Despite reports that mortality is increasing, overall case fatality due to hepatitis C virus (HCV) is thought to be low. Given the variability in published rates, we aimed to synthesize estimates of liver-specific case fatality and all-cause mortality in chronic HCV according to follow-up duration, sustained viral response (SVR) to treatment, and liver disease severity. A systematic review was conducted of studies published in English from 2003 to 2013, reporting liver-specific case fatality estimates from HCV-infected samples. Thirty-five eligible articles were identified; 26 also presented estimates of all-cause mortality. Among community-based samples, liver-specific case fatality ranged from 0.3% over 5.7 years to 9.2% over 8.2 years of follow-up; and of all-cause mortality, from 4.0% over 5.7 years, to 23.0% over 8.2 years of follow-up. Estimates were higher among clinic-based samples and those with more severe liver disease. Among treated patients achieving SVR, liver-specific case fatality was low: up to 1.4% over 11.5 years of follow-up among samples with any severity of liver disease. Estimates were higher among those without SVR: up to 14.0% over 10 years of followup among samples with any severity of liver disease, and higher still among samples with more severe liver disease. The proportion of deaths attributable to liver-specific causes ranged from 55 to 85% among those with severe liver disease. Published estimates of fatality are high among certain populations of chronic HCV patients, with liver-specific causes being an important contributor. Understanding current HCV mortality rates is important for quantifying the total burden of HCV disease.
The number of deaths due to chronic hepatitis C virus (HCV) infection is on the rise: recent estimates from the United States (US) have demonstrated a 1.5-fold increase in mortality between 1999 and 2007.1 However, most deaths attributed to HCV occur among those with end-stage liver disease,1 the progression to which occurs over a long period of time.2 As a result, chronic HCV-related case fatality, defined as the number of condition-specific deaths from the total population of those afflicted,3 is generally considered to be low.4,5 Although early estimates from the World Health Organization suggested that 5 to 7% of those with HCV will die at some point due to the condition,6 and HCV is a key causal factor in 366,000 deaths annually, globally,7 a robust synthesis of contemporary estimates is lacking. This is particularly important given the changing epidemiology of HCV, and the potential impact of emerging therapies.8,9
Published estimates of case fatality in HCV demonstrate substantial variability, due to both patient-specific, and methodological factors. Estimates of case fatality can be impacted by study design, the sampling methodology and frame (i.e., if selection occurred from HCV clinics or the community), disease severity or duration, and causes of death considered.10–12 This variability in estimates of case fatality among populations with chronic HCV, and the evidence of increasing attributable mortality,1 may contradict the belief that case fatality due to HCV is ‘low’. Despite challenges in synthesizing the evidence, understanding the case fatality rate due to HCV is important, as even a small apparent increase in the rate could translate into a substantially higher numbers of deaths due to the high prevalence of HCV. Additionally, understanding the importance of liver-specific causes in contributing to all-cause mortality in HCV, would help contextualize how risk factors and other comorbidities impact an individual’s likelihood of death.
The objectives of this study were to synthesize and compare reported estimates of liver-specific case fatality and all-cause mortality in HCV, according to time since study enrolment, treatment response, baseline liver disease severity, and sampling frame. Findings from this synthesis will allow a better understanding of the true mortality risk among those with chronic HCV.
Material and MethodsA systematic review was conducted of studies reporting on estimates of liver-specific case fatality in HCV, indexed in EMBASE and Medline. Case fatality estimates were stratified according to duration of follow up, baseline liver disease severity, sampling frame (community- or clinic-based), and treatment response (i.e., whether a sustained viral response (SVR) was achieved). We considered all-cause liver-specific estimates of case fatality, but did not synthesize estimates within individual liver disease-related fatality categories, as the categorizations of those varied between studies. Estimates of all-cause mortality were also identified and synthesized, where available.
Search strategy and study selectionA customized search strategy was developed using a combination of index and free terms, and study design filters, to identify studies reporting on liver-specific case fatality in HCV (Appendix 1-4).
Inclusion criteria were developed to identify:
- •
Peer-reviewed original articles published in English from 2003 to 2013 (the most recent ten years at the time of the search).
- •
Randomized studies reporting on long-term mortality outcomes; or, observational studies enrolling either a population-based sample, or a clinic-based sample of consecutively-selected patients; and
- •
Articles presenting liver-specific case fatality estimates over a specified duration of time, with or without estimates of all-cause mortality, among individuals with chronic HCV.
Studies were excluded if:
- •
They selected individuals on the basis of an HCV risk factor that would modify the case fatality rate (such as human immunodeficiency virus (HIV) infection or injection drug use); or
- •
Results were from a duplicate publication or sample.
