes.
In addition to the described short term regulation of hepatocyte AQP8 by vesicle trafficking, the water channel can also be modulated on the long term basis by modifying its gene expression. Thus it was recently found that fasting induces a remarkable down-regulation of hepatic AQP8 mRNA and protein. Interestingly, the reduction of AQP8 paralleled the expected depletion of glycogen liver content. The hepatic AQP8 levels returned to be normal after refeeding.22
Aquaporin-9 (AQP9)Hepatocyte AQP9 is an approximately 32 kDa protein. Immunohistochemical, confocal immunofluorescence as well as immunoelectron microscopy studies localized AQP9 on hepatocytes surface, specifically on the sinusoidal plasma membrane domain, with no significant intracellular expression.14,19 AQP9 is an aquaglyceroporin, i.e., a water channel membrane protein also permeable to certain small uncharged solutes such as urea and glycerol. This AQP is thought to allow the rapid cellular uptake or exit of metabolites with minimal osmotic perturbation. Hepatocyte AQP9 expression was found to be dependent on the nutritional status.25,26 Thus, AQP9 was observed to be markedly induced in fasted rats; a state in which glycerol is actively taken up by liver for gluconeogenesis. No changes in liver AQP9 levels were observed in rats fed ketogenic or high-protein diets. Hepatocyte AQP9 expression is also affected by the circulating insulin levels.25,26 Hence, liver AQP9 levels were found to be elevated in diabetic rats and decreased after administration of insulin. The presence of a negative insulin response element in the AQP9 promoter gene25 suggests an insulinmediated suppression on AQP9 transcription. Interestingly, hepatic expression of AQP9 seems to be gender-dependent. Male rats have higher levels of AQP9 protein and mRNA than female rats.20
Aquaporin-0 (AQP0)Similar to AQP8, this AQP is mainly localized intracellularly in hepatocytes, although its precise subcellular localization has not been determined yet. AQP0 seems not to be regulated by vesicle trafficking,14 based on its lack of responsiveness to dibutyryl cAMP. The physiological role of AQP0 in hepatocytes remains to be elucidated.
Water transport by hepatocytes: Canalicular bile formationCanalicular bile formation is an osmotic secretory process. The biliary excretion of bile salts, via the bile salt transporter Bsep, glutathione, via the organic anion transporter Mrp2, and HCO3-, via the Cl-/HCO3- exchanger AE2 are thought to be the major osmotic driving forces for canalicular bile flow.1 Conceptually, the generation of bile flow is ultimately dependent on the molecular and functional expression of these transporters in the canalicular plasma membrane domain as well as on the canalicular membrane water permeability determined by AQPs. This is assuming that water flows across hepatocyte epithelial barrier by a predominantly transcellular route, with minimal paracellular contribution. In fact, existing evidence suggests that the transcellular pathway accounts for most of the water entering the bile canaliculus. Our recent experimental observations provide additional support for this view. Studies in polarized hepatocyte couplets showed that AQP blockers totally prevented osmotically-driven water transport into bile canaliculus.14 Moreover, theoretical calculations based on the osmotic basolateral and canalicular membrane water permeability values, support a predominant transcellular route for water movement during choleresis.27 Thus, the transcellular pathway is thought to account for most of the water entering the bile canaliculus.
As mentioned in the previous section, AQPs are present in hepatocytes at both apical and basolateral plasma membrane domains as well as in intracellular vesicle compartments. Two of these AQPs can account for the water permeability of both hepatocyte plasma membrane domains, AQP8 modulating mainly the canalicular transport of water, and AQP9 facilitating its basolateral movement. Glucagon (via cAMP) regulates the amount of canalicular AQP8.
Canalicular bile flow is known to be modulated by glucagon.28,29 Although the actual osmotic gradients involved in glucagon-induced choleresis are unknown, these transient gradients are most likely created by the facilitated transport of HCO3- via the canalicular AE2.28,29 There is evidence to suggest that glucagon (via cAMP) is able to stimulate the microtubule-dependent vesicle trafficking of AE2 to hepatocyte plasma membrane.29,30 This mechanism, together with direct activation of the exchanger, could account for the bicarbonate-rich choleresis induced by glucagon. Whether AQP8 is packaged along with AE2 in the same population of vesicles, as has been observed for AQP1 in cholangiocytes,31 or they are in separate vesicles that share a common microtubule-dependent, cAMP-related mechanism, needs to be determined. Moreover, as we observed that AQP8 is targeted to the canalicular plasma membrane domain,14,23 we believe that AQP8 can facilitate the osmotically driven water transport during glucagon-stimulated hepatocyte bile formation. Thus, our findings in isolated cells may be relevant to the in vivo situation.
