Psychosocial stressors related to the coronavirus-19 (COVID-19) pandemic increased alcohol consumption. The effect on patients with alcohol-related liver diseases remains unclear.
Materials and MethodsHospitalizations at a tertiary care center due to alcohol-related liver disease from March 1 through August 31 in 2019 (pre-pandemic cohort) and 2020 (pandemic cohort) were reviewed retrospectively. Differences in patient demographics, disease features, and outcomes were estimated in patients with alcoholic hepatitis utilizing T-tests, Mann-Whitney tests, Chi-square and Fisher Exact Tests and Anova models and logistic regression models in patients with alcoholic cirrhosis.
Results146 patients with alcoholic hepatitis and 305 patients with alcoholic cirrhosis were admitted during the pandemic compared to 75 and 396 in the pre-pandemic cohort. Despite similar median Maddrey Scores (41.20 vs. 37.45, p=0.57), patients were 25% less likely to receive steroids during the pandemic. Patients with alcoholic hepatitis admitted during the pandemic were more likely to have hepatic encephalopathy (0.13; 95% CI:0.01, 0.25), variceal hemorrhage (0.14; 95% CI:0.04, 0.25), require oxygen (0.11; 95% CI:0.01, 0.21), vasopressors (OR:3.49; 95% CI:1.27, 12.01) and hemodialysis (OR:3.70; 95% CI:1.22, 15.13). On average, patients with alcoholic cirrhosis had MELD-Na scores 3.77 points higher (95% CI:1.05, 13.46) as compared to the pre-pandemic and had higher odds of experiencing hepatic encephalopathy (OR:1.34; 95% CI:1.04, 1.73), spontaneous bacterial peritonitis (OR:1.88; 95% CI:1.03, 3.43), ascites (OR:1.40, 95% CI:1.10, 1.79), vasopressors (OR:1.68, 95% CI:1.14, 2.46) or inpatient mortality (OR:2.00, 95% CI:1.33, 2.99) than the pre-pandemic.
ConclusionsPatients with alcohol-related liver disease experienced worse outcomes during the pandemic.
Within months of the first documented case of the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), rapid transmission resulted in millions of cases worldwide [1,2]. In order to reduce transmission of SARS-CoV-2, many countries implemented a societal lockdown, which closed non-essential businesses and transitioned essential activities to home if possible [3,4]. Countries that were successful at social distancing were able to significantly decrease the number of coronavirus-19 (COVID-19) cases; however, this resulted in other complications including financial and economic distress [5]. Perhaps one of the most understated and poorly understood complications of societal lockdown was the psychological distress, specifically in patients with alcohol use disorder [6,7].
Alcohol use disorder affects approximately 6% of adults in the United States and is typically linked to other psychiatric diagnoses, including depression and anxiety [8,9]. Relapse and/or escalating alcohol use are associated with social isolation, stressors including job loss, financial insecurity, and lack of support by medical providers and rehabilitation groups [10,11]. Previous studies have reported an increased incidence of alcohol-related liver disease as a result of heightened alcohol consumption during the pandemic, but the subsequent effect on disease severity and patient outcomes remains unclear based on current literature [12–14].
In this study, we aim to determine the frequency of admissions, disease severity and outcomes of alcohol-related liver disease during the COVID-19 pandemic compared to the pre-pandemic population.
2Materials and Methods2.1Data source and study designThis was an observational cohort study using data from patients hospitalized at The Ohio State University Wexner Medical Center and The Ohio State University East Hospital. The two cohort delineations include pre-pandemic without lockdown restrictions (March 1, 2019, through August 31, 2019) and pandemic with lockdown restrictions (March 1, 2020, through August 31, 2020).
2.2Data collection and quality assuranceDe-identified data were collected by clinicians and directly entered into an electronic data collection form. Missing, inconsistent or conflicting data was reviewed by a transplant hepatologist for completion and accuracy.