Studies reporting results according to person-years of follow up were tabulated separately (Appendix 1-4). Two independent investigators reviewed the title, abstract, and full-text of potential articles; and reached consensus before confirming final study inclusion (Figure 1).
Data extraction and evidence synthesisFollowing best practices published elsewhere,4 duplicate data extraction into a customized Microsoft® Excel® form was performed. Extracted data included baseline characteristics, liver-specific case fatality or all-cause mortality according to duration of follow-up, and whether the sample was selected based on HCV liver disease severity. Case fatality was defined as the number of deaths due to liver disease, and all-cause mortality as the number of deaths due to any cause.3 Two separate measures of death were extracted, depending upon how the data were presented in the original article:
- •
The proportion of the sample that died over a mean or median follow up time, or
- •
The cumulative number of deaths, extracted at the one-, two-, and five-year time points, using Engauge Digitizer v2.14.
Although the first measure was more frequently reported, the second measure was included because it provides estimates over various durations of follow up that can be easily compared between studies with similar inclusion criteria.
The severity of liver disease within a sample was classified into two categories, according to the sampling strategy of the original investigators. Samples were classified as having ‘any severity of liver disease due to HCV’ if enrollment was not restricted based on underlying liver disease severity. Samples were classified as having ‘HCV with severe liver disease’ when the sample was selected based on the presence of any cirrhosis, hepatocellular carcinoma, or other unspecified severe liver disease.
Estimates of liver-specific case fatality or all-cause mortality were plotted according to the duration of follow-up, and stratified according to severity of HCV and treatment response. For studies reporting on both liver-specific case fatality and all-cause mortality, the proportion of mortality attributable to HCV was calculated. This calculation was restricted to estimates presented by time since enrolment, and of known treatment status.