Direct assessment of osmotic water permeability in isolated canalicular and basolateral plasma membrane vesicles by stopped-flow spectrophotometry,27 indicates the presence of both lipid and AQP-mediated pathways for basolateral and canalicular water movement across hepatocyte plasma membrane barrier. The canalicular membrane domain has lower water permeability and becomes more permeable to water when hepatocytes are exposed to cAMP-mediated choleretic agonists.
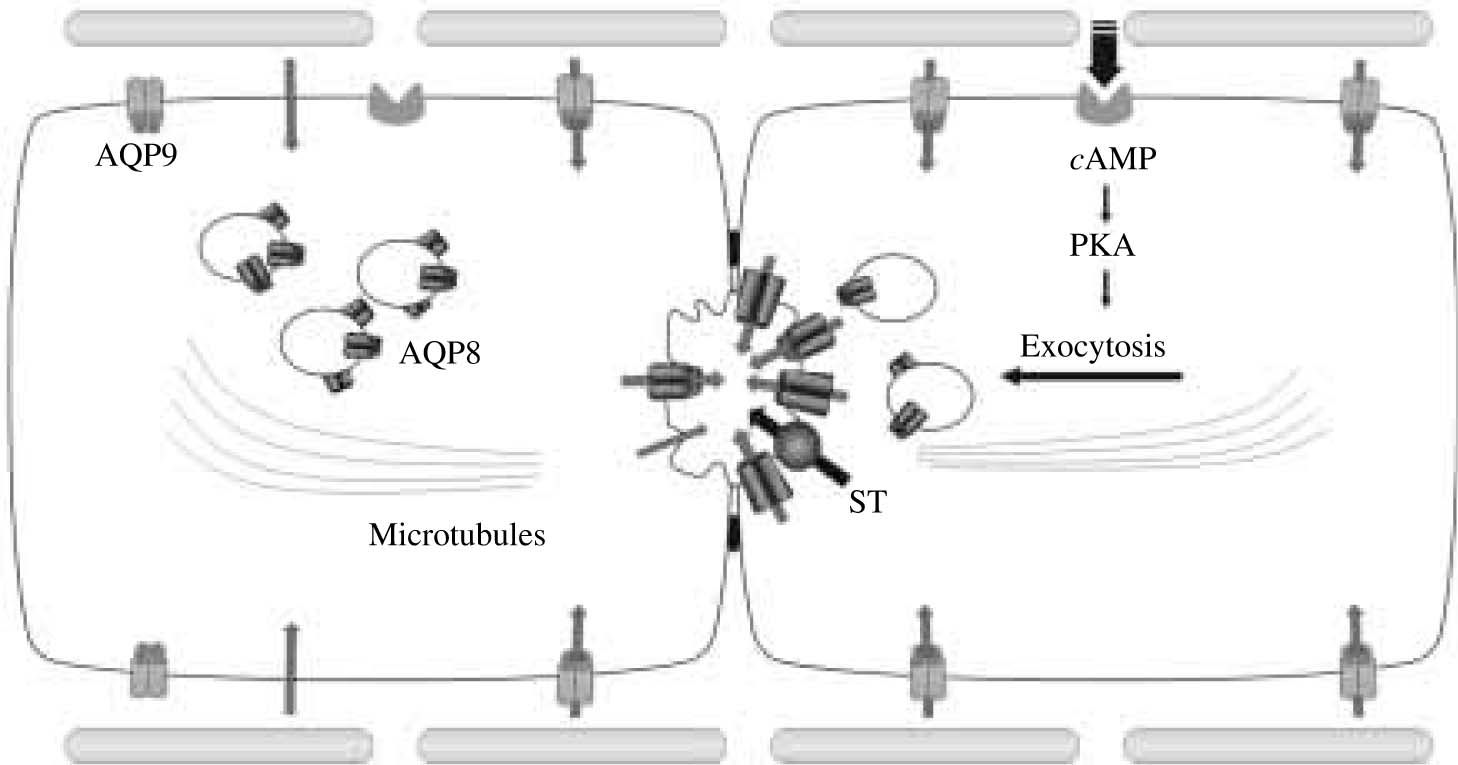
In light of these new observations, it seems plausible that water can move across hepatocyte membranes by both lipid-and AQP-mediated pathways. They also suggest that the canalicular plasma membrane domain is rate limiting for transcellular water transport in hepatocytes, and that under cAMP-mediated stimulation, this domain becomes highly permeable to water by insertion of AQP8 water channels facilitating transcellular osmotic water transport. Thus, hepatocytes are able to modulate their canalicular membrane water permeability, providing a molecular mechanism for the efficient coupling of osmotically active solutes and water transport during canalicular bile formation. In figure 2, we show a proposed model for the mechanisms of water transport in hepatocytes.
