Liver transplantation (LT) for acute liver failure (ALF) still has a high early mortality. We aimed to evaluate changes occurring in recent years and identify risk factors for poor outcomes.
Material and methodsData were retrospectively obtained from the Argentinean Transplant Registry from two time periods (1998–2005 and 2006–2016). We used survival analysis to evaluate risk of death.
ResultsA total of 561 patients were listed for LT (69% female, mean age 39.5±16.4 years). Between early and later periods there was a reduction in wait-list mortality from 27% to 19% (p<0.02) and 1-month post-LT survival rates improved from 70% to 82% (p<0.01). Overall, 61% of the patients underwent LT and 22% died on the waiting list. Among those undergoing LT, Cox regression analysis identified prolonged cold ischemia time (HR 1.18 [1.02–1.36] and serum creatinine (HR 1.31 [1.01–1.71]) as independent risk factors of death post-LT. Etiologies of ALF were only available in the later period (N=363) with indeterminate and autoimmune hepatitis accounting for 28% and 26% of the cases, respectively. After adjusting for age, gender, private/public hospital, INR, creatinine and bilirubin, and considering LT as the competing event, indeterminate etiology was significantly associated with death (SHR 1.63 [1.06–2.51] and autoimmune hepatitis presented a trend to improved survival (SHR 0.61 [0.36–1.05]).
ConclusionsSurvival of patients with ALF on the waiting list and after LT has significantly improved in recent years. Indeterminate cause and autoimmune hepatitis were the most frequent etiologies of ALF in Argentina and were associated with mortality.
Acute liver failure (ALF) is a complex medical condition defined as a rapid development of liver failure characterized by impaired hepatic synthesis, especially coagulation factors, associated with encephalopathy [1]. Due to the substantial advances in medical management of ALF, a significant improvement in spontaneous survival of 10–20% to about 40% has been achieved [2–4]. Epidemiological studies have shown a marked geographical difference in relation to prognosis, etiology and evolution of ALF [5].
A recent study from the Acute Liver Failure Study Group (ALFSG) described that paracetamol toxicity and idiosyncratic drug reactions remain as the most common causes of ALF in the United States [2]. In the UK, paracetamol related admissions to liver units fell 40% after implementing drug sales-restriction [3]. Information on the etiology and outcome of patients with ALF in Latin America is scarce. In Argentina we described acute hepatitis B virus (HBV) infection and autoimmune hepatitis as the two most common causes of ALF and, remarkably, no cases of paracetamol poisoning were reported [6]. This study only included six liver transplant centers for a 6-year period. The liver transplantation field in Argentina underwent major changes after the expansion in the number of liver transplant programs with a disproportionate increase in the number of patients on the waiting list compared to the number of donors. Moreover, the vaccine schedules were modified with the introduction of the vaccine against hepatitis A virus (HAV) and HBV but the long-term impact of this measure in the adult population has not been investigated.
Therefore, the aim of this study was to evaluate whether changes in outcomes and etiologies have occurred in patients listed with ALF using the Argentinean national institute for organ allocation and to identify risk factors for poor outcome in this population.