ResultsThirty-five of 859 articles identified in the systematic search presented estimates of liver-specific case fatality (Figure 1);7–42 26 ( 72%) also estimated all-cause mortality.12–37 Half of the studies included samples selected based on severity of liver disease; eleven provided estimates specific to cirrhosis, and three provided estimates specific to transplanted patients.28,35,37 One article was included in the ‘HCV with severe liver disease’ category where 27% of the cohort had advanced hepatic fibrosis (Ishak score 4), while the remaining members of the cohort had cirrhotic disease.36 Half of the studies presented estimates specific to European populations, and more than half (n = 22) sampled from HCV clinics (Table 1). All studies that included only patients with severe liver disease were clinic (rather than community) based samples.
Characteristics of the 36 studies included in the systematic review.
| Liver-specific case fatality n = 35 n (%) | All-cause mortality n = 26 n (%) | |
|---|---|---|
| Sample severity | ||
| Those with any severity of liver disease due to HCV | 20 (55.6) | 13 (48.1) |
| SVR status-specific* | 7 (19.4) | 5 (38.5) |
| Only HCV with severe liver disease included | 15 (42.9) | 13 (50) |
| SVR status-specific* | 8 (53.3) | 4 (30.8) |
| Sampling frame | ||
| Clinic-based | 21 (60) | 18 (69.2) |
| Community-based | 14 (38.9) | 8 (29.6) |
| Asymptomatic blood donors | 2 (14.3) | 2 (25) |
| Country | ||
| North America | 6 (17.1) | 4 (15.4) |
| Cuba | 1 (2.8) | 1 (3.7) |
| Europe | 19 (52.8) | 13 (48.1) |
| Japan | 7 (19.4) | 7 (25.9) |
| Multinational | 1 (2.8) | 1 (3.7) |
| Taiwan | 1 (2.8) | 0 (0) |
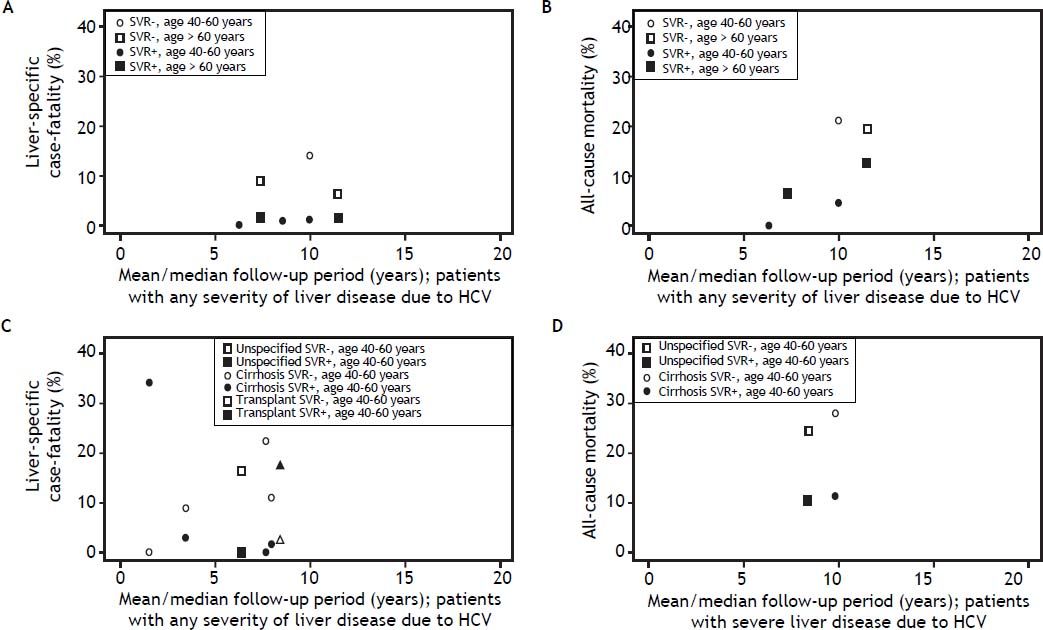
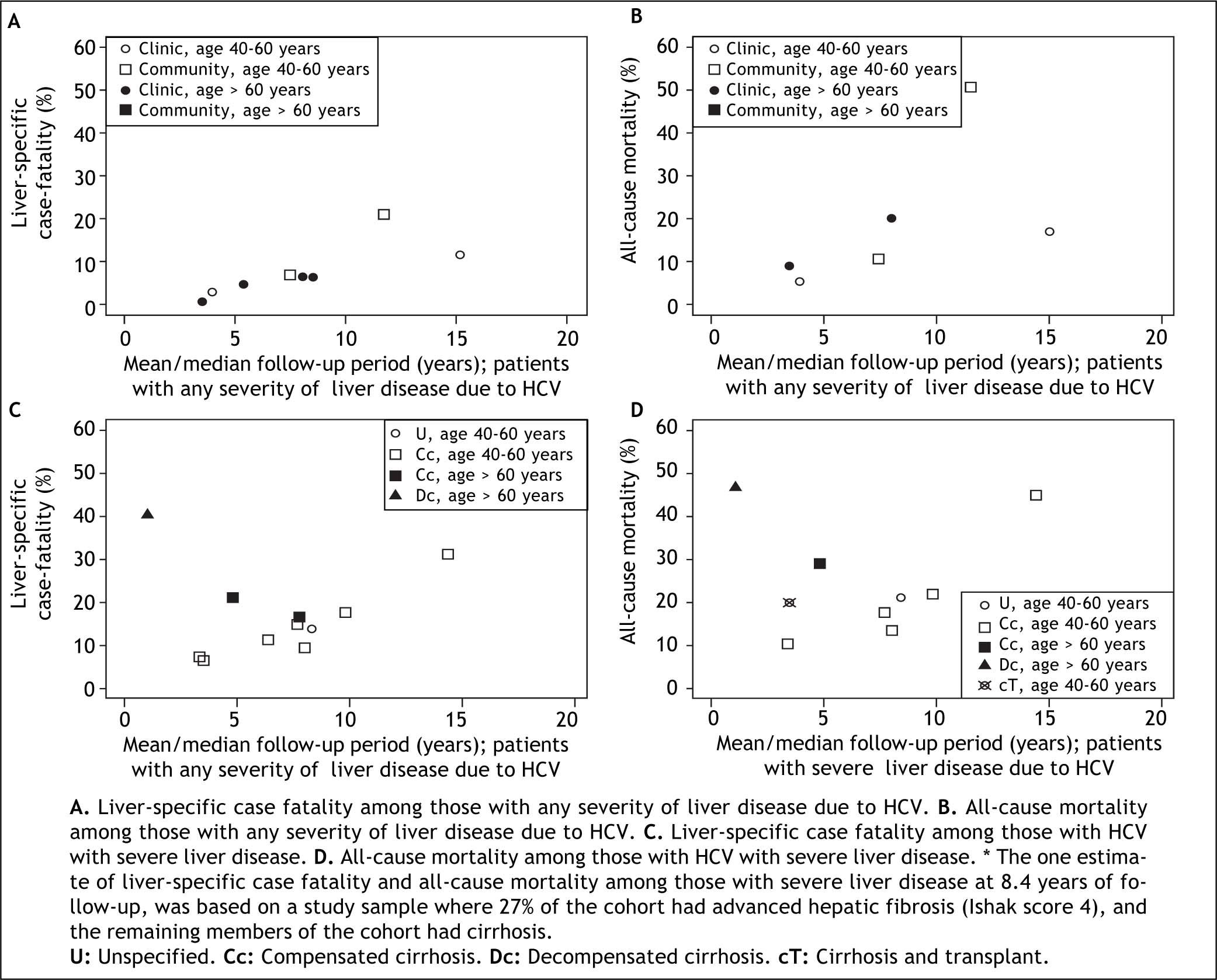
As expected, liver-specific case fatality and all-cause mortality estimates were higher in studies with longer duration of follow-up (Figure 2). Among studies including patients with any severity of liver disease, estimates of liver-specific case fatality from community-based samples (n = 12) ranged from 0.3% over 5.7 years14 to 9.2% over 8.2 years of follow up (Figure 2A).21 Estimates from clinic-based samples (n = 5) were higher, ranging from 2.7% over 3.9 years22 to 21.2% over 11.5 years of follow up.23 As expected, estimates of all-cause mortality from the same studies were higher (Figure 2B). The proportion of all-cause mortality accounted for by liver-specific causes ranged from 28.9 to 81.1% in studies with patients of any severity (Table 2).
