Hepatic cavernous hemangioma accounts for 73% of all benign liver tumors with a frequency of 0.4-7.3% at autopsy and is the second most common tumor seen in the liver after metastases. Patients affected by hemangioma usually have their tumor diagnosed by ultrasound abdominal examination for a not well defined pain, but pain persist after treatment of the hemangioma. The causes of pain can be various gastrointestinal pathologies including cholelithiasis and peptic ulcer disease.The malignant trasformation is pratically inexistent. Different imaging modalities are used to diagnosis liver hemangioma including ultrasonography, computed tomography (CT), magnetic resonance (MR) imaging, and less frequently scintigraphy, positron-emission tomography combined with CT (PET/CT) and angiography. Imaging-guided biopsy of hemangioma is usually not resorted to except in extremely atypical cases. The right indications for surgery remain rupture, intratumoral bleeding, Kasabach-Merritt syndrome and organ or vessels compression (gastric outlet obstruction, Budd-Chiari syndrome, etc.) represents the valid indication for surgery and at the same time they are all complications of the tumor itself. The size of the tumor do not represent a valid indication for treatment. Liver hemangiomas, when indication exist, have to be treated firstly by surgery (hepatic resection or enucleation, open, laproscopic or robotic), but in the recent years other therapies like liver transplantation, radiofrequency ablation, radiotherapy, trans-arterial embolization, and chemotherapy have been applied.
Hepatic cavernous hemangioma accounts for 73% of all benign liver tumors1 with a frequency of 0.4-7.3%2 at autopsy and is the second most common tumor seen in the liver after metastases.3 These lesions consist of large vascular spaces lined by a single layer of endothelial cells and several authors agree that this vascular tumour is a benign, congenital haematoma or tissue malformation that grows slowly from birth. The pathogenesis of this tumor is not still completely understood, but abnormal vasculogenesis and angiogenesis have been speculated to be involved.4 The cavernous hemagioma endhotelial cell differ from sinosoidal endhotelial cell both derived from the human liver. Particularly the first cells exibiting more activated angiogenesis capacity and forming abnormal capillary-like structures in vitro. The grown pattern of the cavernous hemangioma in still not understood. Ectasia rather than hypertophy or hyperplasia usually is recorded.
Some hemangiomas have the receptor for the estrogens and they grown during the puberty, pregnancy (following ovarian stimulation therapy with clomiphene citrate and human chorionic gonadotrophin), or oral contapceptive use, androgen or/and steroid administration.5 These tumors are most commonly found in women, with a female: male ratio of up to 5:1, emphasizing the importance of excess of female sex hormones in these tumours.6,7 In fact enlargement and rupture of these tumors have been reported in pregnancy and in menopausal women submitted to ormonal treatment.8
The size increase have been suggested from many studies, mainly case report.9–20 The biggest study reported in literature compare 94 female patients that receiving hormonal treatment comparing with a group of patient untreated. In the treated group the increase in size was 22.7% compared with untreated patients that was only 9.7%.19 But natural hystory is not completely defined; Moser et al. reported a large family of Italian origin in which three female patients in three successive generations had large symptomatic hepatic haemangiomas without any risk factor.21
In relation to their dimension the hemangiomas are defined as normal or giant. Different mesures are present in the literature and the majority of Authors call giant the hemangioma more than 4 cm.22–26
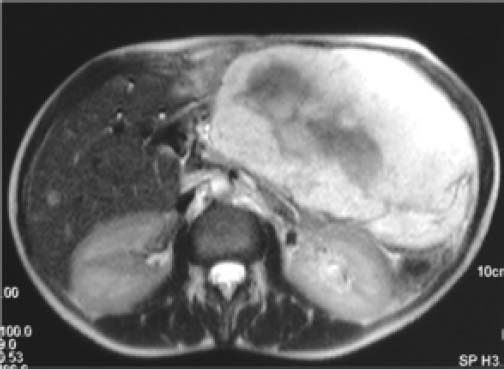