2Materials and methodsThis was a retrospective study of adult patients with ALF listed for orthotopic liver transplantation (OLT) in Argentina from January 1998 to September 2016. The study included adult patients (16 years or older) listed with ALF as emergency status. All enrolled patients met entry criteria for ALF: presence of coagulopathy (international normalized ratio [INR] ≥1.5 or prothrombin index <50%) and any grade of hepatic encephalopathy within 26 weeks of the first symptoms without known underlying liver disease [7]. Patients with Wilson disease, vertically-acquired HBV or autoimmune hepatitis with cirrhosis were included if their disease was recognized for <26 weeks. Etiologic diagnosis was made according to international guidelines at each study center based on clinical history, imaging studies, laboratory values and, in some cases, histopathologic characteristics [1,7]. To confirm the diagnosis of autoimmune hepatitis, the simplified criteria was retrospectively applied, those patients who presented a score ≥6 were identified as autoimmune hepatitis [8]. However, autoantibodies were only considered positive at significant titers (≥1/80). ALF was considered to be indeterminate when extensive clinical and laboratory evaluation (including toxicology screens, autoantibodies and serologic markers for viral hepatitis), as well as imaging studies were inconclusive. Even though patient management and candidacy for OLT was determined at each site. Listing for transplantation criteria were uniform and followed published clinical guidelines [1,7]. Argentina has 21 liver transplant centers, of which 15 are private institutions. A single transplant list is used. The actual donation rate is 10.77 donors per million inhabitants [9]. During both periods, patients meeting criteria for ALF and who were admitted in an ICU were eligible for “Emergency Status” which gave them preference in organ allocation over all forms of chronic liver disease irrespective of time spent waiting for an organ. Also eligible for this category were patients with primary non-function or acute hepatic thrombosis (<7 days following OLT).
Data were obtained from the database of the Instituto Nacional Central Unico Coordinador de Ablación e Implante (INCUCAI), which is the national institute for organ allocation. Since July 2005, after MELD allocation implementation, an internet-based collection system, Sistema Nacional de Información y Trasplante de la República Argentina (SINTRA), was established to capture directly pre- and post-OLT data from all centers.
During the pre-SINTRA era data collection was limited to the following variables: recipient age, gender and type of donor (cadaveric or living). After the implementation of SINTRA, the donor variables were age, gender, type of donor; recipient variables were: age, gender, total bilirubin, serum creatinine, INR, weight, height, etiology of ALF and donor/recipient blood group matching (compatible, incompatible); graft variables were: type of graft (full size, split liver, living donor); variables related to liver transplant technique were: cold ischemic time, warm ischemia time, type of transplant (orthotopic, heterotopic and auxiliary). These parameters were compared in survivors and non-survivors to identify independent predictors of death on the waiting-list and following OLT. The SINTRA database does not include variables related to intensive care management (i.e. ventilation, renal support and intracranial pressure monitoring). Model for end-stage liver disease (MELD) score was determined as described [10]. Although SINTRA has a systematic quality control for data capture, we contacted each center and validated all data presented for the study. Validation included replacement of missing data, review of inconsistencies, and update of dates of follow up. All corrections were returned to SINTRA database.
Survival and transplant outcomes were estimated in two time periods, early period (January 2, 1998 to July 11, 2005), and later period (July 12, 2005 to September 16, 2016). This cut-off was selected according to the pre- and post-SINTRA implementation given that in the SINTRA era we account for more detailed information regarding donor and recipient characteristics.
The study was conducted according to the principles of the Declaration of Helsinki. The local institutional review board of each center approved the study. All procedures followed were in accordance with STROBE guidelines for cohort studies and complied with the ethical standards of the responsible committee on human experimentation and with the Helsinski Declaration 1975, as revised in 2008 [11]. The Institutional Review Board at the Hospital Universitario Austral approved the study.
2.1Statistical analysisData were presented as percentages or means and standard deviations with 95% confidence intervals. Comparison of baseline parameters between patient groups was performed with Student t test, Mann Whitney for continuous variables and x2 test for categorical variables. A competing risk regression analysis for cumulative mortality with subhazard ratios (SHR) estimation was done considering OLT as a competing event. The model only evaluated patients from the later period (n=363) and included the following variables: age, gender, etiology, public or private hospital, INR, bilirubin and creatinine. Given the small proportion of patients, for the competing risk analysis etiologies were divided into three groups: indeterminate, autoimmune and others. To test the effect of other predictors of mortality following OLT we used multivariate Cox proportional hazards models. Significant univariate predictors of mortality (p<0.1) were incorporated into the multivariate models in a stepwise fashion. Only well-represented variables (with <20% of the data missing in the registry) were included in the analysis. Tests were two-sided and significance was accepted at p<0.05. Statistical analysis was performed by MedCalc (Ostend, Belgium) and STATA version 13.