Estimates of case fatality and mortality according to follow up time and severity of HCV: A. Liver-specific case fatality among those with any severity of liver disease due to HCV. B. All-cause mortality among those with any severity of liver disease due to HCV. C. Liver-specific case fatality among those with HCV with severe liver disease. D. All-cause mortality among those with HCV with severe liver disease. * The one estimate of liver-specific case fatality and all-cause mortality among those with severe liver disease at 8.4 years of follow-up, was based on a study sample where 27% of the cohort had advanced hepatic fibrosis (Ishak score 4), and the remaining members of the cohort had cirrhosis.
Proportion of all-cause mortality attributable to liver-specific case fatality (according to time since enrolment), from samples according to selection criteria for HCV severity and treatment status.
| Author | Year | Country | Sampling frame | Treatment status | Liver-specific case fatality (%) | All-cause mortality (%) | Follow-up period | Attributable mortality (%)* |
|---|---|---|---|---|---|---|---|---|
| All HCV | ||||||||
| Di Martino* | 2011 | France | Community | Not treated | 6.4 | 19.9 | 8 years/person | 33.7 |
| Maruoka | 2012 | Japan | Clinic | Not treated | 20.8 | 25.7 | Mean of 9.9 y | 81.1 |
| Neal | 2007 | England | Community | Not treated | 2.3 | 7.9 | Mean of 6.7 y | 28.9 |
| Uto | 2009 | Japan | Community | Not treated | 9.2 | 23 | Mean of 8.2 y | 39.8 |
| Yamasaki | 2012 | Japan | Community | Not treated | 21.1 | 50.5 | Median of 11.5 y | 41.8 |
| Arase | 2007 | Japan | Clinic | Treated | 6.8 | 10.6 | Mean of 7.4 y | 64.2 |
| Kasahara | 2004 | Japan | Clinic | Treated | 2.6 | 3.7 | Mean of 5.8 y | 68.3 |
| Innes | 2011 | Community | Treated | 4.5 | 7.2 | Mean of 5.3 y | 62.5 | |
| Severe HCV** | ||||||||
| Bruno | 2009 | Italy | Clinic | Not treated | 31.3 | 44.9 | Median of 14.4 y | 69.6 |
| Gomez | 2013 | Cuba | Clinic | Not treated | 7.5 | 10.4 | Median of 3.4 y | 71.4 |
| Shiratori | 2005 | Japan | Clinic | Not treated | 14.8 | 20 | Median of 6.8 y | 73.9 |
| Toshikuni | 2009 | Japan | Clinic | Not treated | 21.1 | 28.9 | Median of 4.9 y | 72.7 |
| Braks | 2007 | France | Clinic | Treated | 15 | 17.7 | Mean of 7.7 y | 84.7 |
| Bruno | 2007 | Italy | Clinic | Treated | 9.6 | 13.6 | Mean of 8.0 y | 70.8 |
| Mallet | 2008 | France | Clinic | Treated | 17.7 | 21.9 | Median of 9.8 y | 80.8 |
| Morgan | 2010 | USA | Clinic | Treated | 4.2 | 7.6 | Median of 6.6 y | 55.3 |
| Van der Mer | 2012 | Europe and Canada | Clinic | Treated | 13.8 | 21.3 | Median of 8.4 y | 64.6 |
Estimates of liver-specific and all-cause mortality were higher among patients with severe liver disease at baseline (Figure 2). Liver-specific case fatality among samples of patients with severe liver disease ranged from 4.2% over 6.5 years31 to 31.3% over 14.4 years of follow up.26 The highest liver-specific case fatality estimate of 40.2% over 1.1 years of follow up was observed in the one study of individuals with decompensated cirrhosis (Figure 2C).32 All-cause mortality estimates were up to 44.9% over 14.4 years of follow up among individuals with non-specific severe liver disease or cirrhosis,26 20.0% over 3.5 years among those who had had a liver transplant,28,35 and 46.8% over 1.1 years of follow up among those with decompensated cirrhosis.32 The proportion of mortality attributable to liver-specific causes was generally higher among samples with more severe liver disease (55.3 to 84.5%) (Table 2).