Proposed mechanisms of water transport in hepatocytes. On the left, it is illustrated a hepatocyte in the basal (unstimulated) state. AQP9 is expressed at the basolateral plasma membrane, while AQP8 is expressed at the canalicular membrane as well as in intracellular vesicles. The canalicular membrane domain has lower water permeability than the basolateral, i.e. it is rate limiting for transcellular water transport. Water secretion by hepatocyte into the canaliculus occurs mainly transcellularly via lipid and AQP-mediated pathways. On the right, it is illustrated a hormone-stimulated hepatocyte. After binding to its receptor, glucagon, via cAMP, activates protein kinase A which in turn induces the microtubule-dependent canalicular targeting of AQP8-containing vesicles. Canalicular membranes become highly permeable to water by insertion of AQP8. The activation of preexisting or newly inserted solute transporters (ST), such as the Cl-/HCO3- exchanger AE2, provides the osmotic driving forces for transcellular water transport into bile canaliculus.
It has been recently shown that rat cholangiocytes express message and protein for AQP132,33 and AQP4.34 AQP8 was found to be expressed in cholangiocytes at message level,35 but its protein expression needs to be confirmed.
Aquaporin-1 (AQP1)Rat cholangiocytes express the water channel AQP1, which is present as a non-glycosylated protein of 28 kDa.32,33 Subcellular fractionation as well as light and immunoelectron microscopy analysis showed that AQP1 is present mainly in the cholangiocyte apical plasma membrane domain and in an intracellular vesicular pool.31,33,34 Studies in highly purified freshly isolated cholangiocytes as well as in intact rats, indicate that secretin induces the microtubule-dependent redistribution of AQP1 from intracellular vesicles to the apical plasma membrane domain with a concomitant increase in cholangiocyte membrane water permeability.33,34
AQP1 is expressed in erythrocytes and in fluid transporting epithelia throughout the body at sites of constitutive (not regulated) water transport.4 Liver appears to be an exception in that AQP1 is present in cholangiocytes in a pool of AQP1 carrying-vesicles able to traffic to the plasma membrane in a regulated fashion. Thus, it may be that a protein that is constitutively expressed in one cell type is regulated in another. Proteins are under the control of mechanisms that include interactions between signals within the protein itself and the cellular sorting machinery. These signals can be differentially interpreted by various cell types.
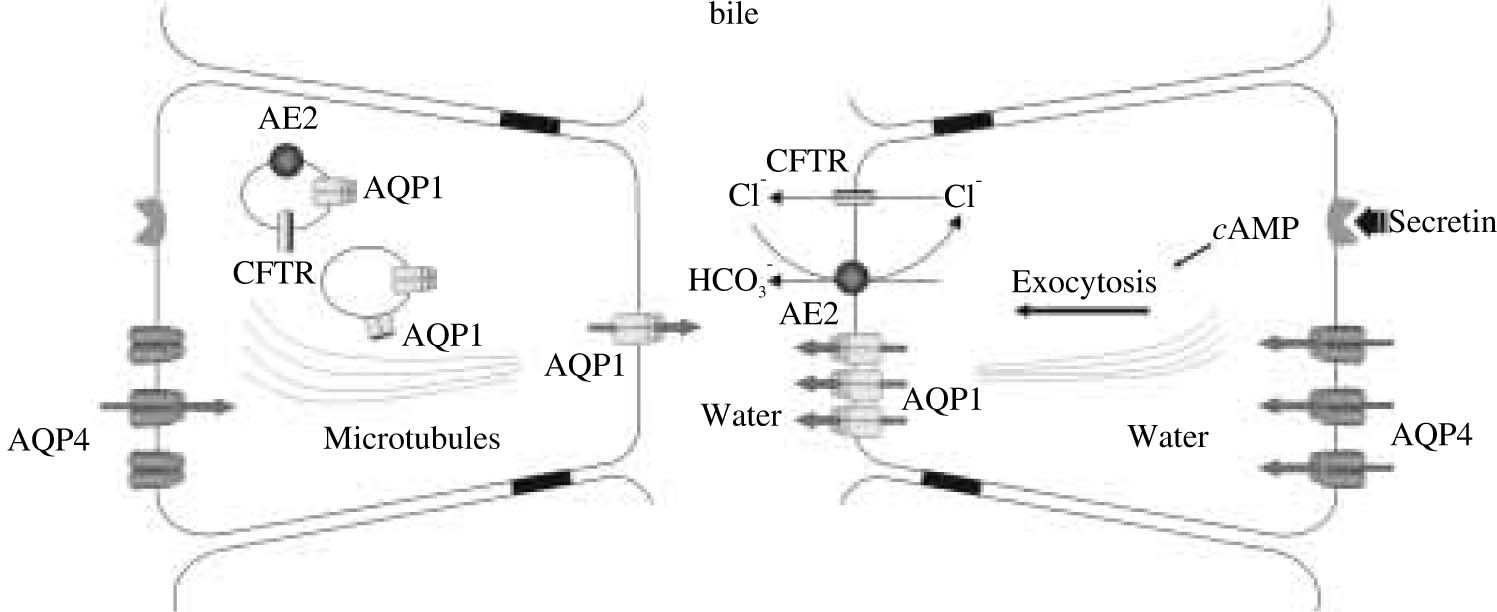
It was recently found in cholangiocytes a specialized population of intracellular vesicles containing AQP1 as well as CFTR Cl- channels and the Cl-/HCO3- exchanger AE2. After exposure of cholangiocytes to dibutyryl AMP in vitro or after secretin infusion in vivo, these three proteins co-redistribute to the apical cholangiocyte plasma membrane.31 It is expected that this process plays a key role in hormone-induced ductal bile secretion.
Aquaporin-4 (AQP4)Cholangiocyte AQP4 is a protein of approx. 31 kDa.34 AQP4 is not responsive to secretin and it seems to be constitutively localized to the cholangiocyte basolateral membrane, mediating water transport across that plasma membrane domain.
