Background and aims. The histologic hallmarks of chronic HCV include inflammation and fibrosis. The impact of interferon therapy on liver histology was evaluated.
Material and methods. The study population consisted of 348 patients with chronic HCV who underwent a baseline liver biopsy, received either no treatment or a single course of interferon based therapy, were followed for 5 years without any treatment or additional treatment and then underwent a repeat liver biopsy. The patients were divided into 3 groups; deferred treatment (NoTx = 47), received interferon based therapy but failed to achieve SVR (NoSVR = 189) and achieved SVR (SVR = 112).
Results. Patients with NoTx and NoSVR had significant increases in mean inflammation scores (from 4.3 to 6.3 and 5.4 to 6.7 respectively; p < 0.001 for both) and fibrosis scores (from 0.9 to 1.8 and 1.9 to 2.5; p < 0.001 for both). The amounts by which inflammation, fibrosis and rate of fibrosis progression increased were not significantly different between the two groups. Increases in total inflammation and the piecemeal necrosis sub-score over time were strongly associated with fibrosis progression. Patients with SVR had a significant decline in mean inflammation and fibrosis scores (from 6.7 to 2.2 and 3.3 to 1.8; p < 0.001 for both); 40% of patients resolved all fibrosis and 50% of patients resolved cirrhosis.
Conclusion. Increases in inflammation are associated with fibrosis progression and in the absence of SVR interferon treatment does not appear to affect the long term natural history of this process. Patients with SVR have resolution of inflammation and fibrosis and many resolve cirrhosis.
The progression of hepatic fibrosis to cirrhosis in patients with chronic hepatitis C virus (HCV) is a highly variable process which occurs over decades.1–4 Several clinical features have been shown to affect the rate of fibrosis progression including age at the time HCV is acquired,1,4 the serum level of liver transaminases,3,4 co-infection with human immune deficiency virus,4,5 co-existent liver disorders such non-alcoholic fatty liver disease,4,6,7 insulin resistance7 and the excessive use of alcohol.1,4,8 Specific polymorphisms within several host genes in patients with HCV have been associated with advanced fibrosis and cirrhosis suggesting that host genetics plays an important role in this process.9,10 The severity of hepatic inflammation, especially the piecemeal necrosis component is also associated with fibrosis progression.11–13
Interferon based therapy has been the primary treatment for patients with HCV for nearly 2 decades.14–16 Therapy has evolved rapidly since 2011 and it now appears unlikely that interferon and ribavirin will continue to be utilized to treat HCV in the future.17 However, understanding how interferon based therapy impacts hepatic inflammation and fibrosis in patients with HCV can be applied to future non-interferon based treatments. Approximately 80% of all patients treated with peginterferon have a virologic response and approximately half become HCV RNA undetectable during treatment. Several studies have demonstrated that a virologic response is associated with a decline in hepatic inflammation.13,14,16,18,19 Although many of these studies have suggested that this may slow the rate of fibrosis progression a recent study has suggested that fibrosis progression may actually be accelerated in patients who fail interferon based therapy.20 The long term impact of interferon therapy on liver histology in patients who do not achieve a SVR remains undefined. In contrast, achieving a SVR is associated with eradication of HCV, a reduction in hepatic fibrosis and a reduced risk for developing hepatic decompensation and hepatocellular carcinoma.14,16,19,21–24 In a recent study which monitored patients by fibroscan and serum fibrosis markers over 10 years 49% of patients had fibrosis regression and 56% resolved cirrhosis.25
The current study was performed to help understand how changes in inflammation affected fibrosis progression in patients with ongoing chronic HCV and fibrosis regression in patients who achieved SVR.
