Bleeding from gastroesophageal varices (GEV) is a serious event in cirrhotic patients and can cause death. According to the explosion theory, progressive portal hypertension is the primary mechanism underlying variceal bleeding. There are two approaches for treating GEV: primary prophylaxis to manage bleeding or emergency treatment for bleeding followed by secondary prophylaxis. Treatment methods can be classified into two categories: 1) Those used to decrease portal pressure, such as medication (i.e., nonselective β-blockers), radiological intervention [transjugular intrahepatic portosystemic shunt (TIPS)] or a surgical approach (i.e., portacaval shunt), and 2) Those used to obstruct GEV, such as endoscopy [endoscopic variceal ligation (EVL), endoscopic injection sclerotherapy (EIS), and tissue adhesive injection] or radiological intervention [balloon-occluded retrograde transvenous obliteration (BRTO)]. Clinicians should choose a treatment method based on an understanding of its efficacy and limitations. Furthermore, elastography techniques and serum biomarkers are noninvasive methods for estimating portal pressure and may be helpful in managing GEV. The impact of these advances in cirrhosis therapy should be evaluated for their effectiveness in treating GEV.
Portal hypertension frequently occurs in cirrhotic patients. Studies have demonstrated that increased portal inflow and increased resistance to portal outflow are responsible for portal hypertension.1 Increased portal inflow is induced by mesenteric arterial vasodilatation, and its underlying mechanism involves the enhanced expression of vasodilators, including nitric oxide (NO) and glucagon.1 Increased resistance to portal outflow is caused by mechanical obstruction of flow by intrahepatic fibrous tissue and intrahepatic vasoconstriction; the mechanism for the latter involves the synergistic effect of the increased expression of vasoconstrictors, such as endothelin, angi-otensinogen and eicosanoids, and the decreased expression of vasodilators, including NO and carbon monoxide.1,2 Portal hypertension plays a key role in the development of cirrhosis-associated complications, such as ascites, hepatic encephalopathy, and gastroesophageal varices (GEV).2
Measurement of the hepatic venous pressure gradient (HVPG) is standard for estimating portal pressure.3 Portal hypertension is defined as HVPG > 5 mmHg. HVPG ≥ 10 mmHg is considered clinically significant portal hypertension (CSPH) and can result in the formation of GEV.4 According to the explosion theory, HVPG ≥ 12 mmHg is the critical value at which bleeding due to GEV can oc-cur.5 Furthermore, HVPG, ≥ 20 mmHg is reported to be significantly associated with failed treatment of acute variceal bleeding, rebleeding, and poor survival.6,7
Studies have reported that GEV develops in approximately 50% of cirrhotic patients8 and that bleeding from esophageal varices (EV) and gastric varices (GV) occurs in approximately 25% of patients at 2 years9 and 10 to 16% at 1 year.10 The mortality rate in cirrhotic patients with a first bleed from GEV is 20 to 35%,11 and patients who survive the first bleed are at high risk of rebleeding (greater than 60% at 1 year), with a mortality rate of approximately 20%.12 A study of hepatitis C virus (HCV)-related compensated cirrhosis found that the presence of EV is a predictor of hepatic decompensation and mortality.13 Thus, establishing methods for prophylaxis and emergent treatments for GEV bleeding is imperative to improve the outcomes of cirrhotic patients.
In this review, we outline the current status of GEV management in these patients and suggest future directions for research.
Treatment Situations and PrinciplesIn medical practice, clinicians should determine the optimal treatment for GEV based on treatment situations and principles. Two possible treatment situations are primary prophylaxis for bleeding and emergent treatment for bleeding followed by secondary prophylaxis. Treatment principles can be classified into two categories: decreasing portal pressure and obstructing GEV. Methods for decreasing portal pressure include medications [i.e., non-selective β-blockers14], radiological intervention [i.e., transjugular intrahepatic portosystemic shunt (TIPS)15], and surgery [i.e., portacaval shunt16]. In contrast, methods for treating the obstruction of GEV include endoscopic approaches [endoscopic variceal ligation (EVL),17,18 endoscopic injection sclerotherapy (EIS),19 and tissue adhesive injection,20,21] or radiological intervention [balloon-occluded retrograde transvenous obliteration (BRTO)22]. Moreover, the treatment approaches for EV and GV differ. Thus, it is important that clinicians understand the application, efficacy, safety, and limitation of each treatment method.
