Liver diseases are a major cause of morbidity and mortality globally. In the Philippines, a lower middle-income country in Southeast Asia, liver diseases accounted for 27.3 cases per 1000 deaths. In this review, we discussed the prevalence, risk factors, and management of hepatitis B, hepatitis C and other viral hepatitis, non-alcoholic fatty liver disease, alcohol-associated liver disease, liver cirrhosis, and hepatocellular carcinoma. The true burden of liver disease in the Philippines is likely underestimated due to limited epidemiological studies. Thus, surveillance of liver disease should be strengthened. Clinical practice guidelines tailored to the local needs of the country have been developed for important liver diseases. Multisectoral cooperation among different stakeholders is needed to manage the burden of liver disease in the Philippines.
Liver disease is a major public health problem globally. The latest estimates from WHO state that liver diseases caused approximately 2 million deaths in 2019 [1]. Liver cirrhosis and liver cancer are currently the 11th and 24th leading causes of death worldwide, respectively. Around 354 million people globally live with hepatitis B or hepatitis C infection [2].
In the Philippines, a lower-middle-income country, accurate statistics on the burden of liver diseases are lacking due to the lack of wide-scale epidemiological studies. According to recent data from the Philippine Statistics Authority, liver diseases accounted for 27.3 cases per 1000 deaths in the country in 2020 [3]. Liver cirrhosis accounts for 31.8% of liver-related deaths in the country, while the malignant disease of the liver comprises 35.8%. Viral hepatitis comprises 5.3% of liver-related deaths [3].
In this paper, we review the clinically and epidemiologically important liver diseases in the Philippines. We present available data on the prevalence and risk factors of viral hepatitis, non-alcoholic fatty liver disease, alcohol-associated liver disease, liver cirrhosis, and hepatocellular carcinoma. We also share the country's clinical practices in the management of these diseases.
2Hepatitis B2.1PrevalenceThe Philippines remains highly endemic to hepatitis B virus infection. The Philippines, together with China, India, Nigeria, and Indonesia, account for 57% of infections globally [4]. The latest modeling study estimates 10.4% HBsAg seropositivity, while the most recent national epidemiological survey found 16.7% seroprevalence in the Philippines [4,5]. Various studies suggest regional and socioeconomic disparities in hepatitis B prevalence. HBsAg seroprevalence appears to be lower in urban (5.6%) compared to rural areas (8.4%) [6,7]. Moreover, lower annual income and low education levels were found to be associated with higher HBsAg seroprevalence [5]. These findings may indicate inequities in health care resulting in higher HBV infection rates among disadvantaged groups. The most common genotypes in the country are genotypes A and C. More studies need to be done as the clinical significance of the HBV genotype could not be evaluated due to the small sample sizes of the studies [8,9].
2.2Risk factors and transmissionTransmission of HBV is through perinatal, percutaneous, or sexual contact. Mother-to-child transmission is a significant cause of chronic hepatitis B in highly endemic countries like the Philippines. Current hospital data showed that chronic hepatitis B among Filipino pregnant patients is at 9.6% [10]. Hepatitis B can also be transmitted through blood transfusion. Patients with a history of blood transfusion have fourfold increased odds of infection in a local study of gynecologic patients [11]. However, this is likely lower in recent years as the implementation of safety procedures such as mandatory blood screening has significantly reduced HBV transfusion transmission [12–14].
Needlestick injuries are a risk for hepatitis B, especially among healthcare workers [15]. Estimates of needlestick injuries among hospital workers in Manila range from 55%−60% [16,17]. High workload, working under pressure and long working hours were cited as top reasons for injuries [16]. Sharing of needles among injection drug users (IDUs) is another important risk factor for HBV infection. In the Philippines, only 63.6% of IDUs reported safe injection practices [18]. Both high rates of needlestick injuries and low rates of safe injection practices pose risks for hepatitis B infection in the relevant population.