Material and MethodsPatient populationAll patients had chronic HCV, underwent a baseline liver biopsy, were subsequently treated with a single course of either standard or pegylated interferon alfa-2a or alfa-2b with or without ribavirin between 1991-2004 or deferred treatment, received no additional treatment for chronic HCV and underwent a repeat liver biopsy at least 5 years after the initial biopsy. The last patient to be included in this series underwent liver biopsy in 2009. Patients were excluded if they had any other cause of chronic liver disease in addition to chronic HCV based upon serologic studies and liver histology with the exception of benign steatosis and mild-moderate non-alcoholic fatty liver disease;26 were anti-HIV positive. Patients were also excluded if they opted to receive treatment or retreatment for HCV with either interferon, peginterferon (with or without ribavirin), or any other agent being investigated as a possible treatment for chronic HCV during the 5 year follow-up period. Other patients were excluded if they developed recurrence of HCV RNA in serum after achieving SVR (HCV RNA undetectable 6 months after completing treatment); if they developed chronic renal failure and required hemodialysis; developed a complication of cirrhosis including variceal hemorrhage, ascites, hepatic encephalopathy or hepatocellular carcinoma; or if they received an organ transplant during the 5 year follow-up and before undergoing the second liver biopsy. Patients with no fibrosis on the initial liver biopsy specimen who achieved SVR were not included because there was no reason for these patients to undergo a second liver biopsy. The use of over the counter herbal or other supplements was permitted.
During the 5 years or more of follow-up patients were monitored at regular intervals. The frequency of follow-up ranged from every 3-12 months based upon the degree of fibrosis present on the initial liver biopsy and the virologic response to the initial treatment. This included patients who achieved an SVR. At each of these visits routine liver chemistries and hematology were performed. Patients with advanced fibrosis or cirrhosis had an ultrasound and alfa-fetoprotein performed at periodic intervals to screen for hepatocellular carcinoma. Patients with SVR also had serum HCV RNA assayed at least once yearly to ensure they remained HCV RNA undetectable in serum. During the early years of the study this was performed by the Roche Amplicor polymerase chain reaction assay (PCR). By the end of the study HCV RNA was assayed by Taqman PCR with a lower limit of detection of 10 IU/ml. In all patients with SVR the most recent specimen(s) were assessed for HCV RNA by Taqman PCR.
The patients were grouped into three cohorts as follows:
- •
Patients who deferred treatment after the initial liver biopsy (NoTx).
- •
Patients who received a single course of interferon based therapy but failed to achieve SVR (NoS-VR); and
- •
Patients who achieved SVR following the initial course of HCV treatment with the exception of those who had no fibrosis on the initial liver biopsy (SVR).
All liver biopsy slides were read in real time by a dedicated liver pathologists (MJC) who was blinded to the clinical, biochemical and virologic status of each patient. The stained slides of any liver biopsy which had been performed at an outside facility were obtained and also scored by this same pathologist. None of the slides were reread specifically for this study. All histologic specimens were scored for the degree of inflammation according the method of Knodell, et al.27 Fibrosis scores were assigned based upon the method of Knodell prior to 2000 and by both Knodell and Ishak, et al.28 thereafter. Fibrosis scores assigned by the Knodell method only were converted to Ishak scores as follows: no fibrosis = 0 by both systems; a portal fibrosis score of 1 by Knodell was assigned a value of 1.5 for Ishak (mid-way between the scores for portal fibrosis [1-2] with Ishak); a bridging fibrosis score of 3 by Knodell was assigned a value of 3.5 for Ishak (mid-way between the scores for bridging fibrosis [3-4] with Ishak); and a score of 4 for cirrhosis by Knodell was assigned an Ishak value of 5.5 (mid-way between the scores for cirrhosis [5-6] with Ishak). This modification affected only 86/716 (12%) biopsies. The conversion from Knodell to Ishak scores was validated in 183 biopsies scored for fibrosis by both methods. The true Ishak score of these specimens was 1.39. The assigned Ishak score was 1.38; CI -0.37-0.075; r = 0.977 (p < 0.0001). The AST-platelet ratio index (APRI) was calculated as described by Wai, et al.29
Study designThe study was originally conceived in the mid-1990s to be a prospective analysis to evaluate the effects of interferon therapy on liver histology. The study was approved by the Investigational Review Board (IRB) at the Virginia Commonwealth University Medical Center and informed consent was obtained from all patients either prior to undergoing the initial liver biopsy or initiating treatment. Over the next several years many patients who enrolled in the study, had failed to achieve an SVR after receiving interferon therapy and were to be followed prospectively for 5 years without retreatment dropped out of the study so they could be retreated with newer and/or investigational agents. In addition, many other patients who were being followed in the Hepatology clinical practice but had not enrolled in the study prospectively underwent a repeat liver biopsy after 5 years of follow-up as part of their clinical care to determine if they had developed fibrosis progression and should either receive an initial course of interferon based therapy or be retreated for chronic HCV. It became apparent that a prospective study could not be conducted to answer this question at our Center because of the manner in which we clinically managed HCV during this time. The protocol was therefore amended to a retrospective longitudinal cohort analysis. This change was approved by the IRB and allowed all patients who had undergone two liver biopsies at least 5 years apart and satisfied all inclusion and exclusion criteria to be included in this analysis.