2.3Study sampleAdult patients with a hospitalization related to alcoholic cirrhosis or alcoholic hepatitis were reviewed for inclusion in this study. Alcoholic cirrhosis was defined as the presence of cirrhosis on imaging or biopsy with a history of alcohol use and lack of other known causes of cirrhosis. Alcoholic hepatitis was defined by The Nation Institute on Alcohol Abuse and Alcoholism (NIAAA) with a history of heavy alcohol use prior to the development of jaundice (total bilirubin at least 3mg/dL), elevated transaminases and no other known cause of acute hepatitis. Each hospitalization during the studied time was reviewed, noting that patients could be admitted multiple times during the studied time. Hospital admissions were excluded if the patient received a planned intervention or surgery unrelated to liver disease. Patients with prior liver transplantation were also excluded.
2.4Outcomes of interestThe primary study outcome was to determine the frequency and type of hepatic decompensation, disease severity, and outcomes in patient with alcohol-related liver disease during the pandemic compared to pre-pandemic. This study had multiple secondary outcomes including a comparison in the number of hospitalization and alcohol use habits in the pandemic cohort compared to the pre-pandemic cohort.
2.5Variables and definitionsDemographics, marital and employment status, disease severity [Model for End Stage Liver Disease Score-Sodium (MELD-Na)] in patients with alcoholic cirrhosis and Maddrey's Discriminant Factor (DF) in patients with alcoholic hepatitis, features of hepatic decompensation (prior, new or worsening ascites, anasarca, hepatic encephalopathy, hepatorenal syndrome and/or gastrointestinal bleeding), interventions (paracentesis, diuresis and endoscopy), presence of other infection, critical care needs (increased oxygen requirements, intubation, vasopressor support, initiation of renal replacement therapy) and outcomes were recorded for each patient as previously described. COVID-19 infection and treatment utilized was recorded for each patient in the pandemic cohort.
The patient's alcohol consumption was assessed by the physician, nursing staff, or social worker during the admission and was self-reported by the patient as abstinent, active but unchanged alcohol intake, or increased alcohol consumption.
2.6Statistical analysisPatient demographics were compared using Chi-square tests, Fisher's exact tests, or two sample t-tests. For the analysis of patients admitted with alcoholic cirrhosis, the outcomes were adjusted for employment status, given statistically significant imbalance between the cohort during pandemic and pre-pandemic cohort. We include multiple admissions for patients with alcoholic cirrhosis. When comparing clinical outcomes among patients with alcoholic hepatitis in the pandemic cohort compared to the pre-pandemic cohort, few patients had multiple admissions; therefore, only the index hospitalization was included in the analysis.
2.7Ethical statementInstitutional Review Board approval (Study Number: 2020H0430) was obtained prior to data collection for this retrospective review.
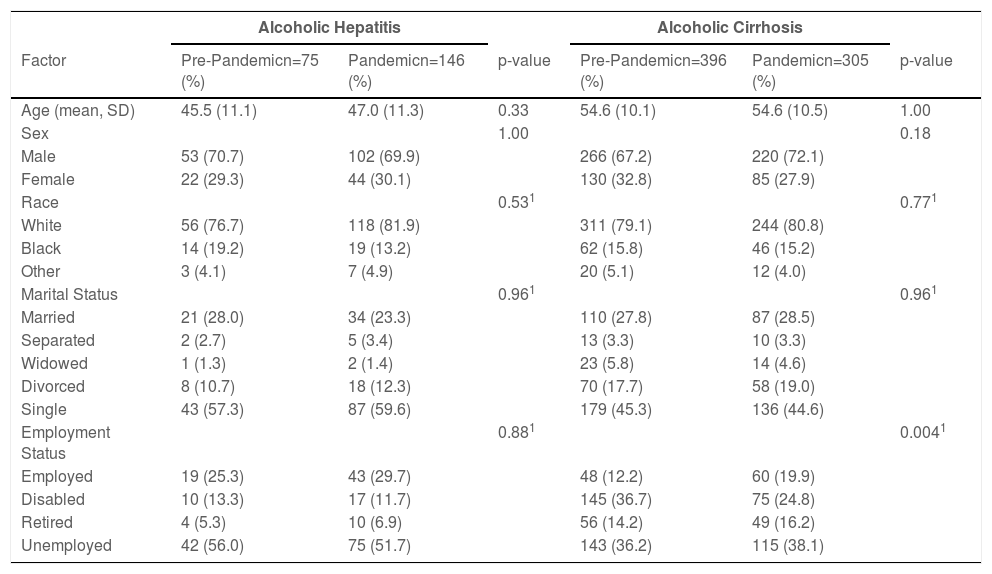
3Results3.1Alcoholic hepatitis admissions during the pandemic compared to pre-pandemicPatient demographics: A total of 146 patients with alcoholic hepatitis hospitalized during the pandemic were compared to 75 patients hospitalized pre-pandemic. Patient demographics were similar between the cohorts, with most patients being middle-aged, Caucasian, male, single and unemployed (Table 1).