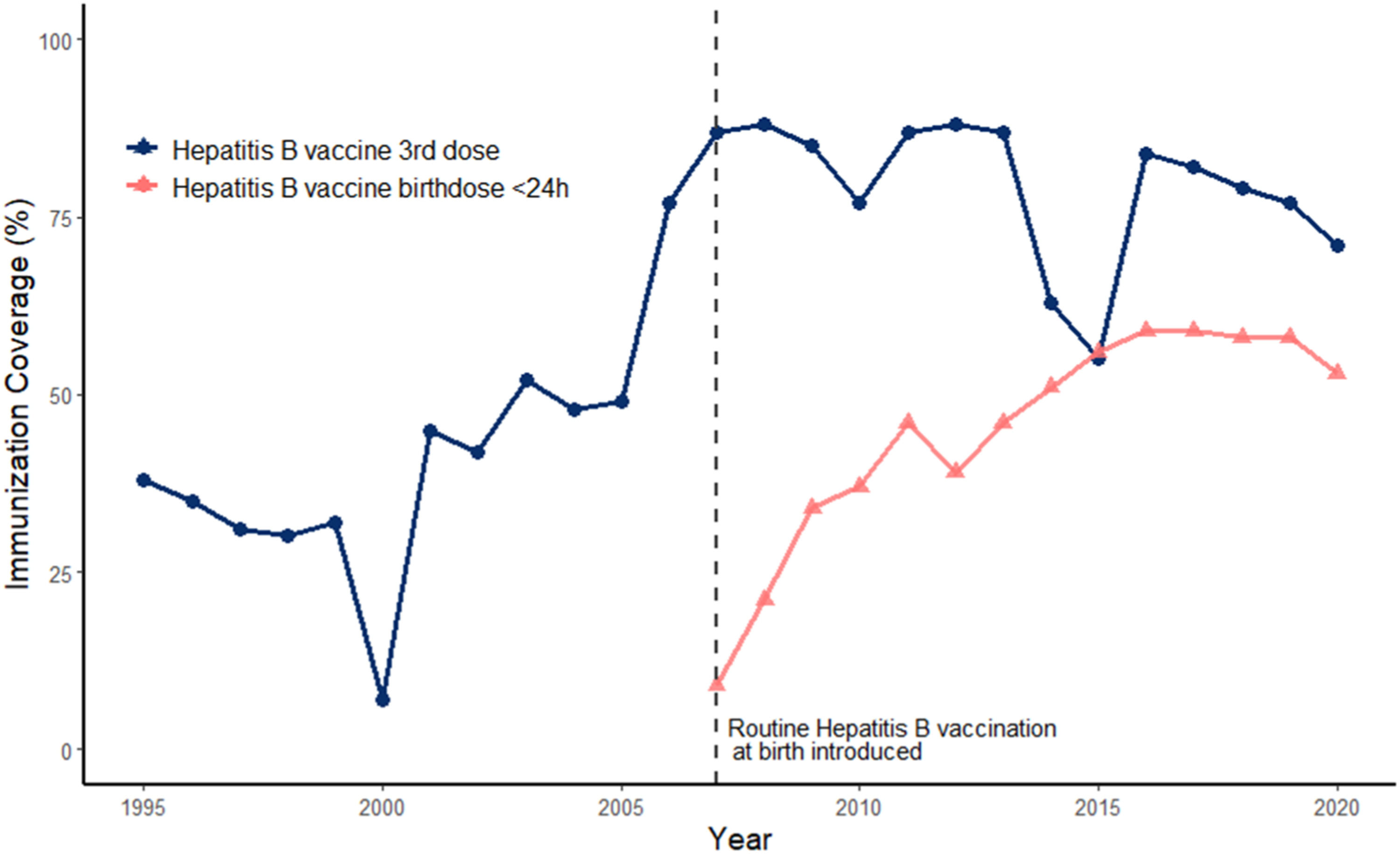
2.3VaccinationThe hepatitis B vaccination program in the Philippines was instituted in 1992, and routine hepatitis B vaccination at birth was fully implemented in 2007(Fig. 1) [19]. The Philippines fulfilled the WHO's timely hepatitis birth dose goal for 2020 (53% in the Philippines vs.>50% WHO goal) but failed to meet the hepatitis B vaccine coverage goal (71% in the Philippines vs. 90% WHO goal)) [2]. Curiously, timely hepatitis birth dose is lowest in private hospitals, and thus engagement of the private sector is crucial for achieving vaccination goals in the country [20].
Improvement in HBsAg seroprevalence is evident due to vaccination efforts. Estimates of prevalence among children after the institution of the national vaccination program revealed a significant drop in HBsAg prevalence (0.8% among 5–6 years old in 2018) compared to adults born in the prevaccination era (16.7% among adults in 2003) [5,21].
2.4Screening and treatmentThe latest national clinical practice guidelines recommend screening for all Filipino adults and adolescents [22]. However, facilities capable of HBV tests are limited, especially in geographically isolated areas. Moreover, there is a stigma associated with positive tests for Hepatitis B. It is important to note that screening recommendations for HBV are incumbent on the availability of pre-and post-test counseling and linkage to care [22–25]. Efforts at simplification the algorithms for the screening, diagnosis, and triage of HBV patients to treatment to allow more widespread identification and treatment of HBV patients have been made to meet the 2030 WHO elimination goals. This includes the use of a single elevated ALT in combination with HBV DNA ≥ 2000 IU/mL to guide the initiation of antiviral treatment in adults with chronic hepatitis B infection.
The national guidelines recommend Tenofovir disoproxil fumarate (TDF), Tenofovir alafenamide (TAF), and Entecavir (ETV) as first-line antiviral therapy for chronic hepatitis B patients [22]. TDF is recommended for pregnant patients with high viral load (>200,000 IU/mL) to prevent mother-to-child transmission of hepatitis B [22–25]. At the present time, most of the screening, diagnosis, and treatment activities for Hepatitis B are largely concentrated in the private sector. With the establishment of the viral hepatitis initiatives within the Department of Health in 2017 through Administrative Order (AO) 2017–0011, a service delivery model for HBV was piloted in 2019 and expansion was planned for 2020 [26–28]. While the COVID-19 pandemic slowed the expansion efforts, the slowdown in the pandemic in 2022 should hopefully see an acceleration in the various efforts for HBV in the public sector. It is hoped that the expansion of the service delivery model in the public sector should allow the rapid scale-up of efforts to screen, diagnose, and treat patients with HBV to achieve the WHO elimination targets.
3Other viral hepatitis3.1Hepatitis c virusHepatitis C is a major cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma worldwide [29]. Data on screening among blood donors and overseas Filipino worker candidates showed prevalence rates between 0.3% to 0.9%, while the latest mathematical modeling estimates 0.4% viremic infection in the Philippines for 2020 [30–33]. This parallels the 0.7% - 1.8% global HCV prevalence estimates [30,34,35]. The most common genotypes in the country are genotypes 1 and 2. Genotype 3 is very uncommon, which allows for the simplification of HCV treatment by eliminating the need for genotyping before treatment [36,37].