Statistical analysisDemographic, clinical, laboratory and histologic data are presented as mean and standard deviation for continuous variables and proportions for categorical data as indicated. Differences in continuous variables were compared by two-tailed Student’s t-test and analysis of variance (ANOVA) as appropriate. Differences in categorical variables were compared by the χ2 test. The main outcomes were changes in inflammation and fibrosis between the groups. The rate of fibrosis progression/regression was defined as the change in Ishak fibrosis units/ year. Univariate regression analysis was utilized to assess for factors associated with fibrosis progression. Variables included were gender, race, age, serum ALT, serum HCV RNA, the baseline total inflammation score and the sub-scores for the various components of inflammation and the fibrosis score. Factors found on univariate analysis to be statistically significant (p < 0.05) were then evaluated with multiple logistic regression analysis utilizing MedCalc software.
ResultsBaseline characteristicsThe demographic, biochemical, virologic and histologic features of the patients at baseline are summarized in table 1. Forty-seven patients did not receive HCV treatment, 189 were treated with interferon or peginterferon with or without ribavirin but failed to achieve a SVR and 122 patients achieved a SVR. The mean age for all patients was 45 years, 65% were male and 65% where Caucasian. The time between the two liver biopsies for each of the three groups differed by less than 1 year.
Demographic, biochemical, hematologic, virologie and histologic findings at baseline.
| No treatment | No SVR | SVR | |
|---|---|---|---|
| N | 47 | 189 | 122 |
| Age (years) | 44 ± 9 * | 45 ± 8 | 45 ± 6 |
| Sex (% male) | 66% † | 64% | 66% |
| Race (% Caucasian) | 57% | 60% | 84% |
| AST (IU/L) | 67 ± 54 | 83 ± 70 | 93 ± 64 |
| ALT (IU/L) | 83 ± 60 | 115 ± 92 | 134 ± 92 |
| WBC (/cc3) | 6.8 ± 2.3 | 6.6 ± 2.2 | 6.7 ± 2.6 |
| Platelets (/cc3) | 245 ± 98 | 206 ± 59 | 194 ± 74 |
| Genotype 1 (%) | 95% | 89% | 74% |
| Log HCV RNA (IU/mL) | 5.7 ± 0.7 | 5.7 ± 0.5 | 5.6 ± 1.0 |
| Inflammation Score | 4.3 ± 2.1 | 5.4 ± 1.9 | 6.7 ± 2.4 |
| Fibrosis Score | 0.7 ± 0.8 | 1.7 ± 1.4 | 2.6 ± 1.1 |
| No fibrosis (%) | 47% | 21% | 0% |
| Portal fibrosis (%) | 47% | 37% | 28% |
| Bridging fibrosis (%) | 6% | 32% | 56% |
| Cirrhosis (%) | 0% | 10% | 16% |
| Years between biopsies | 5.9 ± 1.5 | 6.1 ± 1.7 | 6.8 ± 1.1 |
The impact of interferon therapy on fibrosis progression was assessed by comparing patients who deferred treatment to those who received treatment but failed to achieve SVR. Since no patients in the “No treatment” group had cirrhosis, the 20 patients with cirrhosis in the “No SVR” group were excluded from these calculations. Figure 1 illustrates the change in mean inflammation and mean fibrosis scores over time in these two patient groups. In patients without treatment mean inflammation score increased significantly from 4.3 ± 2.1 to 6.3 ± 2.5 (p< 0.0001). In patients who received prior therapy the inflammation score also increased significantly from 5.4 ± 1.9 to 6.1 ± 2.5 (p < 0.0001). Although the mean change in inflammation scores were somewhat greater in patients without prior treatment (1.9 ± 2.1 vs. 1.3 ± 2.9), these differences were not significant (p = 0.23). Fibrosis scores also increased significantly over time in both patient groups; from 0.9 ± 1.0 to 1.8 ± 1.1 (p = 0.0001) and 1.9 ± 1.4 to 2.5 ± 1.1 (p < 0.0001) respectively. The mean change in fibrosis scores were greater in patients without prior treatment compared to patients without SVR (0.1 ± 1.1 vs. 0.4 ± 1.3 respectively). However, this difference was not significant (p = 0.15). Although the mean rate of fibrosis progression (Ishak units/year) was greater in patients without prior treatment this difference was also not significant (0.13 ± 0.21 vs. 0.01 ± 0.21; p = 0.22).