Alcoholic hepatitis and alcoholic cirrhosis patient demographics.
1: Fisher's Exact Test
Abbreviations: Standard Deviation-SD
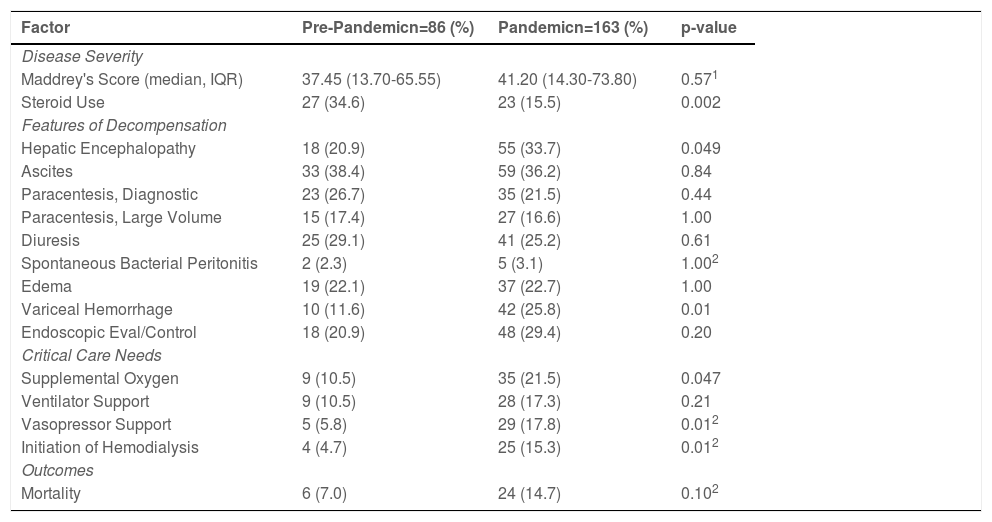
Disease severity: The median DF was similar between the pandemic cohort and the pre-pandemic cohort [41.20 (IQR:14.30-73.80) vs. 37.45 (IQR:13.70-65.55), p=0.57]. Despite similar disease severity, patients admitted during the pandemic were 19% less likely to receive steroids (15.5% vs. 34.6%; p=0.002) (Table 2).
Descriptive statistics of clinical outcomes in patients with alcoholic hepatitis during the pandemic and pre-pandemic.