Some Authors define the hemangiomas giant after 5 cm22,27 and very small number of Authors define giant hemangiomas more than 10 cm.28 The definition of giant hemangioma should be changed to indicate a minimum size of 10 cm. It is easier to accept that a 10 cm hemangioma can cause symptoms than 4 cm tumor. For example, a 10 cm tumor located in the left lobe of the liver compresses the stomach and premature satiety after a meal is a more credible and acceptable indication for surgery (Figure 1). On the opposite it is very difficult to imagine a giant hemangioma a tumor of 4 cm, a size less than a liver segment, that can be responsible of any synptoms in any location of the liver.29
Update in Diagnostic MethodsThe importance of hemangioma, from the point of view of medical imaging comes from its relatively high incidence in comparison to other focal liver lesions, its occurrence as an incidental finding, and the need to differentiate it from other more serious focal liver lesions. The latter consideration is particularly important in two types of patients: patients with primary malignant neoplasm and patients with liver cirrhosis. In the patients with primary liver neoplasm, it is important to differentiate hemangioma from liver metastasis. In the patients with cirrhosis, it is important to differentiate it from hepatocellular carcinoma.
Different imaging modalities have been used in diagnosis of liver hemangioma including ultrasonography, computed tomography (CT), magnetic resonance (MR) imaging, and less frequently scintigraphy and positron-emission tomography combined with CT (PET/CT) and angiography. Imaging-guided biopsy of hemangioma is usually not resorted to except in extremely atypical cases. Specificity ans sensitivity of all these procedures are reported in table 1.
On ultrasonography, hemangioma appears well defined, occasionally lobulated, homogeneously hyperechoic with posterior acoustic enhancement, and frequently located close to a portal or a hepatic vein (Figure 2). In most of the cases, hemangiomas show no change of size or morphological features on follow-up ultrasonography. Most of hemangiomas have no mass effect on the liver surface or adjacent hepatic vessels. However, large hemangiomas may bulge beyond the hepatic contour and exert mass effect on adjacent structures. Occasionally, hemangioma has a slightly hypoechoic center in comparison to its peripheral rim.
Acoustic radiation force impulse (ARFI) elastography is a new adjunct to ultrasonographic examination. Some preliminary studies have shown that stiffness was higher in malignant liver lesions compared to hemangiomas and focal nodular hyperplasia with some overlap,30 while no significant difference was found between hemangioma and solid lesions on other studies.31
Pulse-Inversion Harmonic contrast-enhanced ultrasonography has also been tried for differentiation of hemangiomas and solid hepatic lesions such as hepatocellular carcinoma and metastasis. Most hemangiomas show peripheral globular or rim-like enhancement with progressive centripetal fillin with no fast washout, while hepatocellular carcinoma and metastasis may show arterial enhancement followed by relatively fast washout.32,33
Color-Doppler Ultrasonography shows no flow within most of the heamangiomas. In some hemangiomas, however, there may be an intralesional arterio-portal shunt. In these cases, color Doppler ultrasonography may show flow inside the hemangioma and retrograde flow in the small portal branches around the hemangioma.34
Computed tomography (CT)On CT, hemangiomas show peripheral nodular enhancement, which progresses from the periphery of the lesion to its center until the whole lesion shows homogeneous prolonged enhancement, matching the enhancement of veins (Figure 3). Occasionally the hemangioma shows early enhancement on the arterial-phase CT followed by early filling-in of the lesion. This hyperdynamic behavior of some of the hemangiomas is attributed to intralesional arterioportal shunts. Small hemangiomas with high flow may show complete fill-in on the early phase of the contrast-enhanced CT.35