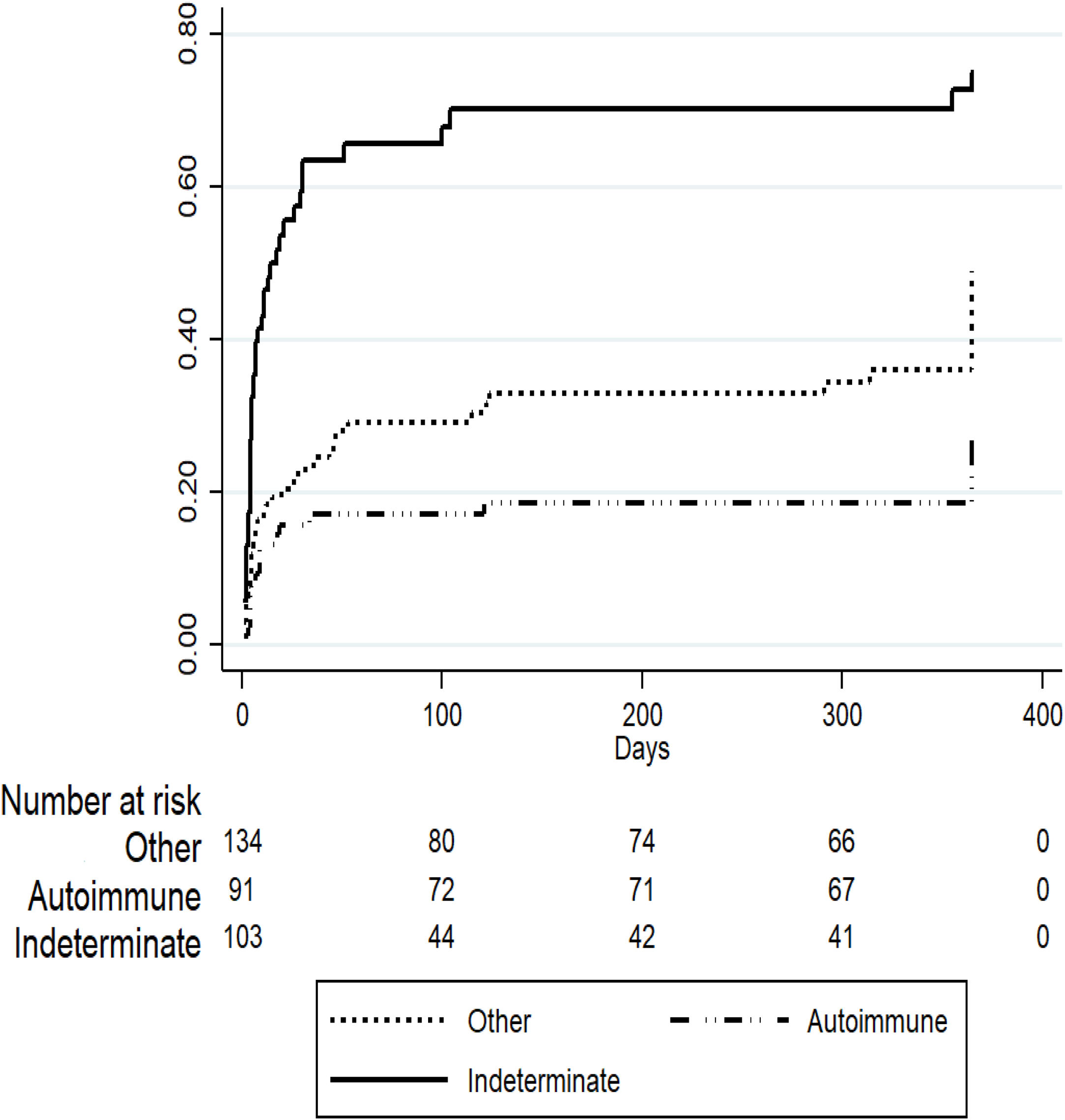
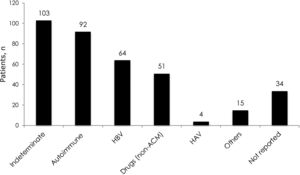
3Results3.1Demographic characteristics and distribution of etiologiesA total of 561 patients with ALF were listed in the INCUCAI database during the study period. During the early and later periods 198 patients and 363 patients were listed, respectively. Overall, 69% were female and mean age at diagnosis was 39.5±16.4 years. Whole cadaveric livers from brain stem dead donors were used in 331 (96.5%) of patients. The split liver technique was used in 3 (0.9%) patients and living donor liver transplantation in 9 (2.6%) patients. No auxiliary transplant was reported during the study period. The mean baseline laboratory parameters were only available for the later period (363 patients) and were as follows: INR 4.1±2.3, creatinine 1.1±1.0mg/dL, total bilirubin 21.2±10.1, body mass index 26.3±5.6 and MELD score 33±7.5. Etiology of ALF was only available for the later period and is presented in Fig. 1. The majority of the cases were due to indeterminate causes involving 103 (28%) patients. Autoimmune hepatitis was the second most common etiology accounting for 92 (25%) of the cases. Acute viral hepatitis infection