Water transport by cholangiocytes: Ductal bile formationCholangiocytes account for only 3% to 5% of the liver cell population; nevertheless, they provide a large surface area for transport between blood to bile and play a significant role in bile secretion producing as much as 40% of total bile volume in some species.37 These observations suggest that the amount of transcellular water movement across an individual cholangiocyte is potentially up to 10 times greater than across an individual hepatocyte.
AQPs are present in cholangiocytes in both apical and basolateral plasma membrane domains as well as in intracellular vesicle compartments. Thus, AQPs can account for the water permeability of both cholangiocyte membrane domains, AQP1 modulating mainly the apical transport of water, and AQP4 facilitating its basolateral movement. Such basolateral transport of water would allow the relative isosmolar status of the cell to be maintained during ductal bile formation and is consistent with the intimate physical association between the basolateral domain of cholangiocytes and the peribiliary vascular plexus, a mesh-like arrangement of blood vessels that surround the bile duct and from which the fluid component of bile originates. High expression of AQP1 in peribiliary vascular endothelia34 may reflect an important functional role of this AQP in facilitating water transport from plasma to bile across cholangiocytes.
It is well known that ductal bile secretion is regulated by secretin. We have recently demonstrated that secretin stimulated ductal bile secretion is associated with the exocytic insertion of AQP1, normally sequestered in cytoplasmic vesicles, into the cholangiocyte apical plasma membrane. Thus, inhibitory studies in isolated rat and mouse bile duct segments suggest that AQP1 plays a key role in osmotically-driven apical water secretion during hormone-regulated ductal bile formation. Indeed, studies in perfused rat bile duct segments in which AQP1 was silenced in vitro using small interfering RNA technology provided additional evidence for a key role for AQP1 in ductal bile formation.38
Although secretin is well known to stimulate ductal bile secretion by interacting with specific cAMP-coupled receptors on the basolateral domain of cholangiocytes,2 little information is available on the stimulus-secretion coupling mechanisms. Based on current knowledge, we propose a model for coupling solute and water secretion in cholangiocytes. In this updated model, secretin is proposed to stimulate the exocytic insertion of vesicles containing AQP1, CFTR Cl- channels, and AE2 (i.e., the Cl-/HCO-3 exchanger) into the apical cholangiocyte membrane. The efflux of Cl- via CFTR provides the luminal substrate to drive the extrusion of HCO3- into the lumen via AE2. The presence of HCO-3 and Cl- molecules in the ductal lumen creates the necessary osmotic force for the movement of water via AQPs into the biliary lumen. The efflux of Cl- would also generate a negative intraluminal potential leading to Na+ and K+ movement through a paracellular pathway; however, the degree to which water follows the efflux of these two ions via a paracellular route is unclear.