Unsafe injection, hemodialysis, and blood transfusion of unscreened blood products are risk factors for HCV infection globally [38]. In the Philippines, metropolitan city surveillance data from 2002 to 2007 showed an 83% (793/960) anti-HCV positivity rate among injecting drug users [39]. On the other hand, the prevalence among hemodialysis patients ranges from 4.5% to 36% in the country [40,41]. Prison inmates have a higher prevalence of HCV infection due to unsafe lifestyles (e.g., IVDU, promiscuity, unhygienic tattooing) prior to incarceration that continues while in prison due to psychosocial problems [42]. A local study in 1996 found 5.0% HCV seroprevalence among 502 prisoners in a medium-security urban prison, with a higher prevalence among MSMs (12.9%) and parenteral drug users (35.0%) [43].
The Hepatology Society of the Philippines (HSP) recommends HCV screening for at-risk populations which include patients with a history of blood transfusion prior to 1995, patients on maintenance hemodialysis, history of injection drug use, incarceration, unsafe sexual practices, and history of sharps injury [44]. Screening is done through anti-HCV antibodies and confirmed through serum HCV RNA PCR [44]. Direct-acting antivirals (DAAs) were recommended as first-line agents for hepatitis C patients in the latest local guidelines [45,46]. The interferon-free treatment approach concurs with management updates from various international liver societies [29,47,48]. Sofosbuvir, in combination with Daclatasvir or Velpatasvir, was the recommended antiviral in the country [45]. Similar to HBV, the screening, diagnosis, and treatment of HCV have largely been concentrated in the private sector, and public health programs have been limited to HIV-coinfected HCV patients [46].
3.2Viral causes of acute hepatitis (Hepatitis a and hepatitis E)Hepatitis A and Hepatitis E viruses cause acute viral hepatitis and are transmitted via the fecal-oral route [49]. The estimated annual hepatitis A incidence rate in the Philippines is 0.4–0.6 cases per 100,000 population [50]. There are no reports of HEV cases in the community, but there is evidence of HEV circulation in the country. HEV RNA was detected in rivers around Manila and HEV IgG was found in household-raised pigs in the Northern Philippines [51,52]
4Non-alcoholic fatty liver disease4.1Prevalence, morbidity, and mortalityThe global prevalence of NAFLD is estimated to be 25.24% [53]. In Asia, NAFLD prevalence ranges from 23.3 to 31.9%, with the incidence of advanced liver fibrosis at 3.7% of NAFLD patients [53,54]. There is no national prevalence data available yet for the country. A study done by De Lusong et al. in 2004 showed the prevalence of NAFLD in a tertiary public hospital in Manila to be 12.2% [55]. Wong et al., in 2018, reported that the prevalence of NAFLD in the country ranges from 10 to 19.9% [54].
Among children and adolescents, the pooled global prevalence rate is likely to be between 5% to 10% [56]. The Study of Child and Adolescent Liver Epidemiology (SCALE) done in San Diego, California, in 2006 showed that the prevalence rate is higher among boys at 11.1% compared with girls at 7.9%. A global meta-analysis of pooled studies by Anderson et al. in 2015 supported this finding revealing a prevalence rate of 9% in boys and 6.3% in girls [57]. Currently, there are no available national prevalence rates for children with NAFLD in the country.
Morbidity and mortality among patients with NAFLD are large because of CVD, followed by extrahepatic cancers and liver-related complications [58]. In the United States, there were a total of 353,234 NAFLD-related deaths from 2007 to 2016 [59]. Cause-specific deaths identified included cardiovascular deaths, cirrhosis, hepatocellular carcinoma, non-liver cancer and complications of diabetes mellitus.
Despite the projected rise in the number of persons affected by NAFLD in the next few years, given the ongoing epidemic of diabetes and obesity, as well as the unique susceptibility of Asian people due to body composition differences in fat and muscle as well as genetic susceptibility, the lack of data in our country undermines its true public health impact and economic burden to Filipinos.
4.2Risk factorsA few studies have looked into the risk factors associated with NAFLD in the Philippines. De Lusong et al. in 2008 enumerated a number of risk factors - female sex, diabetes, and increased BMI (overweight and obesity) were the main risk factors noted to have a significant relationship with NAFLD [55]. Another study also showed the association between obesity and metabolic syndrome with NAFLD [60].
It is widely accepted that obesity/overweight is the most significant risk factor for NAFLD [54]. The pooled overall obesity prevalence among NAFLD patients was 51% globally and the regional prevalence for Asia was 64% [53]. One of the differences in the phenotype of NAFLD in Asia-Pacific versus Western countries is the occurrence of "lean NAFLD." In the study by Tan et al. in 2022, looking at the prevalence of NAFLD in Asia, 21.6% of the NAFLD patients were non-obese. The prevalence of lean NAFLD in the country was evaluated in 2 studies and ranged from 12% to 31% [55,60]. It is important for studies on NAFLD to be conducted in the country to include patients with lean NAFLD.
Insulin resistance is another important risk factor associated with NAFLD. Diabetes has a pooled overall prevalence among NAFLD patients of 22.5% globally and 8.7% regionally [53]. Forty-seven to 69% of NAFLD patients in the Philippines have diabetes [55,60]. In a study among diabetic patients at a tertiary center in the country, there was a significant association between fatty liver and obese diabetic patients [61]. Hyperlipidemia is also a known risk factor among Filipinos [55,60].