Change in mean inflammation score (left panel) and mean fibrosis score (right panel) over 5 years in patients with chronic HCV. Patients are grouped according to whether they received no treatment (No Tx) for chronic HCV or interferon based therapy without SVR (No SVR). Vertical lines on the top of each bar represent standard deviation. An asterisk indicates the change between the initial and follow-up liver biopsies was statistically significant. P values are provided in the text.
The change in fibrosis over 5 years was assessed by comparing patients with the same degree of fibrosis at baseline (Figure 2). In this analysis patients were grouped into 4 categories based upon fibrosis scores; either none (Ishak score of 0), portal (score of 1 and 2), bridging (score of 3 and 4) or cirrhosis (score of 5 and 6). In patients with no fibrosis at baseline (Figure 2A) 59% of those without prior treatment had an increase in fibrosis; 50% progressed to portal fibrosis, 5% bridging fibrosis and 4% to cirrhosis. A similar pattern of fibrosis progression was observed in patients who failed to achieve SVR; 48% progressed to portal fibrosis, 16% bridging fibrosis and 3% to cirrhosis.
Percentage of patients with varying degrees of fibrosis on the second liver biopsy compared to the initial liver biopsy. Patients either deferred treatment (No Tx) or received interferon therapy but failed to achieve a SVR (No SVR). All patients were followed for a minimum of 5 years before they underwent repeat liver biopsy. A. Patients with no fibrosis on the initial liver biopsy. B. Patients with portal fibrosis on the initial liver biopsy.
The change in liver fibrosis over 5 years in patients with portal fibrosis at baseline is illustrated in figure 2B. Fibrosis progression was also similar in patients who deferred treatment and in those who failed to achieve SVR; 32 and 25% of patients respectively progressed to bridging fibrosis or cirrhosis.
Table 2 compares various features from patients in the “No treatment” and “No SVR” groups based upon whether they developed fibrosis progression or not. Progression was observed in 86/ 236 patients (36%). Factors associated with fibrosis progression on univariate analysis included the baseline piecemeal necrosis score, and an increase in the total inflammation and piecemeal necrosis scores over time. Multiple logistic regression analysis identified only an increase in the piecemeal necrosis and total inflammation scores as being independently associated with fibrosis progression.
Factors associated with fibrosis progression in patients with chronic HCV.