Gev Treatment MethodsIn 2007, the practice guidelines for management of GEV were endorsed by the American Association for the Study of Liver Diseases (AASLD) and the American College of Gastroenterology (ACG).23 With reference to the guidelines and evidence published after the guidelines, we describe the current treatment methods for GEV. Furthermore, we refer to some important points regarding therapeutic strategies for GEV recommended by the Baveno VI Consensus Workshop.24Tables 1 and 2 list treatment options for EV and GV according to treatment situations and principles. GV is commonly classified into 4 types based on its relationship with EV and its location in the stomach.25 Here, we mainly discuss treatments for GV in the fundus [i.e., GV as extensions of EV along the fundus (GOV2) and isolated GV (IGV1)].
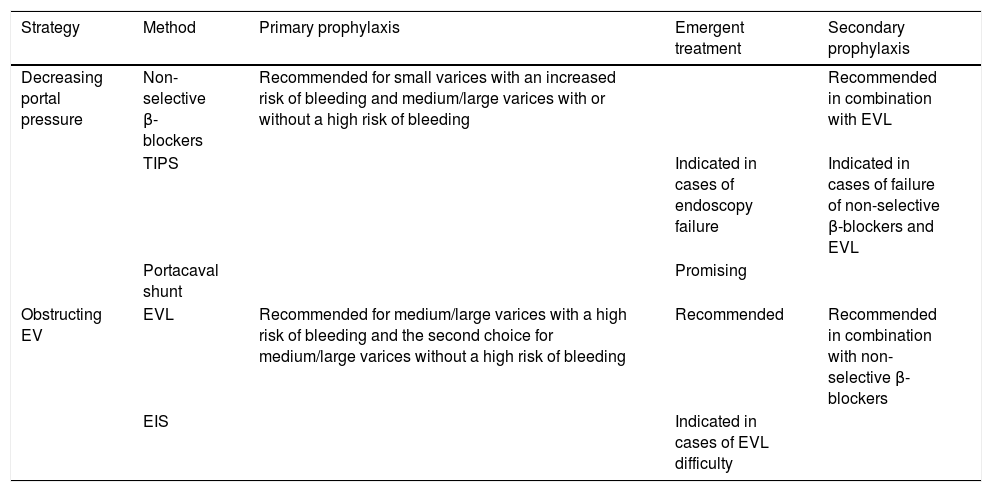
Treatment options for EV.
| Strategy | Method | Primary prophylaxis | Emergent treatment | Secondary prophylaxis |
|---|---|---|---|---|
| Decreasing portal pressure | Non-selective β-blockers | Recommended for small varices with an increased risk of bleeding and medium/large varices with or without a high risk of bleeding | Recommended in combination with EVL | |
| TIPS | Indicated in cases of endoscopy failure | Indicated in cases of failure of non-selective β-blockers and EVL | ||
| Portacaval shunt | Promising | |||
| Obstructing EV | EVL | Recommended for medium/large varices with a high risk of bleeding and the second choice for medium/large varices without a high risk of bleeding | Recommended | Recommended in combination with non-selective β-blockers |
| EIS | Indicated in cases of EVL difficulty |
EV: esophageal varices. EVL: endoscopic variceal ligation. TIPS: transjugular intrahepatic portosystemic shunt. EIS: endoscopic injection sclerotherapy.
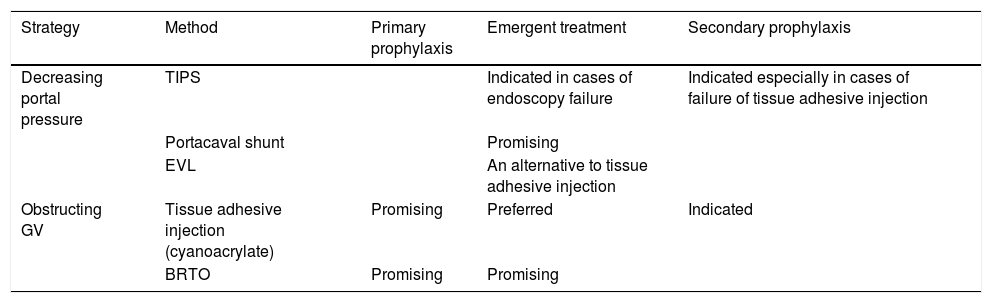
Treatment options for GV.
| Strategy | Method | Primary prophylaxis | Emergent treatment | Secondary prophylaxis |
|---|---|---|---|---|
| Decreasing portal pressure | TIPS | Indicated in cases of endoscopy failure | Indicated especially in cases of failure of tissue adhesive injection | |
| Portacaval shunt | Promising | |||
| EVL | An alternative to tissue adhesive injection | |||
| Obstructing GV | Tissue adhesive injection (cyanoacrylate) | Promising | Preferred | Indicated |
| BRTO | Promising | Promising |
GV: gastric varices. TIPS: transjugular intrahepatic portosystemic shunt. EVL: endoscopic variceal ligation. BRTO: balloon-occluded retrograde transvenous obliteration.