1: Man-Whitney U-Test, 2: Fisher's Test
Abbreviations: Interquartile Range-IQR
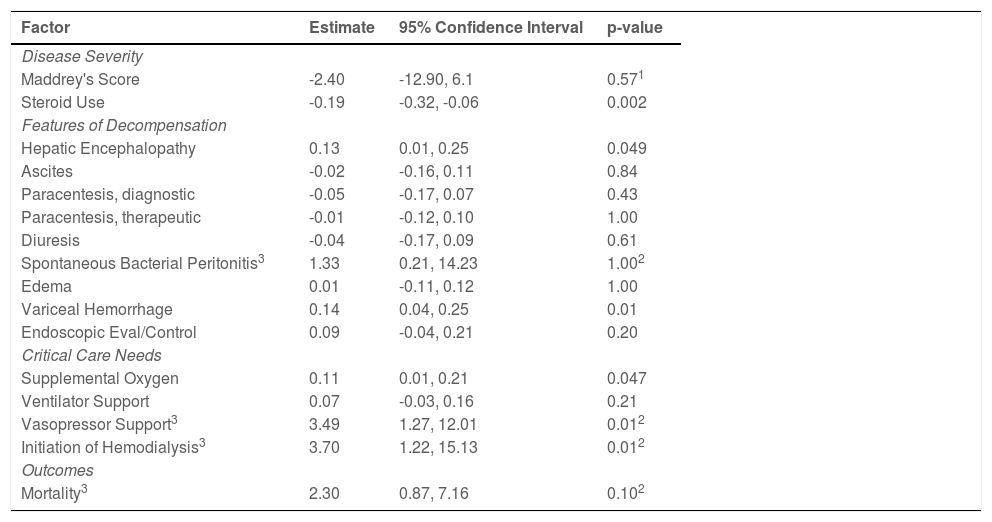
Features of hepatic decompensation: Patients with alcoholic hepatitis admitted during the pandemic were 13% more likely to be admitted with hepatic encephalopathy (33.7% vs. 20.9%, 95% CI:0.01, 0.25, p=0.049) and 14% more likely to have variceal hemorrhage (25.8% vs 11.6%, 95% CI:0.04, 0.25, p=0.01) compared to a patient admitted prior to the pandemic (Table 3).
Clinical outcomes in patients with alcoholic hepatitis admitted during the COVID-19 pandemic compared to pre-pandemic.
1: Man-Whitney U-Test, 2: Fisher's Test, 3: estimates and confidence intervals are odds ratios.
