Steatotic liver disease (SLD), owing to metabolic etiology, is variously defined: nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) and metabolic dysfunction-associated fatty liver disease/metabolic dysfunction-associated SLD (MAFLD/MASLD) [1,2]. NAFLD/NASH and MAFLD/MASLD are strongly associated with accelerated macrovascular disease, whose risk parallels the severity of the stage of liver fibrosis [3,4]. This suggests that appropriate management of SLD owing to metabolic etiology might also prevent such cardiovascular conditions effectively [5].
The retinal neurovascular unit is typically dysfunctional in diabetes and, among patients living with diabetes, clinical manifestations, and epidemiological features of microvascular disease – as opposed to macrovascular damage – vary [6,7]. Therefore, it is important to ascertain whether - among individuals with diabetes – SLD, owing to metabolic etiology and its fibrosis progression, are also a risk factor for microangiopathy, including chronic kidney disease (CKD), retinopathy and neuropathy.
2The paper by Dr. LI, and colleaguesIn this issue of Annals of Hepatology, Doctor Li, and colleagues report on their cross-sectional research conducted among 5413 participants from the NHANES 2005–2008 database [8]. These investigators found retinopathy, categorized into four levels of severity based on retinal imaging, as an independent predictor of significant hepatic fibrosis, assessed with Fibrosis-4 (FIB-4) among individuals with type 2 diabetes [8].
The findings of this study confirm another study recently published in Annals of Hepatology [2]. In this second cross-sectional study, Trivedi et al., globally evaluating 2389 participants from primary care practice, found that the complications of type 2 diabetes (T2D) (namely diabetic nephropathy, retinopathy, or neuropathy) were associated, irrespective of hemoglobin A1c levels, with the stage of liver fibrosis, assessed with FIB-4 evaluated as a continuous and categorical measure using linear and ordinal logistic regression [9].
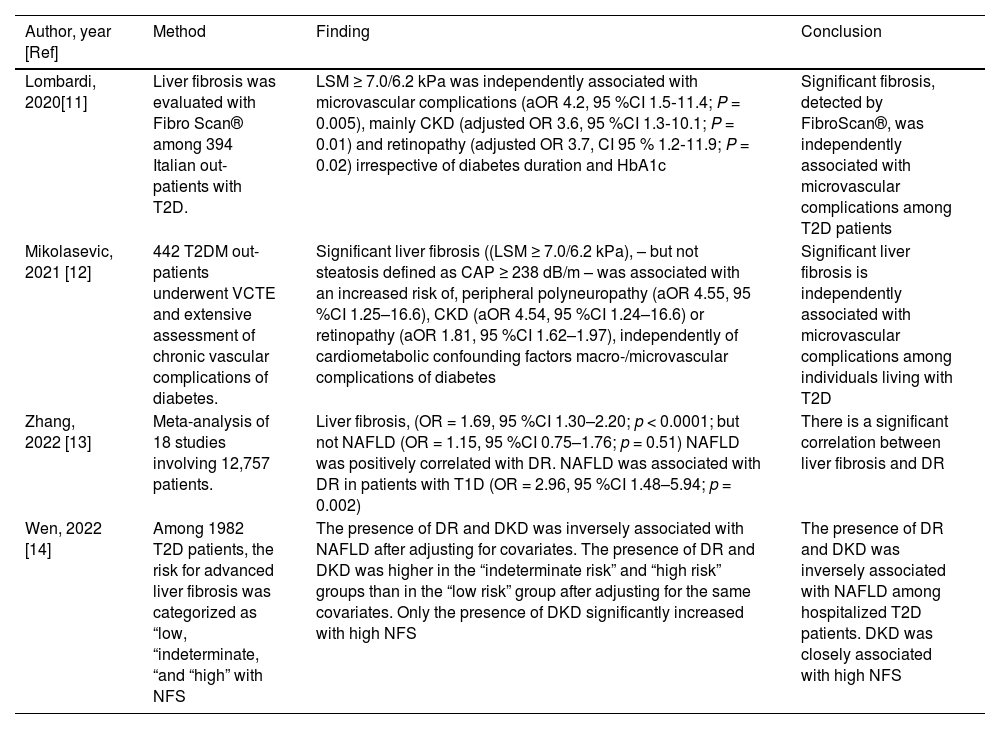
Previous studies have found that liver fibrosis was associated with microangiopathic complications among patients with diabetes. While evidence regarding CKD has extensively been reviewed elsewhere [10], Table 1 specifically focuses on retinopathy (Research strategy: PubMed accessed on Jan 23, 2024, utilizing the following keywords: (liver fibrosis[Title/Abstract]) AND (retinopathy[Title/Abstract]).
