Background. Chronic hepatitis C(CHC) staging is important for therapeutic decision-making. Identification of noninvasive markers can provide alternatives to liver biopsy.
Aim. To assess the value of APRI and FIB4 for CHC fibrosis staging in a cohort of nonselected outpatients from a referral center in Sao Paulo, Brazil.
Material and methods. Medical records of 798 adult outpatients were analyzed retrospectively. For calculations of APRI and FIB4, the original descriptions were considered, and markers were compared with degree of liver injury.
Results. Overall, 49.3% of participants were female, and mean age was 56.9 ± 12.5 years. Genotype 1 was predominant (71.7vs. 23.7% genotype 3); 64% had significant fibrosis, 44% had advanced fibrosis, and 28% had cirrhosis. The areas under the receiver operating curve for significant fibrosis, advanced fibrosis, and cirrhosis, respectively, were 0.809, 0.819, and 0.815 for the APRI marker and 0.803, 0.836 and 0.852 for FIB4. Using the recommended cut off values, approximately 30-40% of the patients could not be classified. In the remainder, either APRI or FIB4 alone correctly diagnosed 80-85% of cases. Concomitant or consecutive use of both APRI and FIB4 increased the number of the cases correctly diagnosed only slightly, but also increased the number of patients not classified within the cutoff values.
Conclusions. In conclusion, use of the APRI or FIB4 markers for detection of hepatic fibrosis may be a viable alternative at referral centers for treatment of CHC in low- and middleincome countries. Despite relatively good accuracy, a significant number of patients could not be assessed by these methods.
Chronic hepatitis C virus infection (CHC)currently affects 185 million people worldwide.1 It is the leading cause of cirrhosis of the liver and hepatocellular carcinoma, the leading indication for liver transplantation in the United States and possibly worldwide, and an indisputably serious public health issue.2,3
Due to high rates of progression to chronic disease and progressive fibrosis,4,5 histological assessment of the degree of liver injury is an important part of the decisionmaking process in evaluating the prognosis of hepatitis C infection and indicating antiviral treatment.
The diagnosis of hepatic fibrosis is primarily morphological, and is established by histological examination of hepatic biopsy specimens. Although it is a relatively safe and reliable procedure, hepatic biopsy is not devoid of risk6 and is subject to sampling error and inter- and intra-observer variation in the assessment of fibrosis.7–9
In developing countries, management of hepatitis C and access to medicines must take into account the financial burden of disease control programs. Both medicines and diagnostic methods are costly, and the promotion of their rational use and prioritization of access to the neediest cases are urgently required. The World Health Organization (WHO) recently proposed that low- and middle-income countries implement the use of AST-to-platelet ratio index (APRI) and Fibrosis-4(FIB4) values for the staging of hepatitis C virus disease, in view of their convenience, easy access, and accuracy.10 The APRI is an indirect biochemical marker of fibrosis,which takes into account the serum level of aspartate aminotransferase (AST) and platelet count for disease staging, with good accuracy, as originally described by Wai, et al.11 The FIB412,13 also uses data routinely available in clinical practice, namely AST, alanine aminotransferase (ALT), platelet count, and patient age; it is non-inferior to the APRI for detection of overall fibrosis and believed to be superior to the APRI for identification of advanced fibrosis (F3-F4). These tests have the advantage of being easy to perform, even at the bedside, and carry no additional costs.
The present study will assess the value of the noninvasive APRI and FIB4 tests as a strategy for identification of hepatic fibrosis in a cohort of nonselected patients seen at a referral center for hepatitis C care in São Paulo, Brazil.
Material and MethodsA retrospective analysis was conducted of the medical records of adult patients from the Chronic Liver Disease Clinic of the Gastroenterology Division, Department of Internal Medicine, Federal University of São Paulo, a referral center for patients with hepatitis C in the city of São Paulo, Brazil. The inclusion criterion was a diagnosis of hepatitis C virus infection confirmed by polymerase chain reaction (PCR)detection of HCV-RNA in peripheral blood (Amplicor-HCV, Roche Diagnostics). HCV genotyping was performed by sequencing of the 5’ non-coding region, and viral load was measured by quantitative RTPCR (Amplicor, Roche).