Critical care needs: Patients with alcoholic hepatitis admitted during the pandemic were 11% more likely to be treated with supplemental oxygen (21.5% vs. 10.5%; 95% CI:0.01, 0.21, p=0.047). Compared to the pre-pandemic cohort, patients during the pandemic had higher odds of requiring vasopressor support (OR:3.49; 95% CI:1.27, 12.01, p=0.01) and initiation on dialysis (OR: 3.70; 95% CI:1.22, 15.13, p=0.01) (Table 3).
Mortality: Inpatient mortality during the pandemic compared to the pre-pandemic cohort was not significantly different (p=0.10) (Table 2).
3.2Alcoholic cirrhosis admissions during the pandemic compared to pre-pandemicPatient demographics: A total of 305 patients with alcoholic cirrhosis required hospitalization during the pandemic compared to 396 patients admitted in the pre-pandemic cohort. The employment status was significantly different between the cohorts with an increased number of hospitalizations during the pandemic in patients that are employed (19.0% vs 12.2%, p=0.004). Otherwise, demographics were similar between the groups (Table 1).
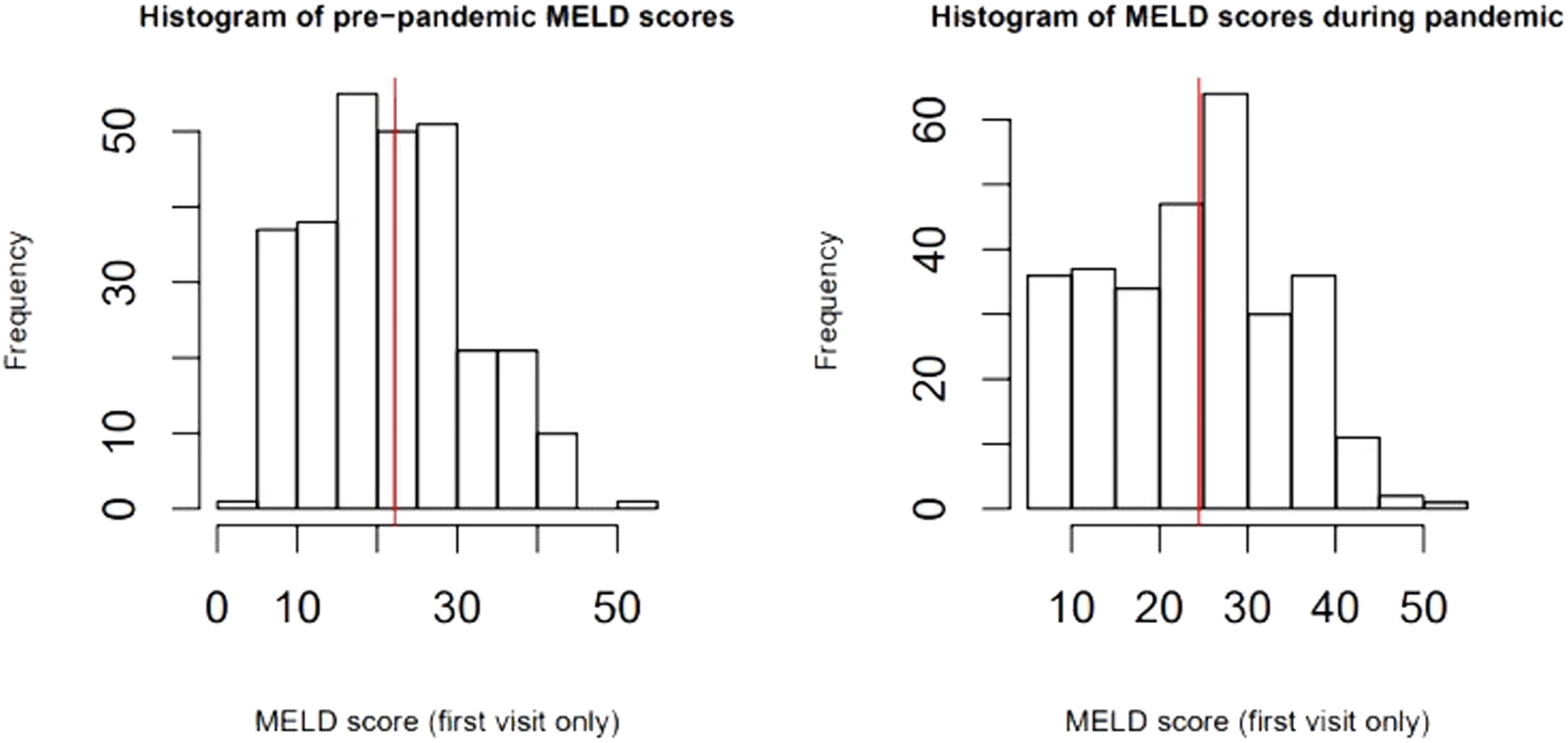
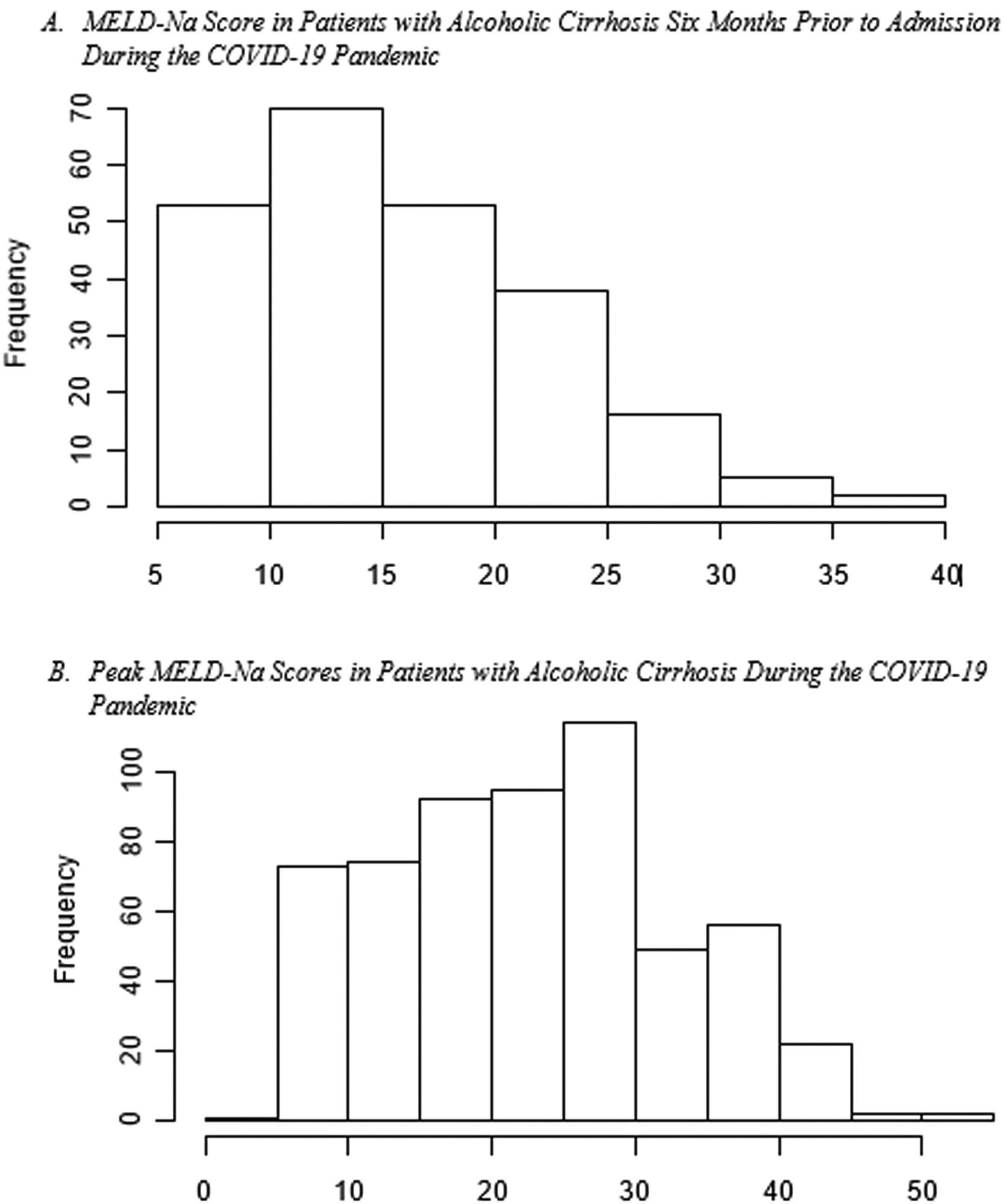
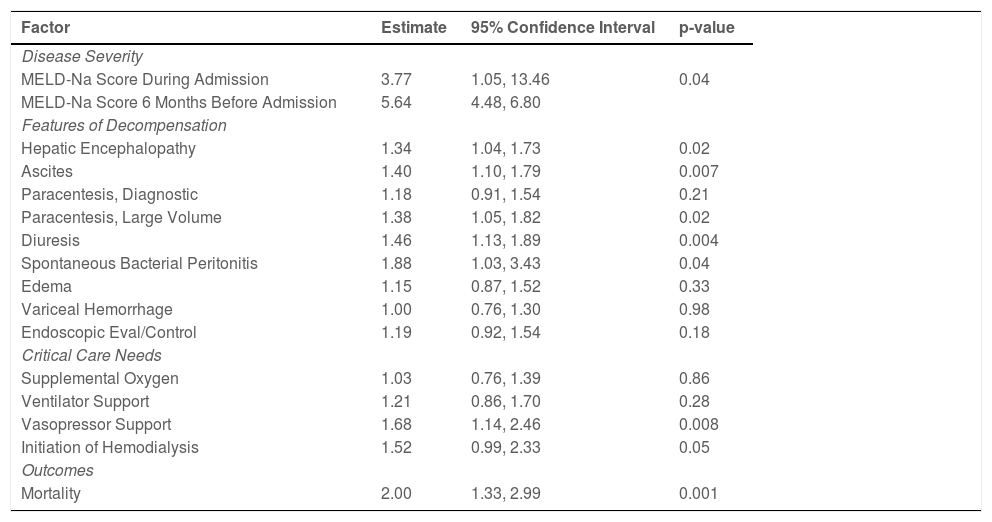
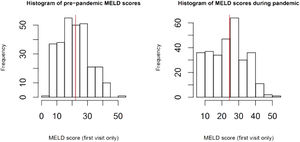
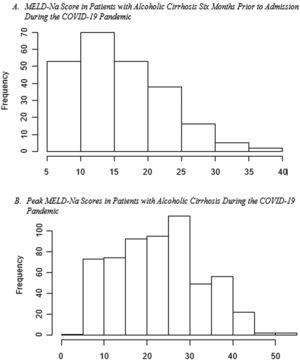
Disease severity: The MELD-Na score during admission was 3.77 (95% CI:1.05, 13.46, p=0.04), points higher during the pandemic compared to the pre-pandemic cohort. When each patient's admission MELD-Na score was compared to their MELD-Na score from 6 months prior to admission, the average MELD-Na score was 5.64 (95% CI:4.48, 6.80) points higher. (Table 4, Fig. 1, Fig. 2).