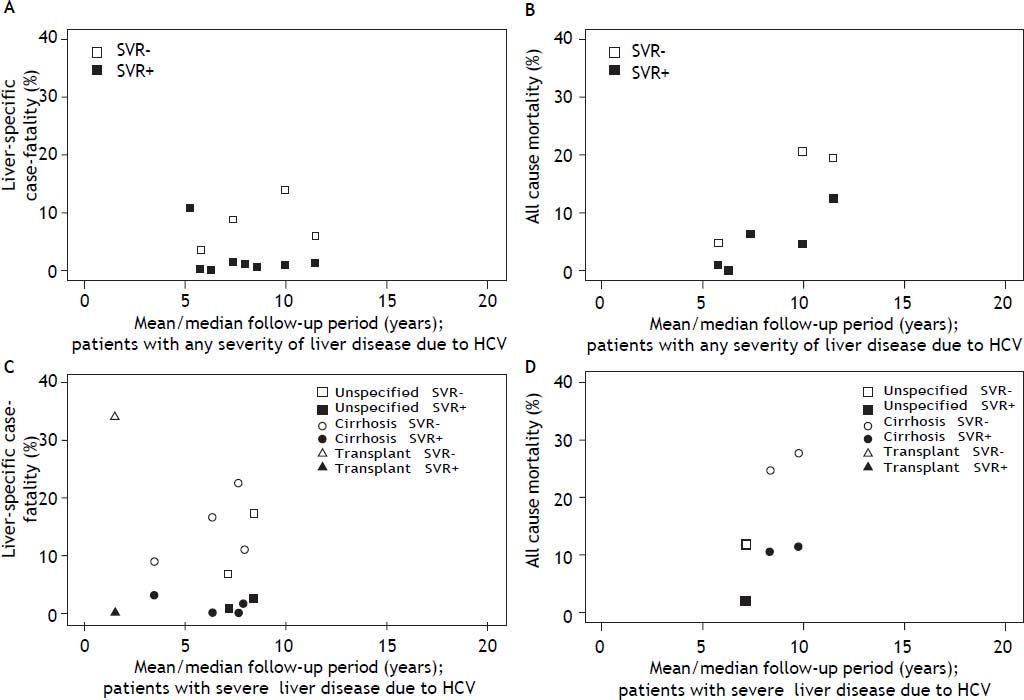
Both liver-specific case fatality and all-cause mortality were relatively constant and low among those who achieved an SVR (Figure 3), independent of liver disease severity. Among those with any severity of liver disease that achieved SVR, the highest estimate of liver-specific case fatality was 1.4% over 11.5 years;19 all-cause mortality was 12.5% over 11.5 years.23 Among those with severe liver disease that acheved SVR, liver-specific case fatality reached 2.9% over 3.5 years;37 and all-cause mortality reached 11.4% over 9.8 years.30 Liver-specific case fatality and all-cause mortality were higher and increased with time among those who did not achieve an SVR (Figure 3). Among those with any severity of liver disease, liver-specific case fatality was up to 14.0%, and all-cause mortality up to 20.8%, over 10 years of follow-up.19 The effect was more dramatic among patients with severe liver disease, where liver-specific case fatality was up to 22.4% over 7.7 years, and all-cause mortality, up to 27.9% over 9.8 years.30 The proportion of mortality attributable to liver-specific causes was approximately twice as high among those who failed to achieve an SVR (56.8%), relative to those achieving an SVR (33.3%) (Table 3).
Estimates of liver-specific case fatality and all-cause mortality according to follow up time and response to treatment: A. Liver-specific case fatality among those with any severity of liver disease due to HCV. B. All-cause mortality among those with any severity of liver disease due to HCV. C. Liver-specific case fatality among those with HCV with severe liver disease. D. All-cause mortality among those with HCV with severe liver disease. * The one estimate of liver-specific case fatality and all-cause mortality among those with severe liver disease at 8.4 years of follow-up, was based on a study sample where 27% of the cohort had advanced hepatic fibrosis (Ishak score 4), and the remaining members of the cohort had cirrhosis.