Magnetic resonance (MR) ImagingOn MR imaging, hemangioma appears well defined, homogeneously hyperintense on T2-weighted images, homogeneously hypointense on T1-weighted images, with peripheral nodular enhancement that progresses to complete and prolonged fill-in on the dynamic gadolinium-enhanced images.36 Hyperintensity of the hemangioma on T2-weighted images has been one of the important features differentiating it from solid neoplastic liver lesions. However, some of these lesions may show also similar hyperintensity on T2-weighted images. To differentiate the hyperintensity of hemangioma on T2-weighted images from these solid liver neoplasms, a useful method is to increase the TE of the T2-weighted images in steps. With such an increase in TE, the signal intensity of solid liver lesions would decrease, while that of hemangioma would remain high.37 Occasionally hemangioma may show central nonenhancing component even on the delayed images of the dynamic gadolinium-enhanced images. This may be attributed to thrombosis, hyalinization, scarring, or cystic changes at the center of the hemangioma.38 Hemangioma may appear similar to simple hepatic cyst on T2-weighted images as both lesions will be extremely well defined and extremely hyperintense on T2-weighted images. However, cysts tend to have lower signal intensity on T1-weighted images, lower signal intensity on proton-density images, no restriction of diffusion on the diffusion-weighted images, and no enhancement on the dynamic gadolinium-enhanced images. On the diffusion-weighted images, hemangioma tends to have less diffusion restriction than metastases and more diffusion restriction than cysts. Recently, tensor diffusion imaging has also been performed for focal liver lesions and has shown that fractional anisotropy (FA) of hemangioma is higher than that of cysts and lower but very close to that of metastases.39
Other imaging modalitiesTc-99m RBC blood pool scintigraphy shows high uptake of the radiotracer in hepatic hemangioma.40 Angiography is seldom used for diagnosis of hemangioma. However, angiographic appearance of hemangioma is recognized from old publications performed before the era of modern sectional imaging, from angiographic studies performed for malignant liver neoplasm with incidentally co-existing hemangioma, and from angiography performed for embolization of giant hemangiomas. On angiography, hemangioma has a characteristic snowy-tree or cotton-wool appearance.41 PET/CT shows no uptake of FDG by hemangioma of the liver, and may be used to differentiate atypical hemangioma from metastasis in patients with primary malignant neoplasm.42 Hemangioma may show uptake and accumulation of lipiodol for a long period after transcatheter arterial chemoembolization of hepatocellualar carcinoma with incidentally co-existing hemangioma.43
Special Situations of Liver Hemangiomas on ImagingHemangioma in the fatty liverHemangioma may appear hypoechoic on ultrasonography if it occurs in fatty liver.44 In this case, the diagnosis of hemangioma can be made by contrast-enhanced CT if the lesion is large enough or MR imaging if the lesion is small. Both CT and MR imaging can show the characteristic imaging features of the hemangioma and the typical appearance of the fatty liver. CT shows the fatty liver as low attenuation of the liver parenchyma on un-enhanced CT. Inphase and opposed-phase T1-weighted MR images are particularly useful in diagnosis of fatty liver.
Giant hemangiomaGiant hemangiomas, larger than 10 cm in diameter are clinically important because they may cause symptoms and may be complicated by hemorrhage, rupture, or intralesional hemolysis. Giant hemangiomas usually have central nonenhancing components and may have central hemorrhagic changes (Figure 4). Surgery or interventional radiological procedures are indicated for the treatment of giant hemangiomas. Ruptured hemangioma needs urgent surgical intervention.28,45
Non-enhancing component within the hemangiomaPersistent non-enhancing component within the hemangioma may be due to thrombosis, sclerosis, scarring, hyalinization, or cystic clefts within the hemangioma. Diagnosis of hemangioma remains to be easy if the hemangioma is large enough to show the characteristic features at the periphery of the lesion. If, however, the lesion is small with non-enhancing central component, it may be mistaken for hypovascular metastasis. In this case, diagnosis can be made by imaging follow-up if no primary malignant neoplasm is present and by PET/CT if there is a primary malignant neoplasm.