Recent studies disclosing an association between liver fibrosis and retinopathy [11–14].
| Author, year [Ref] | Method | Finding | Conclusion |
|---|---|---|---|
| Lombardi, 2020[11] | Liver fibrosis was evaluated with Fibro Scan® among 394 Italian out-patients with T2D. | LSM ≥ 7.0/6.2 kPa was independently associated with microvascular complications (aOR 4.2, 95 %CI 1.5‐11.4; P = 0.005), mainly CKD (adjusted OR 3.6, 95 %CI 1.3‐10.1; P = 0.01) and retinopathy (adjusted OR 3.7, CI 95 % 1.2‐11.9; P = 0.02) irrespective of diabetes duration and HbA1c | Significant fibrosis, detected by FibroScan®, was independently associated with microvascular complications among T2D patients |
| Mikolasevic, 2021 [12] | 442 T2DM out-patients underwent VCTE and extensive assessment of chronic vascular complications of diabetes. | Significant liver fibrosis ((LSM ≥ 7.0/6.2 kPa), – but not steatosis defined as CAP ≥ 238 dB/m – was associated with an increased risk of, peripheral polyneuropathy (aOR 4.55, 95 %CI 1.25–16.6), CKD (aOR 4.54, 95 %CI 1.24–16.6) or retinopathy (aOR 1.81, 95 %CI 1.62–1.97), independently of cardiometabolic confounding factors macro-/microvascular complications of diabetes | Significant liver fibrosis is independently associated with microvascular complications among individuals living with T2D |
| Zhang, 2022 [13] | Meta-analysis of 18 studies involving 12,757 patients. | Liver fibrosis, (OR = 1.69, 95 %CI 1.30–2.20; p < 0.0001; but not NAFLD (OR = 1.15, 95 %CI 0.75–1.76; p = 0.51) NAFLD was positively correlated with DR. NAFLD was associated with DR in patients with T1D (OR = 2.96, 95 %CI 1.48–5.94; p = 0.002) | There is a significant correlation between liver fibrosis and DR |
| Wen, 2022 [14] | Among 1982 T2D patients, the risk for advanced liver fibrosis was categorized as “low, “indeterminate, “and “high” with NFS | The presence of DR and DKD was inversely associated with NAFLD after adjusting for covariates. The presence of DR and DKD was higher in the “indeterminate risk” and “high risk” groups than in the “low risk” group after adjusting for the same covariates. Only the presence of DKD significantly increased with high NFS | The presence of DR and DKD was inversely associated with NAFLD among hospitalized T2D patients. DKD was closely associated with high NFS |
aOR, adjusted odds ratio; CAP, controlled attenuation parameter; CKD, chronic kidney disease; DKD, diabetic kidney disease; DR, diabetic retinopathy; HbA1c, glycated hemoglobin; LSM, liver stiffness measurement; NFS, NAFLD fibrosis score; T1D, type 1 diabetes; T2D, type 2 diabetes.
First, the association linking more advanced stages of liver fibrosis with the risk of DR occurs irrespective of the non-invasive technique used to assess steatosis, namely either liver stiffness measurement or FIB-4. In this connection, it should be emphasized that liver stiffness measurement, in the context of individuals with chronic liver disease, has been found to be an easy-to-use, non-invasive continuous scale tool to rule out clinically significant portal hypertension in >95 % of patients [15]. To this end, also a variety of biomarkers, including aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio (ARR), AST to Platelet Ratio Index (APRI), FiB-4, Forns index, NAFLD fibrosis score (NFS), BARD (body mass index, AAR, Diabetes) score, and Hepamet fibrosis score (HFS), are accurate in ruling out advanced liver fibrosis and positively correlate with scores of cardiovascular risk in patients with chronic liver disease [16]. Of concern, age and diabetes negatively impact the accuracy of non-invasive tests for liver fibrosis [17,18].