- •
Primary prophylaxis for bleeding from EV.
- a)
Decreasing portal pressure. Non-selective β-blockers (i.e., propranolol or nadolol) decrease portal inflow by reducing cardiac output by blocking β1 receptors and producing splanchnic vasoconstriction via the blockage of β2 receptors, resulting in reduced portal pressure.26 Non-selective β-blockers are recommended for patients with small varices that have an increased risk of bleeding, as with Child-Pugh class B/C cirrhosis or varices with red wale markings. Non-selective β-blockers are also the first choice for patients with medium to large varices with or without a high risk of bleeding (i.e., Child-Pugh class B/C cirrhosis or varices with red wale markings).23 To achieve maximum benefits from non-selective β-blockers, the maximal tolerated dosage should be used.23 In a meta-analysis of non-selective β-blockers over a median follow-up of 24 months, the risk of first bleeding was 15% in patients with medication compared with 24% in those untreated. In addition, the risk of mortality was 23% in patients with medication compared with 27% in those without.14 Some studies have attempted to determine which non-selective β-blocker is better for primary prophylaxis for bleeding from EV. Carvedilol is a non-selective β-blocker with a1-blocking property. In a randomized controlled trial (RCT) for cirrhotic patients, carvedilol was found to have a stronger effect on HVPG reduction than was propranolol.27 In a recent study with patients with EV, a greater decrease in HVPG and a lower rate of bleeding from EV were observed in patients treated with carvedilol than in those treated with propranolol.28 These studies suggest that among non-selective β-blockers, carvedilol may be a preferred one for primary prophylaxis, although more evidence is needed.24 The limitations of non-selective β-blockers include possible non-responsiveness;29 contraindications for patients with asthma, insulin-dependent diabetes, or peripheral vascular disease;23 and limited tolerability due to adverse effects, such as general fatigue and lightheadedness. In addition, non-selective β-blockers should not be used for patients with end-stage cirrhosis because administration of these medications can lead to poor patient survival.30 TIPS and portacaval shunts are not recommended for primary prophylaxis because their disadvantages, such as hepatic encephalopathy, are not compensated for by their advantages.23
- b)
Obstructing EV. EVL is the first choice of treatment for patients with medium to large varices and a high risk of bleeding. EVL is an alternative treatment for patients with medium to large varices without a high risk of bleeding when non-selective β-blockers are unsuitable for the patient.23 Repeated EVL may be required to eradicate EV. A meta-analysis of primary prophylaxis for bleeding from EV found that over a follow-up period of up to three years, the bleeding and mortality rates were 14 and 25%, respectively;17 these figures are similar to those reported in studies of non-selective β-block-ers.14 It remains unknown which of these treatments is better for primary prophylaxis. However, a recent meta-analysis reported that the rate of bleeding from EV did not differ significantly between EVL and non-selective β-blockers when only high quality trials were selected for analysis. Severe adverse events following EVL include bleeding from banding ulcers, severe post-ligation pain, and esophageal perforation.31 Another meta-analysis suggested that EVL is superior to non-selective β-blockers for the treatment of primary prophylaxis in patients with large or high-risk EV (risk ratio, 0.69; 95% confidence interval, 0.52 to 0.91).32 Studies of the beneficial effects of the combination of EVL and non-selective β-blockers are controversial.33 EIS is not effective for primary prophylaxis for bleeding from EV.34
- a)
- •
Primary prophylaxis for bleeding from GV. A reported high mortality rate of approximately 50%35 in patients with bleeding GV highlights the need to establish methods for bleeding prophylaxis. Risk factors for bleeding from GV include the large size of the GV, presence of red signs, and poor liver function.10 Compared with bleeding from EV, an HVPG ≥ 12 mmHg is not necessary for bleeding from GV, probably due to the high frequency of spontaneous gastrorenal shunts in these patients.36
- a)
Decreasing portal pressure. In a recent RCT by Mishra, et al., the use of non-selective β-blockers was compared with no primary prophylaxis for bleeding from a large (≥ 10 mm) GV. Between the groups receiving non-selective β-blockers or no treatment, the rates of bleeding from GV did not differ significantly (28 vs. 45% during a median follow-up of 26 months), suggesting that use of non-selective β-blockers may not be effective for primary prophy-laxis.20 As with EV, TIPS and portacaval shunt cannot be used to treat primary prophylaxis for bleeding from GV due to disadvantages, such as hepatic encephalopathy.