Family and twin studies on NAFLD suggest a heritable component of NAFLD. The complex interplay of genetic and environmental factors likely account for the heterogeneity of the clinical presentation of NAFLD patients. The best-studied genetic variants of NAFLD include PNPLA3, TMF6SF2, GCKR, MBOAT7/TMC4, and HSD17B13 [62]. In a study of 32 biopsy-proven Filipino NAFLD patients, Baclig and co-workers found a significant difference in the distribution of PNPLA3 genotypes between NAFLD patients and normal controls. They concluded that genetic variation in PNPLA3 rs738409 C>G seems to be associated with NAFLD among Filipinos and that validation in a larger sample size is needed [63].
4.3Screening and awarenessThere is no recommended screening test or algorithm at present for NAFLD. The American, European, and Asian liver societies do not recommend screening for NAFLD in the general population [54,64,65]. However, recommendations on screening in high-risk populations (type 2 diabetes mellitus, overweight/obese, metabolic syndrome) do exist but differ among societies. Both European and Asian liver societies recommend screening in these populations, while the American liver society does not endorse screening in high-risk groups [54,64–66] This has also been endorsed by endocrinologists [67]. Ultrasonography is an acceptable imaging modality for screening for steatosis in high-risk patients. There are currently no local guidelines on screening for NAFLD.
There is generally a low level of awareness about NAFLD and its risk factors and complications among the general public. This was shown in a large Asian study that included the Philippines [68]. This lack of awareness can even be extended to health care providers, especially primary care providers who are more likely to encounter patients with NAFLD in everyday practice [69]. This general lack of awareness is confounded by the lack of national guidelines as well as the lack of government-initiated and funded strategies on NAFLD [70]. It is important for countries like the Philippines which is witnessing the diabetes and obesity epidemic to come up with a comprehensive public health strategy for NAFLD [71].
4.4Diagnosis and risk stratificationThe AASLD diagnosis of NAFLD requires that (a) there is hepatic steatosis by imaging or histology, (b) there is no significant alcohol consumption, (c) there are no competing etiologies for hepatic steatosis, and (d) there are no coexisting causes of chronic liver disease [64].
Once NAFLD is diagnosed, it is important to determine the extent of liver fibrosis as this has been shown to be an important predictor of adverse outcomes in NAFLD [72]. This can help triage the patient according to the risk of adverse outcomes - primary care for those with mild or no fibrosis or specialist care for those with significant fibrosis [66]. Determination of fibrosis can be easily performed by various non-invasive tests, including biochemical biomarkers and biomarker panels such as AST to ALT ratio, AST to Platelet Ratio Index (APRI), NAFLD Fibrosis Score, Fibrosis-4 (FIB-4) Index, and Enhanced Liver Fibrosis (ELF) test as well as imaging tests such as shear wave elastography and vibration-controlled transient elastography [64]. It is fortunate that most of these non-invasive tests are readily available in the Philippines, although the imaging tests are usually concentrated in large urban centers. However, there are no data on the distribution of fibrosis stages among NAFLD patients in the Philippines.
4.5Management of NAFLDTreatment of NAFLD consists of treating the liver disease and the metabolic comorbidities associated with it, such as obesity, T2DM, hypertension and dyslipidemia.
Lifestyle management is the cornerstone for NAFLD treatment. Lifestyle modification consisting of diet, exercise, and weight loss have been advocated to treat patients with NAFLD [64]. Combined modalities compared to either modality alone have been found to be more effective in reducing liver fat in almost 50% of cases [73]. In a study done by Vilar-Gomez et al., lifestyle changes incurring weight loss are associated with improvement in histologic features of NASH. The highest rates of resolution of NASH and fibrosis regression occurred in patients with weight loss of more than 10% [74].
There is currently no approved pharmacologic treatment addressing NAFLD. There are however ongoing clinical trials for the treatment of NASH. Certain drugs have been shown to improve NASH such as Vitamin E, thiazolidinediones, peroxisome proliferator-activated receptor alpha and delta agonists, glucagon-like peptide-1 receptor agonists and obeticholic acid. These studies, however, are not robust and studies on the Asian population are scarce and none have included Filipino patients.
5Alcohol-associated liver disease5.1Prevalence, morbidity, and mortalityThe prevalence of alcohol-associated liver disease (ALD) has been relatively stable in the past years ranging from 8.1% to 8.8% [75]. However, alcohol-associated liver cirrhosis has increased from 2.2% to 6.6% from 2001 to 2016, with ALD now considered the top indication for liver transplantation in the United States [76]. Heavy drinkers and alcoholics may progress from fatty liver to alcohol-associated hepatitis to cirrhosis, and it is estimated that 10 to 15 percent of alcoholics will develop cirrhosis [77].
In the Philippines, there is no national prevalence of ALD to date. A study in a tertiary hospital in Baguio City showed that the prevalence of ALD among patients enrolled in their substance abuse-use disorder and treatment unit is as high as 40% [78]. In another study looking at the demographics of ALD in another tertiary hospital in Manila from 2005 to 2006, patients with alcohol-associated liver cirrhosis were predominantly male, with a mean age of 52 years old [79].
Acute alcohol-associated hepatitis and liver cirrhosis are associated with high mortality (which can reach 50% in acute alcohol-associated hepatitis), and the median survival time of patients with advanced alcohol-associated cirrhosis can be as low as 1–2 years [80]. In the Philippines, 21.1 deaths per 100 000 men are attributable to alcohol-associated liver cirrhosis, according to the Global Status Report on Alcohol and Health of the World Health Organization in 2018 [81].