It is not known whether a substantial fraction of transepithelial water flow across the tight junctions between cholangiocytes (i.e., the paracellular route) contributes to ductal bile formation. Using established in vitro physiologic models suitable for addressing this question (i.e., enclosed polarized and microperfused bile ducts isolated from normal rats and mice), we observed that osmotically induced transepithelial water movement into the lumen of bile duct units was inhibited by DMSO or HgCl2 (agents that inhibit AQP-mediated water transport) but not by protamine (an agent that alters paracellular water transport).39,40 These data suggest that transepithelial water transport across biliary epithelia occurs principally via water channels and not via a paracellular pathway. Calculated Pf values demonstrating rapid water movement across the biliary epithelia in rat microperfused intrahepatic bile ducts also support this concept.41 Taken together, these data suggest that water transport by intrahepatic bile ducts is predominantly transcellular and AQP-mediated.
Intrahepatic bile ducts not only secrete but also absorb water.2,3 It was found that enclosed polarized intrahepatic bile duct units exposed to hypertonic buffers rapidly decreased their luminal area, reflecting net water absorption. Substantial water movement from lumen to bath was observed if a net outward osmotic gradient was established in microperfused rat and mouse bile duct units.39-41 Addition of glucose to the perfusate of isolated microperfused rat bile duct units was also associated with water absorption (42). In these models, water absorption by biliary epithelia was sensitive to AQP inhibitors, suggesting the involvement of water channels.39,40 AQPs 1 and 4 are the most likely candidates to provide channel-mediated water absorption by biliary epithelia.
Implications of AQPs in liver diseaseBile secretion is compromised in several illnesses, and the pathogenesis of bile secretory failure cannot be understood without a thorough knowledge of how bile is generated. It is known that bile secretion by hepatocytes and cholangiocytes results from the coordinated interactions of several solute membrane-transport systems. As detailed above, recent cumulative evidence indicates that AQP water channels play a key physiological role in canalicular and ductal bile formation. Hence, it is conceivable that defective AQP membrane expression may lead to alterations of normal bile physiology. Currently, there is no conclusive evidence indicating that derangements of normal AQP function are causative of bile secretory dysfunction. Nevertheless, abnormal expression and trafficking of AQPs were found to be present in some pathological conditions in which altered bile secretion occurs. We have recently demonstrated,21 by using immunoblotting, immunohistochemical and immunoelectron microscopy studies, a defective expression of hepatocyte AQP8 in rats with biliary obstruction (i.e., extrahepatic cholestasis). Northern blotting experiments indicated that the levels of AQP8 mRNA were not decreased, suggesting a normal message transcription.21 Thus, although the precise mechanism for AQP8 protein reduction has not been elucidated yet, it seems to be posttranscriptional. It may involve a defective synthesis and/or degradation of AQP8 protein.
We also found that the vesicular translocation of AQP8 to the hepatocyte plasma membrane was compromised in extrahepatic cholestasis.21 As mentioned in previous sections, the microtubules seem to be central for the hormone-regulated vesicle trafficking of liver AQPs. It is known that hepatocyte microtubules undergo significant changes after bile duct ligation,43 which is thought to disturb vectorial vesicular transport of some canalicularly targeted transporters.43 Thus, defective translocation of AQP8 in extrahepatic cholestasis may be due, at least in part, by alterations in the microtubular network. An alteration of the hepatic cAMP level does not seem to be involved in the defective AQP8 translocation, since cAMP was found not to be altered by bile duct ligation.44
The finding that AQP8 expression and trafficking are defective in extrahepatic cholestasis suggests, for the first time, that AQP water channels are involved in the development of bile secretory dysfunction.
Alterations in the functional expression of renal AQPs have also been involved in liver pathology. For example, the levels of AQP2, the vasopressin-sensitive AQP of kidney collecting tubular cells,45 were found to be altered in experimental models of hepatic cirrhosis.45 AQP2 has been observed upregulated in decompensated states of liver cirrhosis which are characterized by sodium retention, edema, and ascites.46,47 It is though that the increased levels of AQP2 would in turn participate in the development of water retention. Interestingly, changes in expression of AQP2 protein levels vary considerably between fundamentally different experimental models of cirrhosis. Thus, AQP2 is downregulated in compensated cirrhosis, characterized by peripheral vasodilatation and increased cardiac output but not water retention.48,49 It has been suggested that the decreased of AQP2 may represent a compensatory mechanism to prevent development of water retention.
ConclusionThe understanding of the physiological mechanisms of bile formation by hepatocytes and cholangiocytes is rapidly evolving because of the recent discovery of the AQP water channels. This knowledge is expected to shed new light on the molecular pathogenesis of certain liver diseases with compromised bile secretion.