- b)
Obstructing GV. Endoscopic intravariceal injection with a tissue adhesive can be employed for obstructing GV. Cyanoacrylates are the most commonly used tissue adhesives. These adhesives polymerize and harden instantaneously upon contact with blood.37 In the RCT by Mishra, et al., en-doscopic cyanoacrylate injection was compared with non-selective β-blockers and no treatment. The rate of bleeding from GV was significantly reduced in the cyanoacrylate injection group compared with the medication and no treatment groups (13 vs. 28% and 45%, respectively).20 The technical success rate of endoscopic treatment was high (100%), and severe adverse events were not observed. Although the rate of developing new EV in the cyanoacrylate injection group was slightly increased compared with the other groups (23 vs. 10%, P = 0.216), bleeding from new EV was not observed. Thus, endoscopic cyanoacrylate injection has been demonstrated to be the most effective primary prophylaxis for bleeding from GV.20 Nevertheless, these results require verification in independent cohorts. Systemic embolisms, such as pulmonary and cerebral emboli, are the main limitation of this therapeutic method.38
- a)
In contrast to GV, cyanoacrylate injection for EV does not appear to have any particular advantage over other methods for managing EV. A recent RCT for patients with medium or large EV, in which cyanoacrylate injection and EVL were compared in terms of therapeutic effects, complications, and outcomes, demonstrated that variceal recurrence was observed more frequently in patients undergoing cyanoacrylate injection (33 vs. 57%, P = 0.04).39 Another RCT in patients with bleeding EV reported that the rebleeding rate after cyanoacrylate injection was higher than that after EVL, although the rates were not significantly different (13.6 vs. 4.7%, P = 0.607).40 Moreover, severe complications associated with cyanoacrylate injection have been reported.41 Thus, cyanoacrylate injection is not a common method for managing EV.
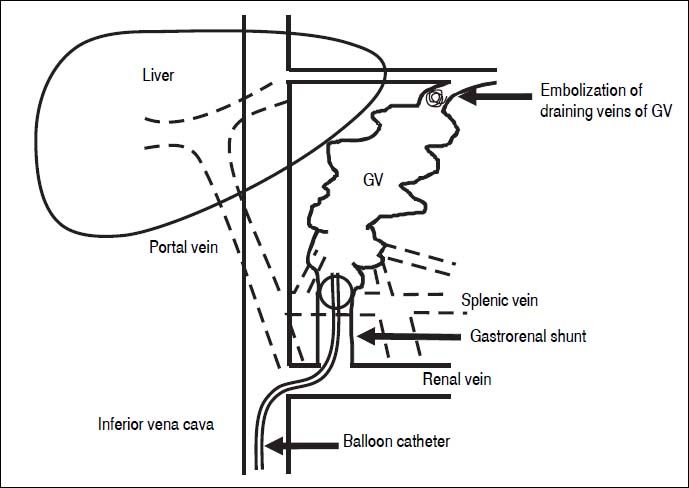
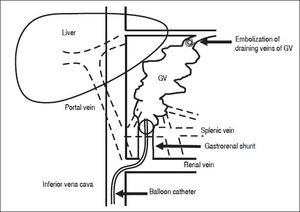
BRTO is another method for obstructing GV.42 Eth-anolamine oleate is commonly used as a sclerosant for BRTO. The procedure of BRTO is illustrated in figure 1. Briefly, a 6-F balloon catheter is inserted into the gastro-renal shunt via the femoral vein. To identify the GV, feeding veins, and draining veins, retrograde venography is performed under balloon occlusion of the gastrorenal shunt. If draining veins of GV are identified, they are embolized with microcoils or gelatin sponge particles. Then, 5% ethanolamine oleate is infused into the space of GV until the space is completely opaque. After maintaining balloon occlusion overnight and disappearance of GV is confirmed by venography, the balloon is deflated and withdrawn. Human haptoglobin is administered to prevent hemolysis and subsequent renal failure.