5.2Risk factors of ALDThe development of alcohol-associated liver disease depends on the quantity and duration of an individual's intake of alcohol. The quantity and duration of alcohol are considered to be the highest risk factors for the development of liver disease [82].
According to the Centers for Disease Control, the standard drink contains 0.6 ounces or 14 g of pure alcohol, and this amount is found in 12 ounces of beer with 5% alcohol content, 5 ounces of wine with 12% alcohol content, and 1.5 ounces of 80-proof distilled spirits or liquor such as gin, rum, vodka and whiskey [83].
Excessive drinking includes binge drinking, heavy drinking, and any drinking by pregnant women and/or people less than 21 years of age. Heavy drinking is defined as consuming 15 or more standard drinks per week for men and eight or more standard drinks per week for women. In the country, patients diagnosed with ALD consumed an average of 117 g or about eight drinks a day, and the mean duration of alcohol intake was 24 years [78].
5.3Screening and diagnosis of ALDThere is currently no single laboratory or imaging study used for screening and confirmation of ALD [84]. Furthermore, patients suffering from ALD may be completely asymptomatic and have no clinical signs, thus making the identification and diagnosis of ALD challenging.
The diagnosis of ALD may be made based on clinical and laboratory features alone in a patient with a significant history of alcohol consumption after other etiologies of chronic liver disease (CLD) have been ruled out. Laboratory and imaging findings seen in ALD include abnormal serum transaminases, particularly if the AST to ALT ratio is >2, hepatomegaly, clinical signs of CLD, radiographic evidence of hepatic steatosis or fibrosis or who have had a liver biopsy showing macrovesicular steatosis or cirrhosis [85].
5.4Management of ALDThe cornerstone of ALD management at any stage of the disease process is abstinence from alcohol. Improvement in liver histology can occur as early as two weeks following the discontinuation of alcohol use [86]. Nutritional support is important in patients with alcohol-associated hepatitis and steroids are recommended in those with severe alcohol-associated hepatitis (Maddrey Discriminant Function >32) without ongoing infection, renal failure, or gastrointestinal bleeding. Liver transplantation is the treatment of choice for end-stage liver disease related to alcohol and is increasingly being applied to highly selected patients with severe alcoholic hepatitis. Unfortunately, liver transplantation as a treatment modality is not widely available in the Philippines. Alcohol use disorder (AUD) should be addressed in patients with ALD. Management should be tailored to the severity of AUD and can include brief counseling to referral to an addictions specialist and pharmacologic therapy when necessary. Reference Singal here
6Liver cirrhosis6.1Prevalence, morbidity and mortalityThe absolute number of CLD cases is estimated at 1.5 billion worldwide. The most common causes of CLD are NAFLD (59%), followed by HBV (29%), HCV (9%), and ALD (2%) [87]. The remaining 1% of cases is divided among genetic and autoimmune diseases, including primary biliary cholangitis, primary sclerosing cholangitis, alpha-1- antitrypsin deficiency, Wilson's disease, and autoimmune hepatitis. An important caveat to these data is that alcohol most likely accounts for a larger proportion of liver disease prevalence and mortality but is underreported because of cultural concerns and is often a secondary (and unreported) liver disease etiology that coexists with viral hepatitis or NAFLD [88].
Cirrhosis is a leading cause of mortality and morbidity across the world [89]. It is the 11th leading cause of death and 13th leading cause of morbidity, accounting for 2.4% of deaths and 1.7% of disability-adjusted life years (DALY) worldwide in 2019 [90]. CLD caused 1.315 million deaths in 2019; approximately two-thirds were men and one-third were women [88].
In the Western Pacific region, cirrhosis of the liver accounted for 216,001 mortalities and 6.5 million morbidities in 2019. The Philippines reported 7076 or 1.05% of total deaths attributable to liver cirrhosis, which has an adjusted death rate of 8.65 per 100,000 population [90] and is currently the 19th leading cause of death in the country. The Philippines recorded 328.4 per 100,000 compensated cirrhosis and 41.8 per 100,000 decompensated cirrhosis [87]. It also reported a total of 229,100 morbidities [90].
6.2Risk factors of liver cirrhosisHepatitis B and hepatitis C are important risk factors for liver cirrhosis. The relative risk of progression to cirrhosis in patients infected with HBV increases as HBV DNA values increase [91]. Meanwhile, the global annual risk of developing HCV-related cirrhosis is approximately 2–6% [92]. Studies on the relationship between viral hepatitis and liver cirrhosis in the Philippines are limited. In 1989, Lingao reported a 58.2% prevalence of hepatitis B among 99 liver cirrhosis patients in a tertiary center [93]. Perez and colleagues, on the other hand, reported an 8.8% HCV positivity rate among 57 cirrhosis patients. In this cohort, 5.3% had HBV-HCV coinfection. [94]).
ALD has a major role in the etiology of non-HBV and non-HCV liver cirrhosis. Alcohol accounts for 30% to 50% of cirrhosis-related deaths globally [89]. According to the WHO, in 2010, of the global deaths caused by cirrhosis, 493,300 were attributed to alcohol (47.9% of all cirrhosis deaths).
NAFLD is an important cause of liver cirrhosis. The natural progression of NAFLD into non-alcoholic steatohepatitis and cirrhosis is somewhat slow, with an incidence of 3.1% over a mean follow-up of 7.6 years [95]. The risk of progression depends on the underlying severity of inflammation and fibrosis, in that about 20% of individuals with NASH may progress to cirrhosis over a period of 2 years [96]. Contemporary studies on the clinicodemographic characteristics of patients with cirrhosis are needed for the country, given that it is likely that, similar to the trends worldwide, ALD and NAFLD are becoming important contributors to the morbidity and mortality associated with cirrhosis.