was diagnosed in 70 (19%) patients. Among these patients, 64 (18% of the studied population in the later period) had an acute HBV infection, 4 (1%) patients had acute HAV and 2 (0.5%) patients had acute hepatitis C infection. No cases of ALF due to hepatitis D or E were identified, as these viruses are not routinely evaluated. Drug or toxic reactions were described in 53 (15%) patients with only 2 patients presenting paracetamol intoxication. In 34 (9%) patients the etiology was not specified.
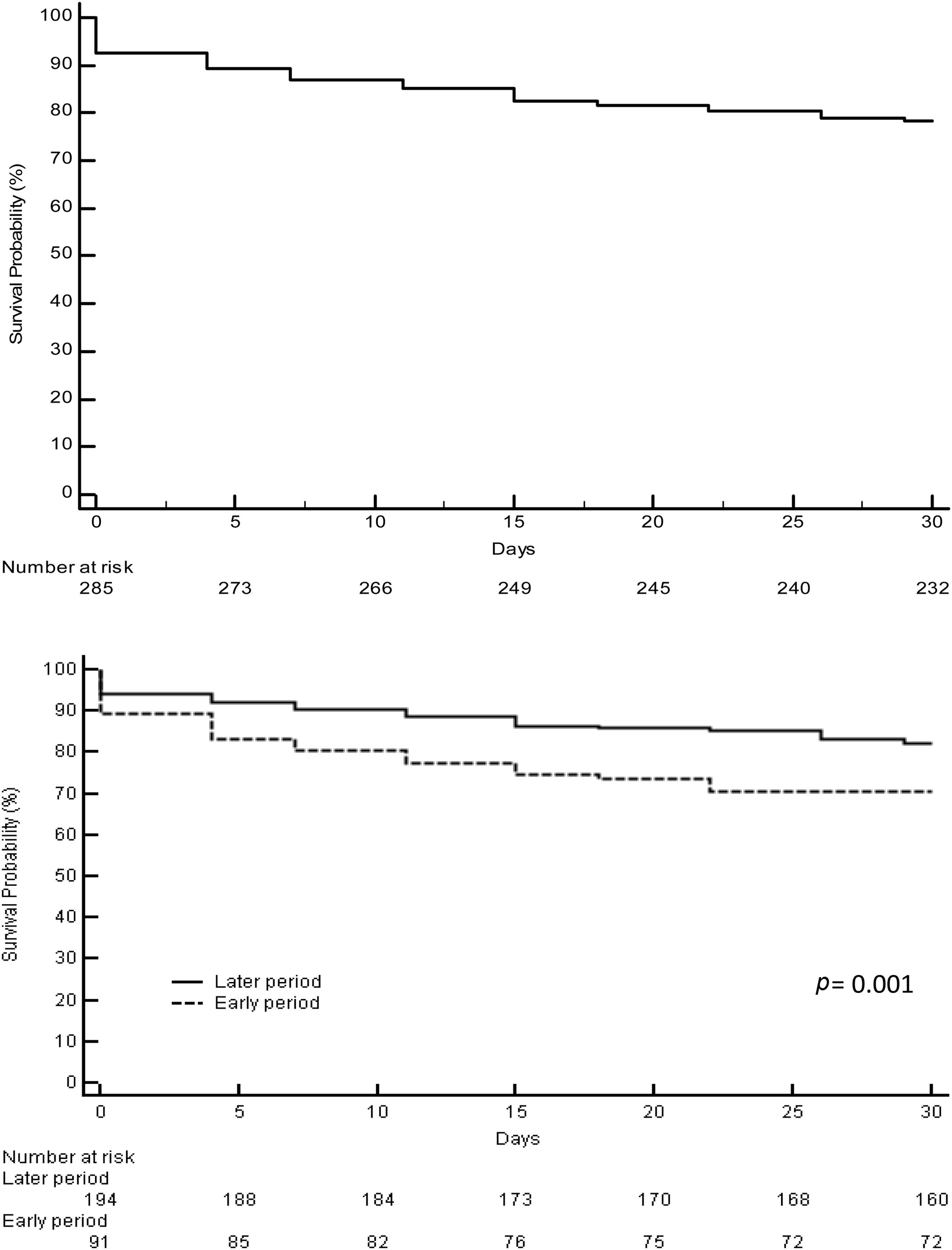
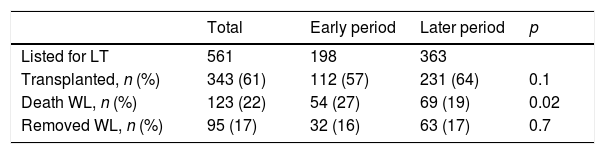
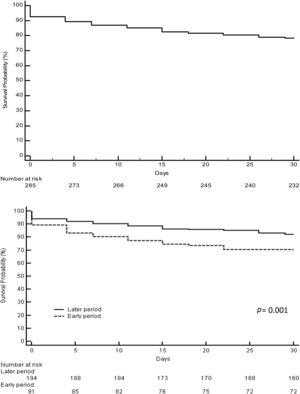
3.2Acute liver failure outcomesOverall, 343 (61%) patients underwent OLT. When comparing the early and later periods there was a reduction in wait-list mortality from 27% to 19% (p=0.02) and an increased access to OLT from 57% to 64% but this was not statistically significant (Table 1). The median time from inclusion in the waiting list to transplantation was 3.5 (IQR 3–4) days and 2 (IQR 2–3) days for the early and later periods, respectively (p<0.001). In the later period, we analyzed time on the waiting list in those patients who finally underwent OLT. Patients with acute viral hepatitis infection presented a significant shorter time to OLT (46 patients, median 2 days [IQR 1–3], p=0.02) when compared to other etiologies; indeterminate (56 patients, median 2 days [IQR 1–4]), autoimmune (67 patients, median 3 days [IQR 3–5]) and others (47 patients, median 2 days [IQR 2–3]). However, this was not associated with worse survival after OLT. Cold ischemia time was significantly different when comparing ALF with other etiologies (mean 6.8h±1.8 vs. 7.6h±2.4, p<0.0001; respectively). Post-transplant survival rates improved between the early and later periods (1-month 70% vs. 82%, p=0.001 and 1-year 67% vs. 76%, p=0.01; respectively) (Fig. 2a and b). During the later period causes of death from ALF were as follows: multiorgan failure 26 (38%) patients, sepsis or infection 7 (10%), neurologic cause 8 (12%) and multifactorial causes 2 (3%). In 26 (38%) cases the cause of death was unspecified or unknown. After 1-year following liver transplantation 63 (27%) patients died. The causes of death were, sepsis or infection 27 (43%) of the cases, multifactorial causes 13 (21%), multiorgan failure 12 (19%), neurologic cause 3 (5%) and it was not specified in 8 (13%) patients.