4Role of the liverSecond, these associations linking liver fibrosis and DR occur irrespective of the duration of diabetes and the degree of metabolic compensation of diabetes, assessed with HbA1c [8], supporting the notion that it is probably the liver (rather than diabetes) that plays a major pathogenic role in the “hepato-retinal” axis [19]. The pathomechanisms underlying this “hepato-retinal” axis, although incompletely understood, are increasingly being elucidated [19]. To this end, lessons from human disease models, such as the so-called ciliopathies [20] and the Senior-Løken Syndrome [21] may shed light on the role of genetics in facilitating, among predisposed individuals with diabetes, the development of eye-kidney-liver complications. In this regard, Olbrich and Colleagues have identified mutations in a novel gene, NPHP3, to cause nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis among teenagers [22]. However, the relevance of such polymorphisms in the NPHP3 gene to the development of DR in adults remains to be ascertained.
5Role of hepatic fibrosisThird, liver fibrosis, teleologically aimed at limiting tissue integrity by circumscribing offending agents, describes an excess accumulation of extracellular matrix components [23]. Liver fibrosis results from persistent liver injury owing to variable insults, including i) chronic viral hepatitis; ii) toxic injury, for example, owing to chronic excess alcohol consumption; iii) injury associated with metabolic dysfunction, such as typically observed in the NAFLD/NASH arena [24]. Whenever liver fibrosis, from a mechanism of organ defense, turns dysfunctional owing to prolonged inflammation and excessive fibrotic response, liver regeneration and function will be compromised, culminating in portal hypertension, risk of complicated cirrhosis and development of hepatocellular carcinoma, which, globally, result in increased clinical burden and healthcare expenditures [23,25]. The grounds underlying the association of liver fibrosis and DR are incompletely defined. On the one hand, both liver fibrosis and DR may result from a “common soil” of insulin resistance, metabolic dysfunction, increased oxidative stress, and sterile metabolic inflammation (i.e., metaflammation), eventually conducive to repeated bouts of cell stress, death, and fibrosing wound response in the liver, the kidneys [26,27], and, by inference, the retinal tissue. On the other hand, the steatotic and chronically inflamed liver, particularly in NASH, and MASH could serve as an amplifier and a perpetuator of systemic insulin resistance and subclinical low-grade chronic inflammatory state [28], eventually conducive to damage of the microvasculature of retina in a proportion of individuals.
6How can these epidemiological associations be used in clinical arena?Probably, one of the most difficult challenges in clinical practice is to push clinicians from different areas of expertise to work together in the setting of multi-disciplinary teams. However, such multi-expert teams are exactly what we would need to utilize the mutually interactive information deriving from the retina and from the liver. For example, the hepatologist should be aware of the risk of DR and refer his/her patients with advanced dysmetabolic SLD to the ophthalmologist. Similarly, the opthalmologist/diabetologist who happens to observe a case of DR should promptly refer the patient for hepatological assessment.
7Conclusion and research agendaIndividuals presenting with NAFLD/NASH, MAFLD/MASLD and concurrent DR are likely to harbor CKD, diabetic neuropathy, and enhanced cardiovascular risk in the context of more advanced liver fibrosis. Prompt recognition of this syndromic picture by expert and dedicated clinicians will trigger comprehensive multi-organ assessment, such as appropriate for a potentially severe systemic disorder.
In this context, strategies aimed at targeting triage for advanced liver fibrosis among those at-risk groups of individuals undoubtedly play a major role [29]. From the therapeutic point of view, these patients exhibiting advanced liver fibrosis in the setting of diabetes may benefit from a variety of marketed drugs and others in the pipeline, including pioglitazone, glucagon-like peptide 1 receptor antagonists, sodium–glucose transporter 2 inhibitors, Fibroblast Growth Factor analogues, Farnesoid X receptor agonists, peroxisomeproliferator–activated receptor agonists, and the liver-directed, β-selective THR agonist resmetirom [30,31].
Additional studies should explore the role of serum levels of mac-2 binding protein and galectin 1 [32,33] as biomarkers of systemic fibrosing disease potentially involving eyes, the kidneys, and the liver, as well as of galectin 1 as a target of selective anti-fibrotic pharmacological intervention [33].
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.