Other associated conditions that are considered possible etiologies of liver cirrhosis include storage diseases such as hemochromatosis and Wilson disease and autoimmune conditions such as primary sclerosing cholangitis, primary biliary cholangitis and autoimmune hepatitis. Other less common etiologies include congestive heart failure and medications.
6.3Screening and diagnosisLiver cirrhosis is usually asymptomatic until decompensation occurs. Patients with compensated cirrhosis can develop clinical signs of decompensation - ascites, hepatic encephalopathy, jaundice, or variceal bleeding - at a rate of 4% to 10% per year [97]. The prognosis changes significantly when decompensation occurs and thus detecting cirrhosis early is an attractive management strategy.
However, there is no existing consensus guideline on the screening of liver cirrhosis in the primary care setting. Although it has been argued that screening for liver cirrhosis among high-risk patients is an accepted step in its management, the lack of an ideal screening test impedes a consensus recommendation [98].
Liver biopsy is currently the gold standard for the diagnosis of cirrhosis which has the added benefit of determining the etiology of cirrhosis and of assessing the histological grade of inflammation and stage of fibrosis to prognosticate the risk of progression to cirrhosis [99]. This method is invasive and carries possible morbidity and a rare but finite mortality risk [100]. It also has the risk of sampling variability error [99]. Currently, there are non-invasive tests being used to determine the fibrosis stage and diagnose cirrhosis. These tests are either blood-based tests (i.e., APRI, FIB-4, ELF test), tests that assess physical properties of liver tissues (e.g., elastography for liver stiffness), and imaging tests such as ultrasound, CT scan, or MRI [101].
6.4TreatmentCirrhosis is a progressive liver disease and the damage can be irreversible. The goal of treatment is to delay or stop further damage to the liver. Elimination of the trigger/s that led to cirrhosis is likely to slow down the progression of fibrosis and reduce the incidence of complications such as variceal bleeding, ascites, encephalopathy, or HCC. There is evidence that treatment of the underlying cause can reverse cirrhosis, although in some cases, sampling variability cannot be excluded [99].
In alcoholic liver cirrhosis, abstinence, particularly early abstinence defined as stopping to drink one month into the diagnosis of liver cirrhosis, has been shown to improve survival [102].
Without antiviral therapies, 6–20% of chronic hepatitis B patients will develop cirrhosis over five years. The cumulative incidence of HBV-related cirrhosis, disease progression, and prognosis are closely associated with serum HBV DNA levels. Antiviral therapy in HBV-related cirrhosis has been documented to decrease disease progression to decompensation and HCC [103].
Oral therapies for hepatitis C have been very successful in clearing the hepatitis C virus [104]. It has been well-documented that viral clearance with antiviral therapy results in reduced morbidity and mortality from chronic liver disease [105].
The ultimate therapy for cirrhosis, especially for those with end-stage liver disease, is liver transplantation [99]. In the Philippines, only a few institutions offer liver transplantation. Most private hospitals are offering liver transplantation to Filipino patients with end-stage liver disease. A total of 59 liver transplants have been performed in the country from 1988 to 2019, of which 21 patients were still alive in 2019, with an approximate survival rate of 56% [106]. There are several limitations to liver transplantation in the country. One is the high cost associated with the procedure compared to nearby Asian countries [106]. Another is the lack of awareness among patients and even healthcare workers about liver transplantation and organ donation. Because of this lack of awareness, organ donation rates have been very poor in the country. Many patients thus seek liver transplantation outside the country. A concerted effort to bring together all the important stakeholders from both the public and private sectors is urgently needed to address organ donation and liver transplantation, which is a huge unmet need in the care of patients with liver disease in the country.
7Hepatocellular carcinoma7.1Prevalence, morbidity and mortalityWorldwide, liver cancer is the sixth most diagnosed cancer and the second leading cause of cancer-related mortality, according to the Global Cancer Statistics 2020 [107]. Hepatocellular carcinoma (HCC) comprises about 75–85% of cases of liver cancer, with the rest due to intrahepatic cholangiocarcinoma and other rare subtypes. Incidence rates of HCC have been decreasing in some high-prevalence areas but increasing in many low-prevalence areas. In the interval between 1978 and 2012, HCC incidence declined in many Asian countries and Italy owing to effective vaccination programs for hepatitis B and antiviral therapies for hepatitis B and C but increased in India, the Americas, Oceania, and most European countries [107,108].
In the Philippines, liver cancer is the fourth most common cancer type after breast, lung and colon cancer and the second most common cause of cancer deaths [90]. The Philippines logged a total of 10,594 new cases and 9953 deaths in 2020, with a 5-year prevalence in all ages at 10.01 per 100,000 population [107]. The calculated incidence rate for liver cancer in the country is 11.4 and the mortality rate of 10.8 per 100,000. The most common cause of HCC in the country is still chronic hepatitis B infection [109].
7.2Risk factors for the development of HCCHepatitis B and C infections have been established as risk factors for the development of HCC due to direct and indirect mutations in hepatocytes. The lifetime risk of developing HCC among patients with chronic hepatitis B infection ranges from 10 to 25%, depending on whether the patient has active HBV infection and/or cirrhosis [108]. In the Philippines, hepatitis B infection was the most common risk factor for HCC infection in a cohort of 222 patients from a tertiary care center [110]. This was similar to a retrospective cohort study at another tertiary care center, with 56% of 346 HCC patients having chronic hepatitis B [111]. Chronic hepatitis C infection, on the other hand, increases the risk for hepatocellular carcinoma by 10 to 20 fold and the annual incidence of HCC ranges from 0.5 to 10% [108]. In the Philippines, chronic hepatitis C accounted for 2–6% of all HCC patients at two tertiary care centers [110,111].