High-flow hemangiomaHemangioma may have intra-lesional arterio-portal or arterio-venous shunts. This type of hemangioma may show total enhancement on the early phases of contrast-enhanced CT or dynamic gadolinium-enhanced images. The early enhancement of this hemangioma may mimic that of focal nodular hyperplasia. Diagnosis of hemangioma can be made in this case by the prolonged enhancement and the high signal intensity of the lesion on T2-weighted images. On color Doppler imaging high-flow hemangioma may show flow signal within the lesion and retrograde flow in the surrounding venous structures.34 Hyperperfusion of the liver parenchyma around the high-flow hemangioma may be demonstrated on the arterial phase of contrast-enhanced CT and gadolinium-enhanced MR imaging.
Very small hemangiomaHemangioma, less than 5 mm in diameter may be difficult to characterize on imaging because it is too small to show the typical peripheral nodular enhancement on contrast-enhanced CT or dynamic gadolinium-enhanced MRI. Such hemangiomas usually show total enhancement from the start. However, the rest of the imaging features, particularly the high signal intensity on T2-weighted images and the prolonged enhancement without significant washout would still be useful in characterization of hemangiomas.
Hemangioma in infants and childrenHemangioma in infants and children are more liable to cause hemolysis and consumption coagulopathy (Kasabach-Merritt syndrome) than hemangiomas in adults. On imaging follow-up, hemangioma in infants and children may show rapid resolution.46
Indications To TreatmentPatients affected by hemangioma usually have their tumor diagnosed by ultrasound abdominal examination for a not well defined pain. A study reported in the literature 58% of the patients had abdominal pain at baseline, and in 50% of cases this pain was the reason for referral which led to the diagnosis of liver hemangioma.47 However, in only 12.6% of the cases could the pain be attributed to the hemangioma; in the other patients, other gastro intestinal diseases, especially inflammatory bowel diseases and peptic ulcer disease, were also present.47 In many studies reported in the literature pain persist after treatment of the hemangioma. In a study by Farges and colleagues,48 pain disappeared in 54% of patients after treatment of associated disorders, and in 4 out of 11 patients, pain persisted even after tumor resection. In this series, pain also diminished in many patients even in those with hepatic hemangiomas, pain is thought to be present as a result of infarct and necrosis of the tumor49 or the result of the tumor pressing on the liver capsule or adjacent organs.50 The latter is especially important in large tumors and left-lobe lesions.48,51 It is important to refer that many patients having pain with small lesion continue to have pain also if the tumor is not growing in size. Many of this symptoms are caused from other diseases especially inflammatory bowel disease at early stage. Furthermore there is no correlation in the majority of the study between the tumor size increase and the pain. But despite what, the abdominal pain remain the indication for surgical treatment, not supported by clinical evidence in a percentage between 48 and 86%.25,48,52 The causes of pain should be critically analized in case of hepatic hemangioma because more than 50% of the patients with abdominal pain and liver hemangioma have been found to have various gastrointestinal pathologies including cholelithiasis and peptic ulcer disease.52 Discomfort and anxiety have been reported as indication for surgery in case of hemangioma.53 These two symptoms should never been considered as indication for surgery especially if tumor is small in size and cannot responsible for any problem.29 The malignant trasformation is pratically inexistent and the patients have to be rassured or adressed to the psichiatrician. Patients operated for hemangioma for stress and anxiety due to the awareness of having a benign tumor, continued to have the same symptoms, even after surgery.51 These patients operated with a wrong indications do not improves their quality of life and they are exposed at all the risks of the surgical operation.
The right indications for surgery remain strictly related to the tumor complications. In fact rupture, intratumoral bleeding, Kasabach-Merritt syndrome and organ or vessels compression (gastric outlet obstruction, Budd-Chiari syndrome, etc.) represents the valid indication for surgery and at the same time they are all complications of the tumor itself. The size of the tumor do not represent a valid indication for treatment.