Outcomes of patients with acute liver failure comparing early and later periods.
| Total | Early period | Later period | p | |
|---|---|---|---|---|
| Listed for LT | 561 | 198 | 363 | |
| Transplanted, n (%) | 343 (61) | 112 (57) | 231 (64) | 0.1 |
| Death WL, n (%) | 123 (22) | 54 (27) | 69 (19) | 0.02 |
| Removed WL, n (%) | 95 (17) | 32 (16) | 63 (17) | 0.7 |
Abbreviations: LT, liver transplantation; WL, waiting list.
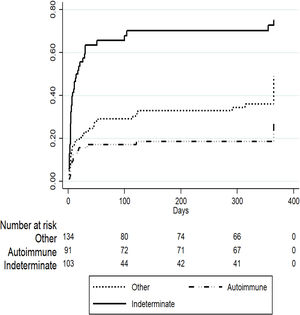
To evaluate predictors of mortality in patients listed for ALF in the later period we performed a competing risk regression analysis considering OLT as the competing event. Table 2 describes the final model where indeterminate etiology of ALF was independently associated with death (SHR 1.63 [95% CI 1.06–2.51], p=0.02) after adjusting for other covariates including age, gender, private or public hospital, INR, creatinine and total bilirubin. On the other hand, patients listed for acute presentation of autoimmune hepatitis presented a trend to improved survival (SHR 0.61 [95% CI 0.36–1.05], p=0.07), however this was not statistically significant (Fig. 3).
Competing risk regression analysis: cumulative incidence of mortality in patients with acute liver failure in the later period (n=363). Competing event: liver transplantation.
| Variables | Adjusted SHR | Standard error | 95% CI | p value |
|---|---|---|---|---|
| Etiology | ||||
| Other (n=168) | [ref] | |||
| Autoimmune (n=92) | 0.61 | 0.17 | 0.36–1.05 | 0.07 |
| Indeterminate (n=103) | 1.63 | 0.36 | 1.06–2.51 | 0.02 |
| INR | 1.06 | 0.34 | 0.99–1.13 | 0.05 |
| Total bilirubin | 0.98 | 0.01 | 0.97–1.01 | 0.2 |
| Creatinine | 0.89 | 0.10 | 0.71–1.12 | 0.3 |
Abbreviations: CI, confidence interval; INR, international normalized ratio; SHR, subhazard ratio.
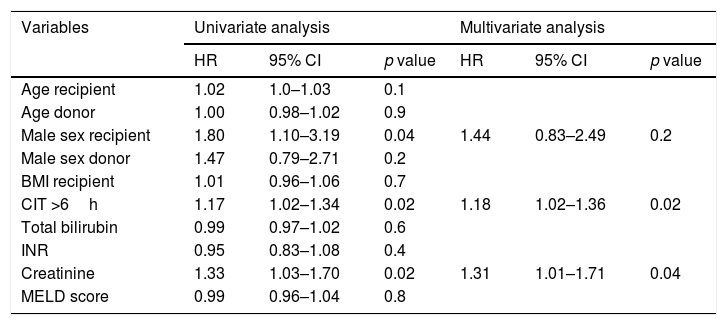
Cox proportional hazard regression analysis comparing the post-transplant survival of patients who underwent transplantation for ALF was only performed in the later period group. Significant 1-year univariate risk factors for post-OLT mortality were male sex (HR 1.80, 95% CI 1.01–3.19; p=0.04), cold ischemia time >6h (HR 1.17, 95% CI 1.02–1.34; p=0.02) and creatinine (HR 1.33, 95% CI 1.03–1.70; p=0.02). However, on multivariate analysis, only cold ischemia time >6h (HR 1.18, 95% CI 1.02–1.36; p=0.02) and creatinine (HR 1.31, 1.31–1.71; p=0.04) remained statistically significant (Table 3).