Alcohol use increases the relative risk of hepatocellular carcinoma by 3- to 10-fold [112]. A Philippine study found two-thirds of HCC patients had a history of drinking alcohol [110].
Metabolic syndrome increases the risk of HCC by 81% [113] and the treatment of metabolic syndrome ameliorates the risk by 37–42% [114]. Diabetes mellitus alone has been associated with a 2–3 fold increased risk of HCC; however, it is unclear what specific severity of sugar control is associated with the increased risk [115]. Between 70 and 80% of NAFLD-related HCCs develop in cirrhotic livers [108]. The presence of concomitant comorbidities such as diabetes, obesity, dyslipidemia, and hypertension increases the risk of progression of NAFLD to HCC [116]. The contribution of metabolic syndrome and NAFLD to HCC development among Filipinos has not been systematically evaluated, although anecdotal reports suggest an increasing prevalence of NAFLD-related HCC in recent years. In a cohort of 180 patients with HCC in a large tertiary care center in Manila, the prevalence of diabetes mellitus and prediabetes was 53% which is higher than in other reported series [117]. Those HCC patients with diabetes mellitus and prediabetes were less likely to have viral hepatitis and more likely to have cryptogenic cirrhosis compared with those who did not have diabetes mellitus and prediabetes.
Aflatoxins, mycotoxins produced by fungi of Aspergillus species, have been shown to have additive or synergistic effects with chronic hepatitis B or alcohol in HCC development [118]. A local case-control study of 90 HCC patients determined the relationship between aflatoxin and alcohol consumption in the development of HCC [119]. They found that heavy aflatoxin exposure with heavy alcohol consumption conferred a two-fold increased risk of HCC development compared with heavy aflatoxin exposure and light alcohol consumption. Current regulatory policy in the Philippines has limited the aflatoxin levels to 20 ug/kg per product for human food consumption [120], leading to the decline of the contribution of aflatoxin exposure to the development of HCC.
The 2014 US Surgeon General's report found that current cigarette smoking was associated with a 70% increased risk of liver cancer, while former smoking was associated with a 40% increased risk [121]. The latest Global Adult Tobacco Survey showed that 22.7% of adult Filipinos (>15 years old) are current tobacco smokers, while 4.9% of adult Filipinos are former smokers [122]. Increased efforts to curb smoking in general and in those with other risk factors for HCC should be undertaken to decrease the morbidity and mortality associated with HCC.
7.3Screening and diagnosis of HCCThe Philippine Clinical Practice Guidelines (CPG) for the Diagnosis and Management of Hepatocellular Carcinoma in 2021 recommended the semi-annual screening of patients at high risk of developing HCC [123]. Those considered to be high risk are those with liver cirrhosis of any etiology and those with hepatitis B who have a family history of hepatocellular carcinoma or who are males aged 40 years and above or females aged 50 years and above. The Philippine CPG suggested the use of ultrasound with or without alpha-fetoprotein as a screening tool.
The data supporting these recommendations, however, were scarce as there was only one randomized control trial that evaluated screening with ultrasound (US) plus AFP every six months versus no screening in patients with chronic hepatitis B. Screening with US plus AFP was associated with a 40% lower risk of HCC-related mortality compared to no screening. The survival advantage was attributed to the significantly higher proportion of HCC detected at an early stage in the screened group [124].
The diagnosis of HCC can be made histologically or noninvasively using contrast-enhanced imaging techniques. The finding of intense contrast uptake on the arterial phase followed by contrast washout on the venous phases on CT scan or MRI in patients with cirrhosis is diagnostic of HCC and biopsy confirmation is not necessary for that situation. (EASL guidelines) The Philippine CPG recommends the use of a multiphasic contrast-enhanced CT scan or MRI in the diagnosis of HCC [123]. The CPG recommends performing a core needle biopsy over a fine needle biopsy to diagnose HCC in those who do not fulfill the imaging criteria for HCC. Both CT scan and MRI scans are available in the country, although CT scan is more widely available compared to MRI and is preferred by most patients because it is easier to do than an MRI.
7.4Treatment of HCCHepatocellular carcinoma staging is used to predict the prognosis of the disease. Among the available staging systems, the Barcelona Clinic Liver Cancer or BCLC staging system is considered the most comprehensive, taking all clinically essential parameters into consideration and providing prognostic guidance to available therapeutic choices [125,126]. This staging system has been adopted by the Philippine CPG [123]. The majority of the HCC cases that are diagnosed in the Philippines are in the late stages and thus not amenable to surgical treatment. In a fairly large cohort from a tertiary care center, 87% of all HCC cases were in the intermediate to advanced stages. [111] Most of the cases (50%) only received the best supportive care. Median survival for HCC for this cohort was 13.7 months but was significantly longer among those who had the early-stage disease (BCLC A, 68.2 months; BCLC B, 26.7 months; BCLC C, 9.9 months; BCLC D, one month). Identifying patients at risk for HCC and enrolling them into surveillance programs is important to diagnose patients at earlier stages and thus improve outcomes. The treatment of HCC in the country has improved in recent years with the adoption of multidisciplinary treatment planning and the availability of the entire armamentarium of therapeutic modalities for HCC. The Philippine CPG also set forth recommendations for the treatment of HCC in the various BCLC stages, favoring resection over local ablation in early-stage disease (BCLC A) and locoregional therapy with TACE over selective internal radiation therapy or external radiation therapy for those with intermediate-stage disease (BCLC B). Combined systemic therapy with Atezolizumab and Bevacizumab was favored over Sorafenib in advanced-stage HCC (BCLC C). The main limitation to the treatment of HCC in the country has been its concentration in large tertiary care centers and the high cost associated with the treatments. Very little data is also available about the outcomes of patients treated with the various treatment modalities. There is a need for increased research on the early identification of HCC among high-risk groups and evaluation of the outcomes of various treatment modalities, especially with the release of the national CPG. Efforts to bring treatments within reach of all Filipinos are important.