The Kasabach Merritt syndrome (also known as hemangioma thrombocytopenia syndrome)54 is a rare but lifethreatening disease and represents a valid indication for immediate surgery in patients with hepatic hemangioma. This syndrome has been described in children in association with cutaneous hemangioma. The main carachteristics of Kasabach-Merritt syndrome are enlarging hemangioma associated with trombocytopenia and microangiopathic hemolytic anemia with acute and massive or chronic and low-grade compsuntive coaugulophaty. Clotting and fibrinolysis within the hemangioma represent the first event of the Kasabach-Merritt syndrome. The patients show in the laboratory test trombocytopenia, anemia, hypofribrinogenimia but over all elevated fibrin degraded products (FDP). In patients with enlarged hemangioma with suspicion of Kasa-bach-Merritt syndome can be monitored every 3 months in case of elevation of 10% of the previous basic value of the FDP this result can represent an indication to surgery.
Another issue concerning the indication to a treatment for hemangioma is still represente by the location of the hemangiomas. In fact some tumors can be located close to the major vascular structures and in this way during their enlargemnet they can compress the major veins causing symptoms like in the case of Budd-Chiari syndrome. This is the reason why some author have suggested a early pro-filactic treatment even in case of asymptomatic patients in case of tumor located close to the confluence of hepatic veins that growing can provoque these problems.55
Traditionally, surgery had been advocated for most hemangiomas due to a concern for possible rupture.22 However, over the last 25 yr this paradigm has been challenged due to the relatively limited number of cases of rupture reported in the literature.56–58 To date, less than 50 cases of spontaneous rupture have been reported,59 while only 5 cases of traumatic rupture are known.57,60–63 Hemangioma ruputure have never been reported in parachuting, this confirm that the presence of hemangiona is not a risk for the spontaneous or traumatic rupture. All the methods to treat an hemangioma are reported in the next paragraph and the cumulative results for each treatment are reported in table 2.
TreatmentSurgical treatmentLiver hemangiomas, if indication exist, have to be treated firstly by surgery (hepatic resection or enucleation, open, laproscopic or robotic), but in the recent years other therapies like liver transplantation, radiofrequency ablation, radiotherapy, trans-arterial embolization, and chemotherapy have been applied.
First hemangioma have been treated by hepatic resection in 1898 by Hermann Pfannestil.64 Since than the hepatic resection have been the most practized theraphy for this tumor. In 1988 almost a century after the first hepatic resection for hemangioma, the enucleation have been reported in the literature.65 Since this second technique have been used more and more. Also if do not exist prospective randomized study comparing the two technique the majority of reports present in the literature are in favour of enucleation. The enucleation is safe, minimize the blood loss, and have a less rate of complications in relation to the hepatic resection, decreasing the risk of biliary fistula and preserving the maximum amount of normal liver parenchima.25,66–69 However, the study populations in these reports were small, and the impact of the hemangioma location was not particularly analyzed. The enucleation is a useful technique that can be performed if the hemangioma has an external plane on the liver surface. The space between the liver and the hemangioma is an avascular plane consisting of liver tissue compressed by the growing hemangioma. Only a few blood vessels traverse the capsule, and these are easily controlled. The tumor can be shucked without blood loss by opening this layer between the capsule of the hemangioma and the liver. The amount of normal functioning liver parenchyma removed is minimal. If the hemangioma is deply located in the liver parenchima, or do not present a free surface from the glisson capsule, or occupe all entire lobe in this case the hepatic resection remain the method of choice.70 The resection remain the choice also in case of multiple hemangiomas of the liver. The largest series concerning enucleation is reported in the literature and is related to 172 patients. In this report also the patients with deep hemangioma located in the liver have been treated by enucleation but with massive blood transfusions and their related risk increased and the procedure that became time consuming.70