| No progression | Progression | p‡ | |
|---|---|---|---|
| N | 150 | 86 | |
| Age (years) | 45 ± 8* | 44 ± 8 | NS |
| Sex (% male) | 64% † | 65% | NS |
| Race (% Caucasian) | 60% | 59% | NS |
| Interferon treatment | 83% | 74% | NS |
| AST (IU/L) | 80 ± 60 | 81 ± 79 | NS |
| ALT (IU/L) | 105 ± 83 | 115 ± 96 | NS |
| Log HCV RNA (IU/mL) | 5.7 ± 0.6 | 5.7 ± 0.5 | NS |
| Inflammation score | 5.2 ± 1.9 | 5.3 ± 0.2 | NS |
| PMN score | 1.0 ± 0.9 | 1.3 ± 1.0 | 0.016 |
| Change in: | |||
| Inflammation score | 1.0 ± 2.6 | 2.2 ± 3.0 | 0.003 |
| PMN score | 0.7 ± 1.3 | 1.4 ± 1.5 | 0.0001 |
| AST (IU/L) | −6 ± 70 | −10 ± 85 | NS |
| ALT (IU/L) | −4 ± 106 | −18 ± 103 | NS |
| Log HCV RNA (IU/mL) | 0.21 ± 1.01 | 0.11 ± 0.72 | NS |
SVR was confirmed in 122 patients by assessing serum for HCV RNA once yearly for at least consecutive 5 years after discontinuing treatment. All patients were repeatedly HCV RNA undetectable. The mean serum AST and ALT in patients who achieved SVR declined significantly (p < 0.0001) from 93 ± 64 and 134 ± 92 to 31 ± 18 and 34 ± 16 respectively. Figure 3 illustrates the inflammation and fibrosis scores at baseline and 5 years after achieving SVR. The inflammation score declined from 6.7 ± 2.4 to 2.2 ± 2.2 (p < 0.0001) and fibrosis score from 3.3 ± 1.3 to 1.8 ± 1.9 (p < 0.0001). Fibrosis regression occurred at a mean of -0.23 Ishak units/year. Of the 122 patients with SVR 16 (63%) had regression in fibrosis by 1 or more stage, 40 (33%) had no change in fibrosis stage and 6 (1%) had more fibrosis 5 years after they had achieved SVR. In the 34 patients with portal fibrosis at baseline 11% had no evidence of fibrosis 5 years after achieving SVR. In the 68 patients with bridging fibrosis at baseline 35% had no fibrosis and 26% portal fibrosis 5 years after achieving SVR. Of the 20 patients with cirrhosis on the baseline biopsy, 2 had no fibrosis, 2 portal fibrosis and 6 bridging fibrosis 5 years after achieving SVR (Figure 4).
Change in mean inflammation score (left panel) and mean fibrosis score (right panel) over 5 years in patients who achieved an SVR following treatment with interferon based therapy. Vertical lines on the top of each bar represent standard deviation. An asterisk indicates that the change between the initial and follow-up liver biopsies was statistically significant. P values are provided in the text.
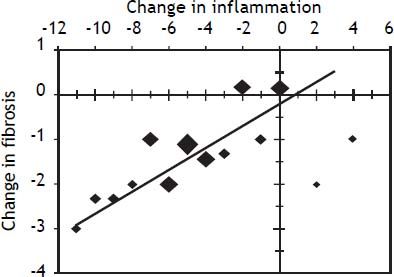
The relationship between the change in inflammation and the change in fibrosis for patients with SVR is illustrated in figure 5. A decline in the inflammation score was associated with a linear decline in hepatic fibrosis. Seventeen percent (20/122) of patients either had no change or an increase of 1-4 points (6 patients) in the hepatic inflammation score after being HCV RNA undetectable in serum for at least 5 years. Only 4 of these 20 patients had a decline in the fibrosis score. No baseline features differentiated patients who failed to have a decline in hepatic inflammation from those that did have a decline in inflammation after achieving SVR. Specifically, there were no significant differences in baseline serum ALT, serum HCV RNA level, the total inflammation score, piecemeal necrosis score or fibrosis score. All patients who failed to have a decline in inflammation also remained persistently HCV RNA undetectable in serum by Taqman PCR.
Relationship between inflammation and fibrosis scores for patients who achieved an SVR. Patients were initially grouped according to the change in inflammation score (inflammation score on repeat liver biopsy - inflammation score on initial liver biopsy). Each point represents the mean change in fibrosis score for each change in inflammation score. The size of each point is proportional the number of patients with that degree of change in inflammation score. The line presents the calculated linear regression fit for the data. The equation of the regression line was: Y = 0.20X - 0.27 (p < 0.001).