Proportion of all-cause mortality attributable to liver-specific case fatality (according to time since enrolment), from samples according to selection criteria for HCV severity and response status.
| Author | Year | SVR status | Liver-specific case fatality (%) | All-cause mortality (%) | Follow-up period | Attributable mortality (%) |
|---|---|---|---|---|---|---|
| All HCV | ||||||
| Arase | 2007 | SVR+ | 1.4 | 6.4 | Mean of 7.4 y | 22.2 |
| Kasahara | 2004 | SVR+ | 0.1 | 1 | Mean of 5.8 y | 14.3 |
| Trapero-Marugan | 2011 | SVR+ | 0 | 0 | Median of 6.3 y | 0 |
| Severe HCV | ||||||
| Morgan | 2010 | SVR+ | 0.7 | 2.1 | Median of 7.2 y | 33.3 |
| SVR- | 6.8 | 12 | Median of 7.2 y | 56.8 | ||
| Van der Meer | 2012 | SVR+ | 2.4 | 10.4 | Median of 8.4 y | 23.1 |
| SVR- | 17.3 | Median of 8.4 y | 70.0 |
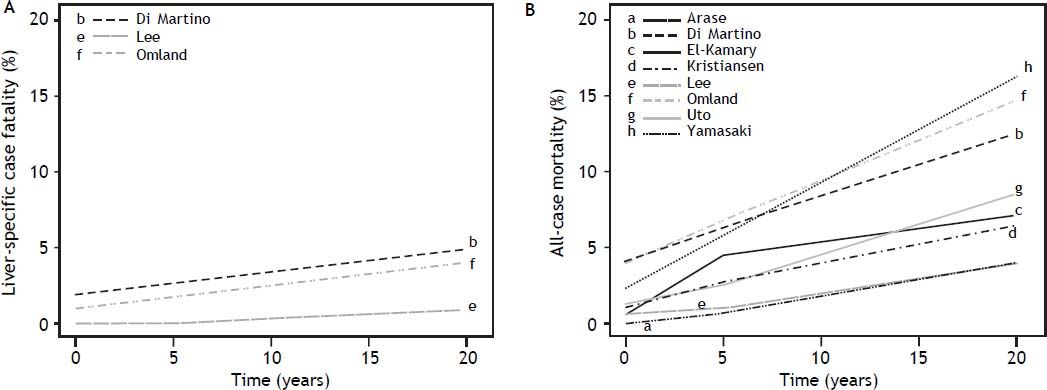
Estimates of the cumulative incidence of liver-specific case fatality among samples of any severity liver disease (n = 3) demonstrated the risk of death due to HCV was not low. Estimates reached 5% case fatality by 5 years since diagnosis (in a study of treatment naïve individuals) (Figure 4A).12 Eight studies reported on cumulative all-cause mortality among those with any severity of liver disease, and estimates ranged from 3.9% among a community-based sample of mixed treatment status,38 to 16.3% in a clinic-based treatment-naïve sample,23 over 5 years (Figure 4B). Although no studies reported cumulative liver-specific deaths among samples with severe liver disease, cumulative all-cause mortality at 5 years was: 15.0% (among liver transplant patients),39 16.2% (cirrhosis),34 and 53.4% (decompen-sated cirrhosis).32
DiscussionRecent reports suggest that deaths due to HCV are on the rise,1 likely due to more patients progressing to severe liver disease;40 hepatocellular carcinoma and cirrhosis are now leading causes of death global-ly.41 Nonetheless, because of the slowly progressive nature of HCV, the overall case fatality rate, and resultant mortality rate, is believed to be low.4,5 Case fatality in the US is approximately 0.7%, given the estimated 2.3 million persons infected,42 and there are 16,000 HCV-related deaths annually.1 Global mortality data also suggest that case fatality rates - although higher than in the US – are still low; there are approximately 366,000 deaths annually,7 out of 180 million cases of HCV globally,43 which equates to a case fatality of approximately 2%.
This critical systematic review of the literature suggests that previous estimates of chronic HCV-related case fatality are underestimated; case fatality rates from population- and community-based studies can be dramatically higher than 1-2%.1,7,43 Even among community-based samples of individuals with all severities of HCV, case fatality reached 10% over ten years of follow-up;21 and estimates from clinic-based samples were more than 20% over the same period.23 Fatalities occurred in up to one-third of individuals with severe liver disease due to HCV over 15 years of follow-up,26 and were higher still among those with decompensated cirrhosis.32 Higher estimates among those who have progressed to more severe liver disease highlight the importance of delaying disease progression to decreasing mortality.
Comparing HCV-specific mortality estimates with the general population quantifies the excess risk of death, from both liver-related and non-HCV-related causes. In a Swedish study, the standardized mortality ratio (SMR; comparing individuals with HCV and the general population) was estimated at 5.8 for all-cause death and 35.5 for liver-specific death.16 SMR estimates from a Norwegian study were comparable at 6.7 for all-cause death and 41.0 for liver-specific death.18 Similar comparisons of mortality from the US, Taiwan, and England suggest at least a two to three-fold excess risk of all-cause death among those with HCV.11,13,38 Despite the importance of liver-specific case fatality, the increased risk of all-cause mortality highlights that non-hepatic causes of death are also important concerns for individuals with chronic HCV infection.