Neonates with an abdominal mass and unidentified congestive heart failure are usually the patients and the symptoms of the diffuse hepatic hemangiomatosis, a rare condition characterized by diffuse replacement of liver parenchyma with hemangiomatous lesions.71 But definition of hemangiomatosis include also hemangiomas of the skin and involvement of at least two visceral organs.72 Hepatic isolatd hemangiomatosis of the liver has been reported as a very rare disease.73 Although several cases of longterm adult survival of diffuse neonatal hemangiomatosis have been reported,74,75 the etiology and natural history of diffuse hepatic hemangiomatosis remains unclear. The histological characteristics of hemangiomatosis includes the presence of large vascular channels in both the normal-appearing hepatic parenchyma and the cavernous tumor region.76 Recognition of the association of hepatic hemangiomatosis in patients with giant cavernous hemangiomas (GCHs) is important because management of hemangioma may depend on the presence and extent of hemangiomatosis. In case of large hamangioma surrounded by hemangiomatosis, the enucleation cannot be possible. In fact in this case the sheath of compressed liver tissue, clearly defining the border between the cavernous tissue and normal liver parenchyma, cannot be identified due to adjacent extensive hemangiomatosis. The enucleation can be avoided because can cause of massive hemorrage, and hepatic resection is a preferable technique.
Surgery of large hemangiomas of the liver both by resection or by enucleation can cause torrential intraoperative hemorrage, difficult to control with the high mortality risk for the patients48,49,77 this is the reason why a vascular control before to start the procedure can be used to render safe the procedure.
Special attention is requested for hemangiomas located in the caudate lobe. Small hemangiomas may enucleated or resected with no difficulty.78 On the opposite resection of giant hemangiomas is more complicated, and isolation and excision of the lobe may be hazardous. The major risk in this second situation is the difficulty of the control of the profuse hemorrage from the undersurface of the liver that can be very difficult to manage. In this case the ligature of the arterial branches to the caudate lobe along the border of the hepatoduodenal ligament, can shrink in size the hemangioma and becaming softer it is more is the manipulation and the dissection.79 This technique is safer is supported by the vena cava and portal vessels encircled and ready to be clamped.
The Louisville consensus statement suggests laparoscopic liver resection as an option for lesions in the left lateral and inferior segments of the right lobe.80 This technique has been described for liver hemangiomas. This technique pose some technical issues that concerning the location of the tumor, the absence of tactile feedback, the ideal technique for parenchimal transection that is not yet standardize and finally in case of large tumor the extraction can be difficult requiring a large incision. Despite the limitations and disadvantages of laparoscopic liver resection, which include a significant learning curve, bleeding that is more difficult to control laparoscopically, inadequate assessment of the liver for additional lesions, and increased risk for gas embolism, the increase in laparoscopic hepatic resections for benign tumors have recorded in the last years based on the fact that these easy procedure are chosen by novice laparoscopic teams because of decreased initial difficulties.81 The role of laparoscopic liver resection for liver tumors is unclear at present82–85 but it can use in experienced groups with the right indications and good results. No case of laparoscopic enucleation has not yet reported.
The robotic procedure have until now very few reports.86,87 It is a technique that like for the pilot for the airplane it will probably replace in the next 50 years the majority of the work of the surgeon. With the possibility to schedule the transection procedure by a computer the role of the surgeon will become just to supervise the procedure. But this is the future, for the present robotic surgery is not standardize for the resection of the hepatic hemangioma and the few experience do not permit to choice this technique as a firts step and it should be performed in specialized center skilled both in hepatic and robotic surgery.