Table 3 compares markers of fibrosis in patients with fibrosis progression, no progression and in patients with SVR. Patients with fibrosis progression had a slight decline in the platelet count, patients with no change in fibrosis had no change in platelets and patients with SVR had a significant (p < 0.01) increase in the platelet count. Similar changes were noted in the APRI.
Impact of sustained virologic response on platelet count and APRI.
| Baseline | 5 years | P | |
|---|---|---|---|
| Platelet count | |||
| Progression | 217 ± 54* | 188 ± 75 | 0.0001 |
| No progression | 212 ± 78 | 212 ± 76 | |
| SVR | 194 ± 74 | 230 ± 85 | |
| APRI index | |||
| Progression | 0.80 ± 1.03 | 1.11 ± 1.20 | 0.0001 |
| No progression | 0.93 ± 0.22 | 0.96 ± 1.03 | |
| SVR | 1.15 ± 1.02 | 0.33 ± 0.31 |
The results of this study have demonstrated that the vast majority of patients with chronic HCV who achieve SVR and remain HCV RNA undetectable in serum over many years have regression in fibrosis. Overall, about 40% of patients had complete loss of fibrosis and 50% no longer had evidence of cirrhosis 5 years after achieving SVR. Significant improvements in non-invasive markers of fibrosis, such as platelet count and APRI were also observed in patients with SVR. The degree of fibrosis improvement was closely linked to changes in the inflammation score. A long term improvement in both inflammation and fibrosis has also been observed in patients who achieved SVR in a previous study.30
The current study also evaluated the impact of interferon based therapy in patients with chronic HCV who failed to achieve SVR. Previous studies suggested that these patients also derive histologic benefit and this may reduce the rate of fibrosis progression.13,14,16,18,19 However, in many of these studies the repeat liver biopsy was performed less than 1 year after discontinuing interferon treatment. None of the previous studies compared the histologic impact of interferon to a control group of untreated patients. In the HALT-C study, over 800 patients with advanced fibrosis or cirrhosis underwent 2-3 liver biopsies over 4-5 years.31,32 No significant reduction in fibrosis progression was observed even in patients who had profound virologic suppression during treatment with full-dose peginterferon during the lead-in phase and with low-dose peginterferon maintenance therapy.32,33 Similar results were observed when a more sensitive technique, morphometric analysis, was utilized to quantitate tissue collagen.34 The unique aspect of the present study was that changes in liver histology in patients who failed to achieve SVR were compared to patients who deferred HCV treatment. The rate of fibrosis progression observed in the NoTx group, 0.12 Ishak units/year, was very similar to that reported for untreated patients in previous studies,1,11,12,35 and similar to that observed in patients with “No SVR”. Thus, although short term improvements in liver histology may be achieved following interferon based therapy in patients without SVR it is unlikely that a single course of HCV treatment significantly impacts the long term natural history of fibrosis progression in the absence of SVR.
The primary limitation of this study was that patients could not be matched for baseline characteristics. Patients with no fibrosis who achieved SVR could not be included because there was no reason for these patients to undergoing a repeat liver biopsy. In addition, patients with more fibrosis rarely deferred treatment or opted for retreatment if they failed to achieve an SVR. This explains why the “SVR” and “No SVR” groups had significantly higher fibrosis scores at baseline than the “No Tx” group (Table 1). As a result, we only evaluated fibrosis progression in patients with the same degree of fibrosis at baseline (Figure 2). Another limitation was that biopsies were not rescored and 2 different systems were utilized during the nearly 15 years required to conduct this study. However, all biopsies were scored by the same pathologist during the entire study and the system utilized to convert Knodell to Ishak fibrosis scores was internally validated and tightly correlated.
Liver injury in chronic HCV is composed of two processes; inflammation and fibrosis. Several studies now strongly suggest that inflammation is the driving force for fibrosis progression in patients with chronic HCV. Inflammation increases with increasing fibrosis in chronic HCV11,12,36 and fibrosis progression is strongly associated with higher inflammation scores.11,12,36,37 The piecemeal necrosis subscore appears to be the most important part of the inflammation score leading to fibrosis progression.12 Similar results were observed in the current study. Why inflammation increases in some patients with chronic HCV but remains stable in others remains undefined. Most likely, this is secondary to host immunologic factors and their response to HCV.38 Specific genetic polymorphisms may be associated with fibrosis progression because they affect the host immunologic response to HCV and the level of inflammation.9,10 This possibility is worthy of additional studies.