Multivariable analysis for clinical outcomes in patients with alcoholic cirrhosis admitted during the COVID-19 pandemic compared to pre-pandemic.
Abbreviations: Model for End Stage Liver Disease Sodium-MELD-Na
Features of hepatic decompensation: Patients with alcoholic cirrhosis during the pandemic have a higher odds of being admitted with hepatic encephalopathy (OR:1.34; 95% CI:1.04, 1.73, p=0.02), having spontaneous bacterial peritonitis (OR:1.88; 95% CI:1.03, 3.43, p=0.04) and ascites (OR:1.40; 95% CI:1.10, 1.79, p=0.007) requiring a large volume paracentesis or titration of diuretic therapy compared to the pre-pandemic cohort (Table 4).
Critical care needs and mortality: Patients with alcoholic cirrhosis admitted during the pandemic have higher odds of requiring vasopressor support (OR:1.68; 95% CI:1.14, 2.46, p=0.008) and experiencing inpatient mortality (OR:2.00, 95% CI:1.33, 2.99, p=0.001) compared to the pre-pandemic cohort (Table 4).
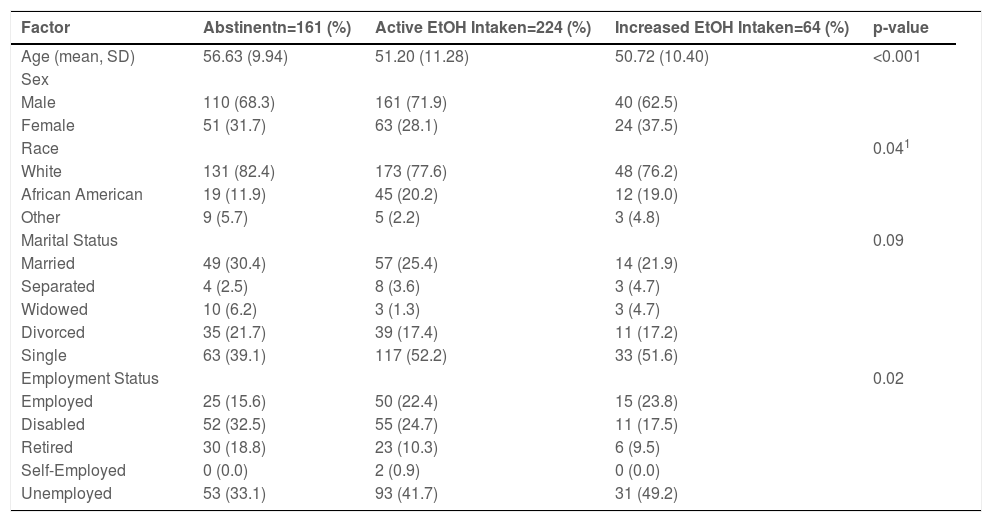
3.3Alcohol intake during the pandemic3.4. Alcohol intake patterns were studied in 449 patients during the pandemic, with the majority (64%) endorsing alcohol intake. While many (88%) of these patients endorse stable alcoholic consumption, 22% of these patients endorsed increased alcohol intake. During the pandemic, increased alcoholic intake and employment status were significantly different between the cohorts (Table 5).