Cox proportional hazard models for predictors of mortality 1-year after liver transplantation in patients with acute liver failure (N=231).
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age recipient | 1.02 | 1.0–1.03 | 0.1 | |||
| Age donor | 1.00 | 0.98–1.02 | 0.9 | |||
| Male sex recipient | 1.80 | 1.10–3.19 | 0.04 | 1.44 | 0.83–2.49 | 0.2 |
| Male sex donor | 1.47 | 0.79–2.71 | 0.2 | |||
| BMI recipient | 1.01 | 0.96–1.06 | 0.7 | |||
| CIT >6h | 1.17 | 1.02–1.34 | 0.02 | 1.18 | 1.02–1.36 | 0.02 |
| Total bilirubin | 0.99 | 0.97–1.02 | 0.6 | |||
| INR | 0.95 | 0.83–1.08 | 0.4 | |||
| Creatinine | 1.33 | 1.03–1.70 | 0.02 | 1.31 | 1.01–1.71 | 0.04 |
| MELD score | 0.99 | 0.96–1.04 | 0.8 | |||
Abbreviations: BMI, body mass index; CIT, cold ischemia time; INR, international normalized ratio; MELD, model for end-stage liver disease.
We used the INCUCAI database to analyze the etiologies and outcomes solely of patients listed for ALF. To the best of our knowledge, this is the largest published cohort of patients listed for ALF in Latin America, and gives a comprehensive overview of the evolution and outcomes of ALF in Argentina. The main etiologies of ALF in Argentina were indeterminate and autoimmune hepatitis. We described that survival of patients with ALF on the waiting list and after OLT has significantly improved over the last years. After performing a competing risk analysis considering OLT as the competing event we found that indeterminate cause of ALF was associated with worse outcome and possible autoimmune hepatitis with might be associated with improved survival. In those who underwent OLT, we identified prolonged cold ischemia time and serum creatinine to be independently associated with poor outcomes.
It is important that physicians taking care of these complex patients know detailed ALF epidemiology in their regions in order to allow a prompt and proper diagnosis and a better management of their patients [12]. We confirm previous reports regarding the low incidence of paracetamol-related ALF in our country, accounting for only 0.5% of cases [6,13]. These results clearly contrast with those reported by the ALFSG and King's College were almost half of the patients presented paracetamol-toxicity [2,3]. Our data showed that indeterminate or cryptogenic cause is the major etiology in Argentina accounting for 28% of cases, similar findings to those reported in Spain (32%) and Germany (24%) but inferior to those described by the European Liver Transplant Registry (43%) [4,14,15]. Efforts should be made to ensure that the etiology is correctly diagnosed in a timely fashion using measures beyond the usual autoimmune and hepatitis serologies. In this sense, the use of paracetamol-cys adducts measurements to confirm or deny that paracetamol is the cause of liver injury might not be necessary in our area. On the other hand, hepatitis E was not routinely evaluated, so the real prevalence of this infection remains unknown. Correct diagnosis of patients with fulminant presentation of autoimmune hepatitis is challenging. These patients often have elevated γ-globulin levels and positive autoantibodies, but these can be absent in a proportion of cases [1,16]. Autoimmune hepatitis is a common cause of end-stage liver disease and ALF in Argentina [6,17]. In accordance with these reports, autoimmune hepatitis was one of the most prevalent etiologies of ALF in our study. Patients with autoimmune hepatitis presented with better spontaneous survival when compare to indeterminate causes. This interesting finding could be associated to the fact that autoimmune hepatitis can benefit from the early use of corticosteroids. Whilst the risk and benefits of corticosteroid administration in this scenario should be weighed carefully, early diagnosis and treatment of these patients might preclude OLT or death [18,19].