The procedure of BRTO. The balloon catheter is inserted into the gastrorenal shunt, and retrograde venography is performed under balloon occlusion of the shunt If draining veins of GV are identified, they are embolized with micro-coils or gelatin sponge particles. Then, 5% etha-nolamine oleate is infused into the space of GV until the space is completely opaque. BRTO: balloon-occluded retrograde transvenous obliteration. GV: gastric varices.
A recent meta-analysis evaluated the effectiveness and safety of BRTO for GV with a high risk of bleeding and for bleeding GV.22 The technical success and clinical success rates (defined as a lack of recurrence or rebleeding of GV or the complete obliteration of varices upon subsequent imaging) were 96 and 97%, respectively. Furthermore, the rate of major complications, such as portal or splenic venous thrombus, was low (5%). This treatment method is attractive, especially for patients with GV and portal-systemic encephalopathy because the splenorenal shunt responsible for encephalopathy is obstructed by BRTO. The development or recurrence of EV after BRTO should be noted; the reported rate of new EV is 33%.22 Unfortunately, almost all studies used in these analyses were retrospective. Future RCTs are required to establish the efficacy of BRTO for GV.
Emergent treatment for bleeding- •
Emergent treatment for bleeding from EV. During acute bleeding, it is essential to maintain volemia and prevent bacterial infection with intravenous antibiotics, such as quinolones.23 Furthermore, lactulose or rifaximin may help prevent hepatic encephalopathy due to bleeding.24
- a)
Decreasing portal pressure. In the acute phase, 3 to 5 days of intravenous splanchnic vasoconstrictors, including terlipressin, somatostatin, and somatostatin analogues (octreotide or vapreotide), can decrease portal inflow, thereby helping to control bleeding; non-selective β-blockers are contraindicated due to their depressor action.23 TIPS is indicated as a rescue therapy when endoscopic approaches have resulted in failure.23 One study reported that an HVPG, ≥ 20 mmHg is significantly associated with uncontrolled bleeding after EIS.6 The success rate of TIPS is 90%.43 As with TIPS, a portacaval shunt is an artificial portosystemic shunt used to decrease portal pressure. This procedure is used to surgically create an anastomosis between the portal vein and inferior vena cava. A recent trial has demonstrated that an emergency portacaval shunt achieved 100% hemostasis for bleeding from EV.44 The longterm outcome after portacaval shunt creation is reported to be excellent. In RCTs by Orloff, et al., the rates of rebleeding and encephalopathy were significantly reduced in a portacaval shunt group compared with an EIS or a TIPS group, and the overall survival rates were significantly increased in the former compared with the latter.45 These results should be verified in independent data sets. Moreover, the biological mechanisms underlying the low rate of portal-systemic encephalopathy after porta-caval shunt should be clarified.
- b)
Obstructing EV. EVL is the first choice for acute he-mostasis; EIS is indicated when EVL is not technically feasible.23 A recent meta-analysis has shown that the success rate of EVL for bleeding from EV is greater than 90%.17
- a)
- •
Emergent treatment for bleeding from GV. As with EV, the initial approach is to maintain volemia and administer antibiotics and splanchnic vasoconstrictors.
- a)
Decreasing portal pressure. TIPS is indicated when bleeding from GV is not controlled by endoscopic approaches combined with intravenous splanchnic vasoconstrictors.23 In a study of TIPS in patients with bleeding EV or GV, hemostasis was successful in 96% of the patients.46 Moreover, a recent RCT of patients with bleeding GV compared the efficacy and safety of the portacaval shunt, TIPS, and endo-scopic approaches. The rate of hemostasis was significantly increased in the portacaval shunt group (97 to 100%) compared with both the TIPS group (82%) and the endoscopy group (83%). Furthermore, the rate of permanent control for up to 5 years or until death was significantly increased in the portacaval shunt group (97 to 100%) compared with the TIPS group (6%) and the endoscopy group (27 to 29%).16 These results should be verified in independent data sets.
- b)
Obstructing GV. Endoscopic cyanoacrylate injection is the preferred method for treating bleeding from GV.23 In a recent large-scale study (n = 121), the rate of hemostasis was 91%.47 EVL is another treatment option.23 An RCT reported that the rate of he-mostasis did not significantly differ between EVL and cyanoacrylate injection groups (94 vs. 94%); however, the rebleeding rate was significantly increased in the former compared with the latter (44 vs. 22%).48 Additionally, BRTO is a promising measure for hemostasis.22
- a)
As mentioned, patients who have experienced bleeding from GEV are at high risk of rebleeding and mortality, which provides a clear rationale of the need for second prophylaxis for bleeding.