Estimating the proportion of deaths due to liver-specific causes is a useful measure for understanding the HCV burden and quantifying the potential survival benefit of achieving SVR. Our findings compliment those of a recent review that demonstrated that SVR decreases liver-specific case fatality in uncomplicated chronic HCV,10 by considering patients with more severe liver disease at baseline, and the contribution of liver-specific death to all-cause mortality. We found that liver-specific causes accounted for the majority of deaths among those with chronic HCV, and, to an even greater extent, among those with severe liver disease. The extent to which specific nonliver-related causes contribute to all-cause mortality in HCV varies depending on the age of the patients and the distribution of other risk factors for deaths.44 Describing the proportion of all-cause mortality due to liver-specific causes may help to facilitate comparisons of mortality estimates across studies.
Strengths of the study included the comprehensive approach to this evidence synthesis, including articles from different geographic areas, study designs, and sampling strategies. We included studies that reported no deaths and individuals with all stages of liver disease, to avoid bias in the fatality rates. Multiple types of case fatality measures were considered to take a broad view in understanding the impact of chronic HCV on case fatality. Finally, samples were required to be population-based or consecutively selected, to collect estimates from studies of higher methodological rigor in their sampling strategy.
The study also suffered from a number of limitations. First, we were limited by the quality of the design and extent of reporting of the original articles. Studies typically reported basic demographic and clinical characteristics of the sample, but other mortality risk factor data were infrequently presented. As such, we were unable to tease out the impact of specific contributors to liver-specific case fatality (i.e. alcohol abuse) from the impact of the underlying HCV infection. We attempted to control for this by excluding articles that selected patients based on the presence of other risk factors for mortality (i.e. HIV infection). Heterogeneity in study designs and samples prevented meta-analysis;45,46 we also chose not to annualize reported estimates of case fatality.10 Instead, the time horizon presented in the original articles was retained, to avoid assuming a constant hazard of death. The inclusion of Kaplan-Meier curves, or age- and sex-specific rates, in future publications of mortality in HCV would facilitate comparisons of data across studies and present a more accurate portrayal of the risks of death.
Second, due to a lack of data availability, we were unable to stratify results by time since infection. Instead, we considered the impact of baseline liver disease severity on case fatality as a proxy. Third, there was variability in the measures of liver-specific case fatality and the categorizations of causes of death between studies. To address this, we did not compare between individual liver disease categories and, instead, focused on aggregated measures of liver-related case fatality. Finally, as studies were selected for inclusion based on presenting liver-specific case fatality estimates, the findings for all-cause mortality may not be reflective of all studies reporting on overall deaths in chronic HCV.
HCV case fatality rates may vary, depending on a number of clinical and methodological factors. Despite this, these estimates highlight that case fatality among those with chronic HCV may have been underestimated, and liver-specific causes of death are an important contributor to mortality in HCV.
Abbreviations- •
HCV: hepatitis C virus.
- •
SVR: sustained viral response.
- •
US: United States.
This study was sponsored by AbbVie. The design, analysis, and financial support of this study were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the presentation.
DisclosuresJennifer C. Samp and David Walker are AbbVie employees and may hold AbbVie stock or options. Stephanie Cline and Katherine Gooch were employees of AbbVie at the time of the conduct of this study.
Suzanne Lane is an employee of ICON plc. At the time of this work, Shelagh Szabo was an employee of ICON plc.
Ricardo Jimenez-Mendez and Adrian Levy are paid consultants to ICON plc.
Search strategy.