8ConclusionsIn this review, we presented available data on disease prevalence, risk factors, available diagnostics and therapeutics of important liver diseases in the Philippines (Table 1). Liver diseases are a major public health concern and a significant cause of morbidity and mortality. However, despite its significance, liver diseases have not received much attention in developing countries such as the Philippines, where the disease burden is high [127,128]. The lack of public and political awareness of the magnitude of the problem results in large part from the paucity of research on liver diseases. More research on prevalence, risk factors, and morbidity and mortality rates in the population are needed to shed light on the burden of liver disease in the country and thus formulate policies to address the problem.
Disease prevalence, risk factors, and available methods for diagnosis and treatment of liver diseases in the Philippines.
| Hepatitis B | Hepatitis C | Non-Alcoholic Fatty Liver Disease | Alcoholic-associated Liver Disease | Liver Cirrhosis | Hepatocellular carcinoma | |
|---|---|---|---|---|---|---|
| Disease prevalence | 10.4% | 0.4% | 10.0–19.9% | No national prevalence data40% of substance abuse-use disorder and treatment unit clients | 328.4 pe 100,000 - compensated cirrhosis41.8 per 100,000 – decompensated cirrhosis | 10.01 per 100,000 |
| Risk Factors* | Being born to infected mother (Main risk factor); Other risk factors: injection drug use;needle stick injury;multiple sexual partners; being an inmate at acorrectionalfacility inmates | Unsafe injection practices;hemodialysis;blood transfusion of unscreened blood products;being an inmate at a correctional facility | Diabetes mellitusOverweight/ObesityInsulin ResistanceHyperlipidemiaMetabolic syndrome | Excessive alcohol consumption (≥15 standard drinks of alcohol/week in men, ≥ 8 standard drinks of alcohol/week in women) | Hepatitis BHepatitis CALDNAFLDLess common: autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, Wilson disease, iron overload | Hepatitis BHepatitis CALDNAFLDMetabolic syndromeCirrhosis from any etiologyCigarette smoking |
| Available methods for diagnosis | HBsAgHBeAgAntiHBe, HBV DNA quantification | Anti-HCVHCV RNA quantification | Imaging test (ultrasound, CT scan, MRI)Controlled attenuation parameter (transient elastography or FibroScan®) | Serum transaminases,Imaging test (ultrasound) | Biomarker panels - APRI, FIB-4Liver stiffness measurement (shear wave elastography, transient elastography or FibroScan®Liver biopsy | Multiphasic contrast-enhanced CT scan/MRILiver biopsy |
| Available treatment | Tenofovir Disoproxil Fumarate (TDF)Tenofovir Alafenamide (TAF)Entecavir (ETV) | Sofosbuvir in combination with Daclatasvir or Velpatasvir | Lifestyle management | Alcohol abstinence | Treatment of underlying chronic liver disease causeLiver transplantation | Surgical resectionLiver transplantationLocoregional therapy (RFA, MWA, TACE, SIRT)Radiation therapySystemic therapy (Atezolizumab with Bevacizumab, Lenvatinib, Sorafenib) |
Efficient monitoring and surveillance systems are needed for liver research and disease burden assessment in the country. Comprehensive, high-quality disease registries and biobanking facilities can improve both research output and health outcomes [129–131]. The government can spearhead efforts on building and integrating these facilities, ensuring the involvement of both the public and private sectors. Recent government initiatives to advance research and development efforts in liver disease include collaborations with the Italian Liver Foundation and the University of the Philippines Manila to develop a liver research agenda, promote basic and translational research in liver diseases, to provide a platform for collaborative research, and to develop comprehensive research programs in liver diseases through the establishment of the Philippine Liver Network [132].
Government should enact policies to promote a healthy lifestyle. Laws that discourage excessive alcohol and high sugar intake must be enacted and properly implemented. The Philippines passed several laws that can help curb lifestyle diseases. In 2019, the Philippines approved an increased excise tax on alcohol products [133]. Taxes on sweetened beverage drinks were instituted in 2017 to curb sugar consumption in the country [134].
Lastly, equitable access to healthcare needs to be ensured. Geographic, cultural, social, and economic barriers to healthcare need to be properly identified and actively addressed. Access to screening and treatment can be cost-prohibitive, and thus they should be included in the government health insurance system. These efforts should be strengthened by the passage of the Universal Health Care Act into law in 2019, which seeks to ensure that all Filipinos are guaranteed equitable access to quality and affordable healthcare goods and services and protected against financial risk.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionsJPO and EDO contributed to the conception and design of the manuscript. EDO and KJM contributed to the initial drafting of the article. EDO, KJM, and JPO edited and approved the final version to be submitted.