- •
Secondary prophylaxis for bleeding from EV.
- a)
Decreasing portal pressure. Following hemostasis, decreasing portal pressure is essential for preventing rebleeding. Non-selective β-blockers are effective for this purpose.23 Studies have examined the relationship between the response of non-selective β-blockers and rebleeding rates. When patients who achieved a reduction in HVPG of at least 20% of baseline values and/or below 12 mmHg were defined as responders, the rebleeding rates were significantly reduced (7 to 13%) in responders compared with nonresponders (46 to 65%).12 In the guidelines, non-selective β-blockers combined with EVL but not non-selective β-blockers alone are recommended for secondary prophylaxis for bleeding23 (see below).
By contrast, TIPS is indicated when combination therapy fails.23 After publication of the guidelines, an RCT suggested that early TIPS after EVL or EIS combined with a splanchnic vasoconstrictor for bleeding EV is a promising method as second prophylaxis to treat bleeding in Child Pugh class B/ C patients who are at a high risk of failure to control bleeding and of mortality. This study revealed that the 1-year bleeding-free survival rate and overall survival rate were significantly increased (97 and 86%, respectively) in the TIPS group compared with those in the non-selective β-blocker plus EVL group (50 and 61%, respectively).49 Another study of TIPS as secondary prophylaxis reported similar re-sults.50 However, a recent network meta-analysis on various treatments as secondary prophylaxis for bleeding from EV demonstrated that TIPS does not contribute to improved overall survival; however, it is the best method for preventing rebleeding and death due to rebleeding.19 Most recently, an RCT was conducted with Child Pugh class A/B patients to determine the more effective treatment method for secondary prophylaxis of bleeding from EV between TIPS with small diameter stents (8 mm in diameter) and medications (propranolol and iso-sorbide-5-mononitrate). In the trial, EVL was performed in patients who were nonresponsive to medications. The results of this study demonstrated that the two-year rebleeding rate was significantly reduced (7%) in the TIPS group compared with the medication only group (18%) and the medication-EVL group (31%), whereas the rate of adverse events, such as encephalopathy, tended to be increased in the TIPS group compared with the other groups. Furthermore, the overall survival rate did not significantly differ among the groups.51 Given these findings, in cases of severe portal hypertension (e.g., HVPG ≥ 20 mmHg) with a high risk of mortality due to rebleeding, TIPS may be the best treatment. The possibility of developing hepatic encephalopathy after TIPS should be considered. Future studies are needed to determine which patients should receive TIPS as the first-line therapy for secondary prophylaxis for bleeding. One drawback of TIPS is shunt dysfunction due to stent occlusion; however, the use of covered stents has reduced the rate of stent occlusion.52
- b)
Obstructing EV. EVL is the most widely used endo-scopic approach to secondary prophylaxis for bleeding. EIS is not recommended as secondary prophylaxis for bleeding because previous studies have failed to show the superiority of EIS over EVL.23 For the eradication of EV, repeated EVL is required in most cases. Current guidelines recommend that to increase the prevention of rebleeding from EV, EVL should be performed in conjunction with non-selective β-blockers.23 A recent meta-anal-ysis was conducted to determine which treatment method - EVL alone or EVL plus non-selective β-blockers - results in better outcomes for cirrhotic patients who had survived bleeding from EV. This analysis showed that the combination therapy significantly lowered the risk of rebleeding (risk ratio, 0.68; 95% confidence interval, 0.54 to 0.85) and of bleeding-related mortality (risk ratio, 0.52; 95% confidence interval, 0.27 to 0.99). However, the overall mortality rates were similar between the two treatment groups.18 Another meta-analysis reported similar results.53 This meta-analysis also compared the outcomes between patients treated with non-selective β-blockers and isosorbide mononitrate, a long-acting venous dilator,54 and those treated with the same medications combined with EVL. The results revealed that combination therapy slightly lowered the risk of rebleeding (risk ratio, 0.76; 95% confidence interval, 0.58 to 1.00) without effecting mortality (risk ratio, 1.24; 95% confidence interval, 0.90 to 1.70).53 Taken together, EVL combined with non-selective β-blockers with or without isosorbide mononitrate appears to be the most effective method for preventing rebleeding followed by medication alone and then EVL alone.
Thus, EVL and treatment with non-selective β -blockers with or without isosorbide mononitrate or TIPS is considered the treatment of choice for secondary prophylaxis for bleeding. Protocols for the use of secondary prophylaxis should be established in the future based on the degree of portal hypertension, response to medications, and liver function.