Previous studies in other liver disorders have demonstrated that reducing the degree of hepatic inflammation can either lead to regression of fibrosis or delay fibrosis progression. Anti-viral therapy in patients with chronic hepatitis B virus,39,40 and suppressing the immune response in patients with autoimmune hepatitis41,42 are both associated with a reduction in inflammation and fibrosis. Suppressing inflammation with maintenance interferon has been shown to prevent fibrosis progression.43 Although very few patients in the HALT-C trial had suppression of HCV RNA with maintenance peginterferon, those patients in whom HCV RNA was suppressed to low or undetectable levels also had a reduction in inflammation scores and evidence of fibrosis regression.44 In the present study, a strong linear relationship was observed between the decline in inflammation and fibrosis scores in patients who achieved SVR. Patients with SVR who had no improvement in inflammation also had no improvement in fibrosis. Why some patients with SVR had no improvement in inflammation remains unclear at this time. This observation may explain why some patients who achieve SVR do not resolve cirrhosis and remain at risk for hepatocellular carcinoma.
In summary, the current study has demonstrated that in patients with chronic HCV a strong relationship exists between hepatic inflammation and fibrosis. In patients with ongoing chronic disease, increases in inflammation are associated with fibrosis progression. In contrast, achieving SVR and eradicating HCV, not just receiving interferon therapy, is associated with a marked reduction in inflammation and fibrosis regression. The future of HCV treatment is to suppress HCV with multiple oral antiviral agents without interferon and/or ribavirin. This approach has already been demonstrated to achieve high rates of SVR with various combinations of anti-viral agents.45–49 The observations of the present study strongly suggest that fibrosis regression including resolution of cirrhosis will occur in the vast majority of patients who will achieve SVR with these future therapies.
AcknowledgementsThe authors would like to acknowledge the assistance of our nursing staff (Charlotte Hofmann, Jennifer Salvatori, Denice Shelton, Paula Smith and Kim Williams), who assisted in the care of these patients for more than a decade.
Financial DisclosureThis study was not funded by any pharmaceutical company, private foundation or governmental agency.
The authors disclose the following relationships they have with various pharmaceutical companies and devise manufacturers which may be perceived as a conflict of interest with this study.
Dr. Shiffman is a consultant for GenProbe, Glaxo-Smithkline, Janssen, and Roche/Genentech; has attended advisor meetings with Achillion, Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb, Gilead, Globeimmune, Janssen, Novartis, Roche/Genentech, Merck and Vertex; has been a speaker for Boehringer-Ingelheim, GlaxoSmithKline, Gilead, Janssen, Roche/Genentech, Merck and Vertex; and has received research support from Abbvie, Anadys, Gilead, Bristol-Myers-Squibb, Globeimmune, Idenix, Intercept, Lumena, Merck, Roche/Genentech, Vertex.
Dr. Sterling has received research support from Abbvie, Boehringer-Ingelheim, Bayer, Bristol-MyersSquibb, Merck, Roche/Genentech and Vertex; and has attended advisor meetings with Abbvie, Bayer, Bristol-Myers-Squibb, Gilead, Merck, Roche/Genentech, Salix, and Vertex.
Ms Hubbard, PA has no conflicts.
Ms Long, NP has attended advisor meetings with Boehringer-Ingelheim, Bristol-Myers-Squibb, Gilead, Merck and Vertex; and has been a speaker for Gilead, Merck and Vertex.
Dr. Luketic has received research support from Abbvie, Bristol-Myers-Squibb, GenFit, Gilead, Idenix, Intercept, Lumena, Novartis, Takeda and Vertex.
Dr. Sanyal has attended advisor meetings with and has received research support from Abbvie, Bayer, Exhalenz, GenFit, Gilead, Gore, Ikaria, Intercept, Merck, Norgine, Roche/Genetech and Salix.
Drs. Contos, Fuchs and Stravitz report nothing to disclose.