Patient demographics based on alcohol consumption during the COVID-19 pandemic.
1: Generalized Fisher's Test for three group comparison.
Abbreviations: Standard Deviation-SD.
A total of 4 patients (3%) had comorbid alcoholic hepatitis and COVID-19 infection. Mean Maddrey's score was 85.3. While the majority of these patients did not have any features of hepatic decompensation during admission, half of these patients required vasopressor, ventilator and hemodialysis support. Only one of these patients experienced inpatient mortality.
A total of 10 patients (3%) had comorbid alcoholic cirrhosis and COVID-19 infection. Median MELD-Na score of 21 during admission. The majority of these patients experienced at least one feature of hepatic decompensation during admission, with the most common being ascites. One patients required critical care support or experienced mortality during admission.
4DiscussionIn this large cohort of patients with alcohol-related liver disease, we determined that patients hospitalized during COVID-19 had an increased frequency of admissions for alcoholic hepatitis and hepatic decompensation. Patients with alcoholic cirrhosis admitted during the pandemic had higher MELD-Na scores, an increased frequency of critical care needs and inpatient mortality compared to pre-pandemic cohort. This correlated with continued and increased alcohol consumption during the COVID-19 pandemic.
The majority of the patients in this study endorsed continued or increased alcohol consumption during the COVID-19 pandemic, which has been noted in previous studies [15]. While the risk of chronic liver disease due to alcohol consumption is typically higher in single, Caucasian male patients, this study revealed a significant increase in those typically thought to have less risk, including employed patients [16,17]. Similar trends have been reported in previous studies and highlight the effect of social isolation and minimized healthcare resources during the COVID-19 pandemic on patient populations thought to be less susceptible to psychosocial stressors [14,18].
While fewer patients with alcoholic cirrhosis were admitted during the pandemic, these patients had higher MELD-Na scores and were more likely to have a feature of new or worsening hepatic decompensation. While patients with cirrhosis are at an increased risk of hepatic decompensation and acute on chronic liver failure if they contract COVID-19, it is crucial to highlight only a small percentage (2.2%) of the patients with cirrhosis in this study had COVID-19 [19,20]. While the increased frequency of hepatic decompensation noted during the COVID-19 pandemic was likely multifactorial, increased alcohol consumption and infections other than COVID-19 could contributed to these findings [21]. These factors may have been further compounded by reduced outpatient resources, specifically laboratory monitoring, interventions such as a large volume paracentesis, in-person appointments with medical providers where early signs of decompensation could be intervened upon, patient's reluctance to pursue medical care until advanced symptoms, delayed liver transplantation evaluations and a reduced number of transplants performed [22,23]. As expected, this study showed patients with alcoholic cirrhosis admitted during the COVID-19 pandemic had increased critical care needs, specifically vasopressor use which is common in patients admitted with hepatic decompensation and acute on chronic liver failure [24,25].
We recognize this study has limitations. This study compared two groups of patients with alcohol-related liver disease one year apart. While we assume that increased alcohol consumption contributed to higher rates of hepatic decompensation and worse outcomes in the pandemic group, we are unable to account for the natural progression of liver disease, which can result in similar findings. In addition, given the retrospective nature of this study, we were unable to accurately quantify the exact amount of alcohol intake as this was not consistently documented. Furthermore, alcohol intake likely varied during the studied pandemic time as varying degrees of social distancing and societal lockdown were in effect during the six months evaluated in this study. Finally, this study only evaluated patients at a single center and may not be applicable to patient populations in different regions. Despite these limitations, our study also has significant strengths, including the significant number of patients evaluated in this single-center analysis.
5ConclusionsIn summary, patients with alcohol-related liver disease had an increased frequency of hospitalization for alcoholic hepatitis, new or worsening hepatic decompensation, critical care needs and in-hospital mortality during the COVID-19 pandemic, which may be partly explained by increased alcohol use. These findings highlight the importance of outpatient resources, counseling and close physician monitoring in patients with alcohol-related liver disease as a relapse in alcohol consumption leading to hepatic decompensation significantly affect the patient's transplant candidacy and life expectancy.