- a)
- •
Secondary prophylaxis for bleeding from GV.
- a)
Decreasing portal pressure. In a recent RCT on secondary prophylaxis, non-selective β-blockers and en-doscopic cyanoacrylate injection were compared among cirrhotic patients who survived bleeding from GOV2 or IGV1. During a median follow-up of 26 months, the rates of rebleeding and mortality were significantly reduced in the cyanoacrylate injection group compared with the medication group (15 vs. 55% and 3 vs. 25%, respectively).21 Another RCT was conducted to determine whether non-selective β-blockers enhanced the efficacy of repeated endoscopic cyanoacrylate injection for preventing rebleeding from GV. The trial revealed that the addition of non-selective β-blockers did not influence the rates of rebleeding or overall survival in patients.55 Thus, non-selective β-blockers may not be effective for secondary prophylaxis.
TIPS is the treatment of choice for secondary prophylaxis. An RCT was conducted to determine whether TIPS or endoscopic cyanoacrylate injection is more effective in preventing rebleeding from GV. After a median follow-up of 33 months, rebleeding occurred at a significantly lower rate in the TIPS group compared with the cyanoacrylate injection group (11% and 38%), whereas the mortality and complication rates, with the exception of hepatic encephalopathy, were similar.56 Collectively, TIPS may be a more effective method to prevent rebleeding from GV compared with treatment by endoscopic cyanoacrylate injection and non-selective β-blockers.
- b)
Obstructing GV. Endoscopic cyanoacrylate injection is widely employed as secondary prophylaxis for bleeding from GV. This endoscopic therapy can be repeated to achieve the eradication of GV. The efficacy of endoscopic cyanoacrylate injection is described above. A recent review of bleeding from all types of GV compared the effectiveness of endo-scopic cyanoacrylate injection and two other endoscopic approaches, EIS and EVL. This analysis suggested that cyanoacrylate injections may be more effective at preventing rebleeding than other methods; however, no definitive conclusions were drawn due to the low quality of the evidence.57 TIPS should be considered when endoscopic cy-anoacrylate injection has failed.
- a)
HVPG measurement is currently considered the most reliable method for estimating portal pressure and can be a predictor of disease progression, including variceal bleeding, in cirrhotic patients.58–60 Although HVPG-guided therapy for GEV can lead to a determination of the optimal treatment,61,62 a major limitation of HVPG measurement is its invasiveness. If a more convenient, simple, and inexpensive method for estimating portal pressure could be developed, it would contribute greatly to the management of GEV in cirrhotic patients. Recently, elastography techniques and serum biomarkers have been introduced into clinical practice. These methods could be promising alternatives to HVPG measurement.
Elastography techniques have been studied to determine their usefulness in predicting EV and determining which cases present a high risk of bleeding. These techniques are referred to as ultrasound and magnetic resonance elastography. Regarding ultrasound elastography, liver and spleen stiffness values are correlated with HVPG and are useful for predicting the presence of EV in cir-rhotic patients.63–67 Some researchers have demonstrated that spleen stiffness values can be used to identify patients with EV or high-risk EV.65,66 Furthermore, a recent study of ultrasound elastography demonstrated that changes in liver stiffness values are correlated with changes in HVPG, suggesting that this technique could be helpful for monitoring portal hypertension-associated conditions, including GEV.68 Studies of magnetic resonance elastography have found that liver and spleen stiffness values are significantly associated with EV grade.69,70
Among serum biomarkers, FibroTestTM (Biopredic-tive, Paris, France) has been used most extensively for noninvasively assessing the status of EV in cirrhotic patients. In an earlier study, a significant correlation between FibroTestTM and HVPG was reported; however, this correlation was weaker in cirrhotic compared with non-cirrhotic patients.71 More recently, a large-scale study of patients with chronic HCV infection demonstrated that FibroTestTM is predictive of the presence of EV as well as of severe complications, including bleeding from EV. The predictive performance of FibroTestTM for severe complications is similar to that of ultrasound elastography.72
As stated in the Baveno VI workshop, further studies are required to establish the use of noninvasive methods for estimating portal pressure and to assess the status of GEV.24 Such methods would minimize endoscopic examination of cirrhotic patients and contribute to the more timely and appropriate planning of therapeutic strategy for the treatment of GEV in such patients.