Identifying patients with ALF at risk of death without OLT is the most challenging assessment in this setting. Multiple prognostic systems have been developed to aid decision making for OLT. In our study we solely focused on patients with ALF who were listed. Thus, using traditional ALF prognostic scores in this population to identify patients who will benefit from OLT can represent a bias. To gain a better understanding of factors associated with death in our cohort we performed a competing risk regression analysis including OLT as the competing event. In the final model we found indeterminate cause to be strongly associated with detrimental outcome. When compared to other etiologies, patients with indeterminate cause have worse survival after being listed, independently if they were transplanted or not. We believe this is an interesting finding that will help transplant teams to specially focus on these patients, not only regarding medical support but also to make all the possible efforts to try to identify the etiology. Recently, the ALFSG described the evolution of patients listed for ALF and stratified the outcomes by their four main etiologies [20]. Despite a great proportion of the ALFSG patients presented paracetamol toxicity they reported that patients with non-paracetamol intoxication were more likely to receive a transplant and less likely to die without transplant. This is in line with our findings. Patients with autoimmune hepatitis presented a trend toward better survival. As aforementioned, we speculate this is a consequence of a more indolent presentation characterized by a prolonged interval between jaundice and encephalopathy allowing physicians to install medical therapies (corticosteroids) and to eventually be transplanted. The risk in this scenario is that patients with autoimmune hepatitis can be “overtransplanted” given the longer time they can remain on the waiting list.
Recipient and donor factors associated with poor post-OLT outcomes are similar in transplants performed electively or secondary to ALF. In the ALF setting, variables better predicting survival without transplant are not the same as those predicting survival after OLT. Recipient clinical condition and graft quality directly impact the outcome of transplantation. Intensive care support, increased age and BMI are well-identified factors associated with poor outcome after OLT for ALF [4,21,22]. In our series, prolonged cold ischemia time and creatinine were independent factors associated with reduced survival after OLT. Prolonged cold ischemia time is a well-established risk factor included in the donor risk index for graft failure [23]. Ischemic injury damages the liver graft at the cellular level and may lead to primary nonfunction, ischemic cholangiopathy and delayed graft function. Each additional hour of cold ischemia time is associated with an additional 1% increased risk of graft loss [23]. On the other hand, renal dysfunction is an indicator of multisystemic disorder triggered by ALF, as well marked activation of systemic inflammatory response that can extend into the posttransplant period. We acknowledge that relevant variables such as encephalopathy grade, vasopressors or ventilatory support are missing in the model. Thus, we recommend careful interpretations of our results given that many confounders were not adjusted in our analysis.
As with all registry-based observational studies, the results of this study are subject to certain limitations. First, our study is retrospective and cannot account for inherent and undocumented patients characteristics. However, we checked for missing values and inconsistencies, and queries were referred to the participating institution. In addition, we only included patients listed for OLT so our results can overestimate the need of liver transplantation. Lastly, using the INCUCAI database, we are dependent on the variables and definitions present in the data set, and important information such as detailed intensive care management and hepatic encephalopathy grade are missing.
In summary, this analysis of the INCUCAI database confirms that indeterminate cause is the most frequent etiology in our population and it is associated with worse outcome irrespective of accessing OLT when compared to autoimmune hepatitis or other etiologies. We consider this information is critical to assist physicians and other healthcare providers in the clinical evaluation and therapeutic decision-making processes. Noticeably, ALF outcomes improved over time, in association with increased survival without transplantation. We speculate this improvement is possibly related to better selection of candidates, a reduction in days from transplantation listing to actual transplantation during the later period as well as improved supportive care. Future studies should focus on more accurate prognostic markers of liver regeneration and on optimizing early transplantation availability in order to prolong survival in these patients.AbbreviationsALF acute liver failure acute liver failure study group hepatitis A virus hepatitis B virus model for end-stage liver disease orthotopic liver transplantation subhazard ratio
The authors have no conflicts of interest to declare.
The authors would like to thank the Academic Department of the Austral University School of Medicine for their assistance in editing this paper, specially to Matias Ricardo Tisi Baña and Martin O’Flaherty for their critical analysis and thoughtful comments.