Radiofrequency ablationRadiofrequency ablation (RFA), used both percutaneously or laparoscopically, is a minimally invasive, safe, and effective treatment for primary and metastatic liver neoplasms.7,88 Recently, percutaneous RFA therapy was also performed successfully for patients with hemangioma of the liver.89–92 The mechanism of the RFA destruction of the hemangiomas is not completely understood. The effect of srinkage of the tumor under the RFA is due to the action of the localized thermal injuries on the flat endothelial cells which constitute the wall of the widely dilated nonanastomotic vascular spaces. The temperature exceding 90 °C damaging the endothelial layer lining the vascular structure may promote thrombosis.91
Both techniques percutaneous or laparoscopic can be used to treat the hemangioma with radiofrequency. Using the percutaneous technique tumor close to the gallbladder ot other viscera cannot be treated, and at minimun 1 cm of distance need to avoid serious complications. Fullthickness burns of the stomach, small bowel, and colon have been observed when the edge of the thermal lesion was less than 1 cm from the surface of the liver in a pig liver model.26
On the opposite the laparoscopic approach due to the pneumoperitoneum eleves the diapragm and the organ are displaced. This increase the operative space with facilitation of needle placement and at the same time avoiding damage of the closest viscera. Furthermore the laparoscopic approach permit the use of intraoperative ultrasonography. This increase the possibility to determine real-time RF electrode placement and evaluate the efficacy of ablation. The success of the hemangioma ablation using radiofrequency depends also from the powerful of the used device and the lenght of the tines; both have to be maximized especially in largest hemangioma ( more than 10 cm).93
Advantages and disadvantaged of the RF ablation depends from the benignity and hypervascularity of this tumor. The main 3 advantages of this techniques include at the first place the benignity of the tumor that does not require a safe margin of the liver parenchima surrounding the tumor. Second advantages is due to the content of the tumor. Infact as the hemangioma is constitute by blood positioned in cavities the effect of radiofrequency can lead to an obvious collapse of tumor tissue around the ablation zone. Third advantage of the hemangioma is strictly related to the benignity of the residual tumor, if it remains after the first treatment, does nor need to be treated as soon as possible because neither develop rapid tumor progression nor metastasize to distant sites.94
The main disadvantage of the radiofrequency technique is the almost certain hemolysis due to the blood supply of this tumor. More is large the tumor and more is the risk and the gravity of this complication. Hemolysis can lead to various degrees of hemoglobinuria, hemolytic jaundice, anemia, or even renal damage accordingly, depending on its different severity.90
Furtermore other complications have been reported as eophageal perforation due to the closeness of the left lobe and ARDS.95
Monoclonal antibodyVascular endothelial growth factor (VEGF) is recognized as a regulator of blood vessel growth and it is postulated that also the hemangioma can grown under the action of the VEGF [96]. In the early 1970s, Folkman, et al.97 identified a tumor-angiogenesis factor that was mitogenic to tumor capillary endothelial cells and suggested that blocking this factor might arrest tumor growth. The use of specific antibodies directed against VEGF abolishes the vascular endothelial growth-promoting activity in vitro.98 Bevacizumab is a recombinant humanized monoclonal antibody (93% human and 7% murine) directed against VEGF which is used for the treatment of metastatic colorectal cancer in combination with 5-fluorouracil (5-FU)-based regimen.96
Bevacizumab have been reported as a medical treatment inducing a biological and clinical response in a patient of symptomatic hereditary hemorrhagic telangiectasia with liver involvement complicated by high-output cardiac failure, portal hypertension and cholestasis. There was a marked diminution of hepatic vascularity, a twofold reduction in liver volume and normalization of cardiac output, thus alleviating the need for a liver transplantation. Therapy was well tolerated. The authors suggested that biological agents targeting angiogenic growth factors represent a novel form of therapy for these patients.99 If the use of bevacizumab will be confirmed can be used not only for the hemangiomas of the liver but also for the hemangiomas in other parts of the body as well.