| Database: Embase <1996 to 2013 Week 29>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present> Search Strategy: |
|---|
| 1. exp hepatitis C/ or hepatitis c.mp. or hepatitis c virus/ (148811). |
| 2. (CHC or hep C or HCV).ab,ti. (92224). |
| 3. exp mortality/ (755904). |
| 4. 1 or 2 (160527). |
| 5. 4 and 3 (6339). |
| 6. Clinical study/ (40632). |
| 7. Case control study.mp. (153527). |
| 8. Family study/ (7319). |
| 9. Longitudinal study/ (140898). |
| 10. Retrospective study/ (771989). |
| 11.Prospective study/ (580619). |
| 12. Randomized controlled trials/ (129382). |
| 13.11 not 12 (575312). |
| 14.Cohort analysis/ (313242). |
| 15. (Cohort adj (study or studies)).mp. (307142). |
| 16. (Case control adj (study or studies)).tw. (124249). |
| 17. (follow up adj (study or studies)).tw. (65221). |
| 18. (observational adj (study or studies)).tw. (97222). |
| 19. (epidemiologic$ adj (study or studies)).tw. (114580). |
| 20. (cross sectional adj (study or studies)).tw. (136156). |
| 21.or/6-10,13-20 (2182035). |
| 22. 21 use emef (963701). |
| 23. Epidemiologic studies/(65528). |
| 24. exp case control studies/(725866). |
| 25. exp cohort studies/(1481507). |
| 26. Case control.tw. (152702). |
| 27. (cohort adj (study or studies)).tw. (179247). |
| 28. Cohort analy$.tw. (7562). |
| 29. (Follow up adj (study or studies)).tw. (65221). |
| 30. (observational adj (study or studies)).tw. (97222). |
| 31. Longitudinal.tw. (264568). |
| 32. Retrospective.tw. (572109). |
| 33. Cross sectional.tw. (332108). |
| 34. Cross-sectional studies/ (267148). |
| 35. or/23-34 (2795840). |
| 36. 35 use prmz (1859318). |
| 37. 22 or 36 (2823019). |
| 38. 5 and 37 (1791). |
| 39. exp animal/ not human/ (5994864). |
| 40. 38 not 39 (1790). |
| 41. limit 40 to yr=”2003 -Current” (1411). |
| 42. limit 41 to english language (1352). |
| 43. limit 42 to “review” (77). |
| 44.42 not 43 (1275). |
| 45.44 use emef (828). |
| 46. limit 45 to (conference abstract or conference paper or conference proceeding or “conference review”) [Limit not valid in Ovid MEDLINE(R),Ovid MEDLINE(R) In-Process; records were retained] (252). |
| 47.44 not 46 (1023). |
| 48. remove duplicates from 47 (859). |
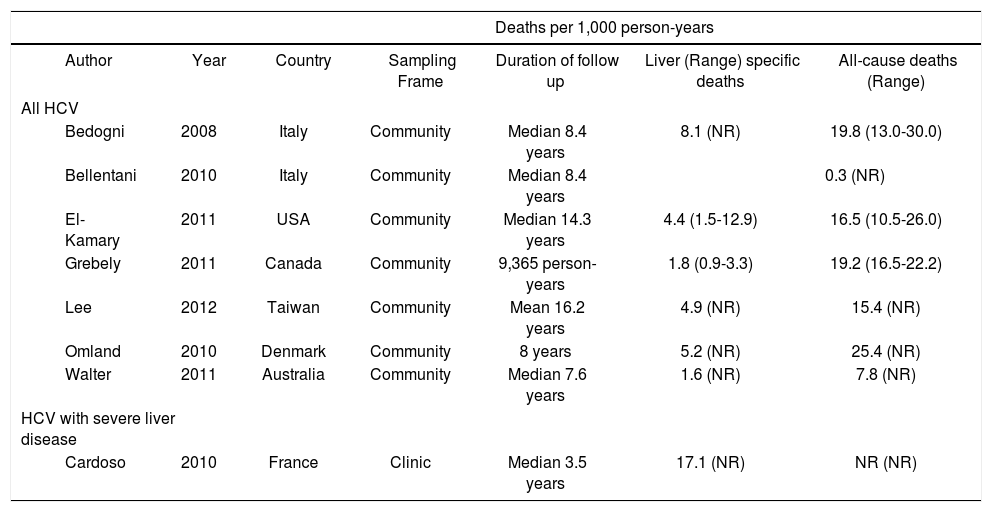
Estimates from studies reporting on liver-specific and all-cause deaths per 1,000 person-years, according the severity of HCV.
| Deaths per 1,000 person-years | |||||||
|---|---|---|---|---|---|---|---|
| Author | Year | Country | Sampling Frame | Duration of follow up | Liver (Range) specific deaths | All-cause deaths (Range) | |
| All HCV | |||||||
| Bedogni | 2008 | Italy | Community | Median 8.4 years | 8.1 (NR) | 19.8 (13.0-30.0) | |
| Bellentani | 2010 | Italy | Community | Median 8.4 years | 0.3 (NR) | ||
| El-Kamary | 2011 | USA | Community | Median 14.3 years | 4.4 (1.5-12.9) | 16.5 (10.5-26.0) | |
| Grebely | 2011 | Canada | Community | 9,365 person-years | 1.8 (0.9-3.3) | 19.2 (16.5-22.2) | |
| Lee | 2012 | Taiwan | Community | Mean 16.2 years | 4.9 (NR) | 15.4 (NR) | |
| Omland | 2010 | Denmark | Community | 8 years | 5.2 (NR) | 25.4 (NR) | |
| Walter | 2011 | Australia | Community | Median 7.6 years | 1.6 (NR) | 7.8 (NR) | |
| HCV with severe liver disease | |||||||
| Cardoso | 2010 | France | Clinic | Median 3.5 years | 17.1 (NR) | NR (NR) | |