Prevention of GEV formation and GEV regressionAs mentioned, various treatment methods have enabled prophylaxis of variceal bleeding as well as emergent he-mostasis for bleeding in cirrhotic patients. However, no methods have been established for preventing the formation of GEV [preprimary prophylaxis73] and for regressing GEV. A randomized controlled study reported that timolol, a non-selective β-blocker, did not prevent the formation of GEV in patients with cirrhosis and portal hypertension.74 If a method for reversing cirrhosis was available, it could decrease portal pressure, thereby preventing the formation of GEV or regressing GEV.
Recent medical advances have eliminated or minimized causes of cirrhosis, with the potential to ameliorate liver fibrosis and reverse cirrhosis. For example, longterm treatment with nucleotide or nucleoside analogues for hepatitis B virus (HBV)-related chronic liver disease resulted in the reversal of advanced liver diseases, including cirrhosis.75,76 Studies of HCV-related cirrhosis indicate that liver fibrosis can be ameliorated in patients who achieved a sustained viral response (SVR) via interferon therapy.77–79 SVR after interferon therapy significantly reduces HVPG in patients with HCV-related cirrhosis.80 Furthermore, a recent study of interferon therapy in patients with HCV-related cirrhosis reported that none of the SVR patients developed EV compared with 31.8% of untreated subjects and 39.1% of non-SVR patients.81 Most recently, newly developed direct-acting antivirals (DAAs) have achieved higher SVR rates (> 90%) in patients with HCV-related cirrhosis82–84 compared with interferon-based therapy,85,86 suggesting a possible method for increasing the reversal of HCV-re-lated cirrhosis. Additionally, a study of alcoholic cirrhosis revealed that abstinence from alcohol led to a decrease in HVPG and the regression of EV and prevented bleeding from EV.59
In contrast, a recent study of patients with chronic HBV infection revealed that 3% of non-cirrhotic patients, including treatment responders, developed cir-rhosis.87 Similarly, a study of interferon therapy in patients with chronic HCV infection reported that 9% of non-cirrhotic patients developed cirrhosis after SVR.88 In another study of patients with HCV-related compensated cirrhosis who had achieved SVR after interferon therapy, 36% exhibited exacerbated EV. This study found that the existence of portosystemic collateral vessels at the initiation of interferon therapy is a risk factor of EV ex-acerbation.89 These findings suggest that causal therapy for cirrhosis is not always a sufficient measure for preventing GEV formation or for regressing GEV, especially when advanced cirrhosis is present. Recently, antifibrot-ic agents have been developed, and some are the subjects of clinical trials for chronic liver disease.90–92 This therapeutic method directly ameliorates liver fibrosis and thus has the potential to prevent GEV formation or promote GEV regression, even in patients with advanced cirrhosis. Future studies should focus on assessing the usefulness of antifibrotic therapy for managing GEV in cirrhotic patients.
ConclusionDue to intensive study, the strategies used to manage GEV have evolved considerably over several decades. However, prophylactic and emergent treatments for GEV have not been sufficiently established. In particular, evidence supporting an optimal treatment method for GV is rare compared with that for EV. Because the clinical course of GEV mainly depends on the degree of portal hypertension, therapies for GEV based on the estimation of portal pressure are preferable in any clinical situation. HVPG measurement is the gold standard for this estimation, but its invasiveness has hampered its introduction into clinical practice. The use of elastography techniques and serum biomarkers might overcome the current difficulty in estimating portal pressure, which would allow for more appropriate treatment of GEV. Furthermore, recent medical advances, including the advent of highly effective antiviral agents for HBV and HCV infection, have enabled the reversal of cirrhosis. These advances might pave the way for preventing (preprimary prophylaxis) and regressing GEV.
Abbreviations- •
BRTO: balloon-occluded retrograde transvenous obliteration.
- •
CSPH: clinically significant portal hypertension.
- •
DAAs: direct-acting antivirals.
- •
EIS: endoscopic injection sclerotherapy.
- •
EV: esophageal varices.
- •
EVL: endoscopic variceal ligation.
- •
GEV: gastroesophageal varices.
- •
GOV2: gastric varices as extensions of esophageal varices along the fundus.
- •
GV: gastric varices.
- •
HBV: hepatitis B virus.
- •
HCV: hepatitis C virus.
- •
HVPG: hepatic venous pressure gradient.
- •
IGV: isolated gastric varices.
- •
NO: nitric oxide.
- •
RCT: randomized controlled trial.
- •
SVR: sustained viral response.
- •
TIPS: transjugular intrahepatic portosystemic shunt.
We declare that there are no conflicts of interest associated with this study.