RadiotherapyRadiation therapy provides partial reduction in hemangioma size and relief of symptoms. Usually the dose of 30 Gy is given in 15 fractions over 3 weeks. With this method a complete clinical response have been reported over a period of 8-14 months.100 This treatment can be effective with minimum morbidity if other treatment are nor advisable, but complications including radiation hepatitis, veno-occlusive disease, and hepatoma have to be taken in due account.101 Although the biological mechanisms underlying the treatment efficacy of RT for haemangioma remain undetermined,102 damage to vascular endothelial cells and smooth muscle cells is generally assumed to play a key role in the radiation effects, leading to vascular thrombosis, necrosis and fibrosis. Three stages can be described in this process.1 Acute stage: interstitial oedema of the vascular endothelium develops;7 transient subacute stage: organising changes occur in the central vein, which may progress to partial obliteration;103 chronic stage: sclerosis or thrombosis of hepatic arterioles and portal tissues takes place against the healing process.
However, radiation therapy is rarely recommended as a first-line therapy for the liver hemangiomas owing to the concern of treatment-related liver toxicity and the long-term potential for secondary malignancies.101 This treatment is reserved for massive hepatic haemangiomas associated with intractable congestive heart failure or hyperconsumptive coagulopathy in paediatric patients,101,104,105 that cannot be treated with other therapies.
TAESuccessful use of trans arterial embolization (TAE) before surgical resection of ruptured hepatic hemangioma was first reported by Yamamoto, et al. in 1991.106 Since than this procedure have been also reported as a pre treatment before the elective surgical procedure, but results of this procedure are controversial107 because of the fear of causing ischemia, intracavitary bleeding or infection.107 The idea was to decrease the size of the hemangiomas, especially the tumors larger than 20 cm, by blocking its arterial supply and consequent shrinkage of the tumor, which facilitated subsequent mobilization of the liver, and consequently decreased intraoperative hemorrhage.108–110 Recently, 98 patients affected by hemangiomas have been treated using trans arterial embolization using pingyangmycin-lipiodol emulsion that have proved to be a useful procedure as the only therapy of hemangiomas of the liver.111 However, TAE may result in severe complications including ectopic embolizations and destructive biliary damage.112
Radiation therapy and TAE are generally considered palliative.
ChemotherapyThe chemotherapy until now have not been extensively reported. Recently a very interesting case report reported by Hascimoto M and co-workers113 have open new perspective concerning this kind of theraphy. A 35 years old women affected by ovarian tumor with 6 hemangiomas of the liver have been submitted, before surgery, to five cycles of bleomycin/etoposide/cisplatin (BEP) therapy [cisplatin (20 mg/m2), etoposide (100 mg/m2) and bleomycin (5 mg/ m2) on days 1–5. After this treatment also the hemangiomas reduced in size. At the intervention one of this tumor have been resected to be sure was not a metastasis. But the resected tumour was diagnosed as a typical haemangioma.
Although it is not clear why the haemangiomas decreased in size after systemic chemotherapy, some speculations can be proposed. First that development of hepatic haemangioma was associated with oestrogen so as the oophorectomy suppressed ovarian function, the absence of stimulation decreases also the haemangioma. Second, the decrease in the size of the haemangioma may have been in part due to the chemotherapy itself, especially to the direct devascularizing effect of bleomycin.111,114 Third, a combination of the devascularizing effect of bleomycin and suppression of ovarian function due to chemotherapy may have contributed to the reduction in size.
Liver transplantationLiver transplantation have been reported for giant hemangioma of the liver and life-treathening coaugulophaty secondary to Kasabach Merritt syndrome115 of for other indications.116 It is an extreme life treatening treatment with very few indications.
ConclusionThe hepatic cavernous hemangioma is the most common liver benign tumor. Liver hemangiomas, when indication exist, have to be treated firstly by surgery (hepatic resection or enucleation, open, laproscopic or robotic), but in the recent years other therapies like liver transplantation, radiofrequency ablation, radiotherapy, trans-arterial embolization, and chemotherapy have been applied. New prospective randomized studies are needed for evaluate which of these techniques is the best when the surgical procedure is not possible.