Patients with hepatitis B virus or HIV coinfection, chronic liver disease of other etiologies, hepatocellular carcinoma, history of antiviral therapy, history of liver transplantation, current immunosuppressant therapy, alcohol intake ≥ 20g/day (male or female), or insufficient tissue for liver biopsy analysis were excluded. All eligible patients, except those with clinical, sonographic, and/or endoscopic manifestations of cirrhosis underwent percutaneous liver biopsy. Specimens were embedded in paraffin and stained with hematoxylin and eosin (H&E), reticulin, and Masson’s trichrome. On specimen analysis, structural injury was assessed using the METAVIR classification.14
ALT and AST levels were measured by automated kinetic methods in blood collected after a 12-h fast and expressed as IU/mL. The platelet count was measured by an automated analyzer and expressed as platelets/mm3. The mean interval between measurement of biochemical parameters and liver biopsy was less than 3 months.
The APRI11and FIB413 were calculated using the following formulas, as originally reported:
- •
APRI = AST(*ULN)/ platelet count (*109/L) × 100.
- •
FIB4 = AST(IU/mL) x age / platelet count (*109/L) × ALT (IU/mL)1/2.
The study protocol was approved by the Federal University of São Paulo Research Ethics Committee.
Statistical analysisReceiver operating curves (ROC) were used to analyze the accuracy of the noninvasive APRI and FIB4 tests for disease staging. The discriminant ability of each test was obtained by calculating the sensitivity, specificity, positive predictive value, and negative predictive value. P-values ≤ 0.05 were deemed statistically significant. All data were analyzed using the SPSS statistical package, version 17.0 (SPSS Inc., Chicago, IL, USA).
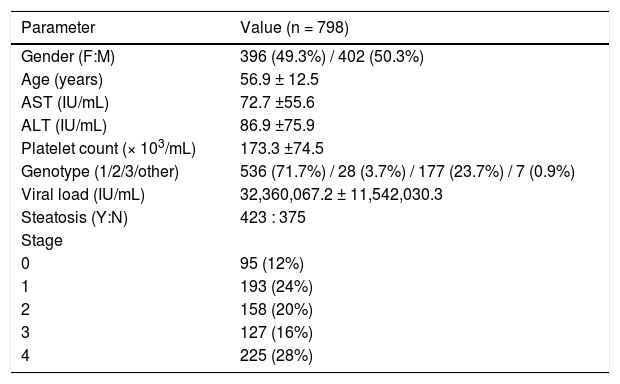
ResultsA total of 798 patients with chronic hepatitis C were assessed. Of these, 396 (49.3%) were female, with a mean age of 56.9 ± 12.5 years. Regarding HCV genotype, 536 (71.7%) were infected with genotype 1 and 177 (23.7%) with genotype 3. The mean AST, ALT, and platelet countvalues were 72.7 ± 55.6IU/mL, 86.9 ± 75.9IU/mL, and 173.3 ± 74.5 platelets/mm3, respectively. On histological examination of biopsy specimens, 288 patients (36%) had no fibrosis or early-stage fibrosis (F0-F1) and the remaining 64% had significant fibrosis (≥ F2), 28% of whom had cirrhosis (F4). Portal/periportal activity was grade ≥ 2in 520 patients (76.2%). Steatosis was present in 423 patients (63%). The demographic characteristics and biochemical and viral parameters of the sample are presented in table 1.
Sample profile.
| Parameter | Value (n = 798) |
|---|---|
| Gender (F:M) | 396 (49.3%) / 402 (50.3%) |
| Age (years) | 56.9 ± 12.5 |
| AST (IU/mL) | 72.7 ±55.6 |
| ALT (IU/mL) | 86.9 ±75.9 |
| Platelet count (× 103/mL) | 173.3 ±74.5 |
| Genotype (1/2/3/other) | 536 (71.7%) / 28 (3.7%) / 177 (23.7%) / 7 (0.9%) |
| Viral load (IU/mL) | 32,360,067.2 ± 11,542,030.3 |
| Steatosis (Y:N) | 423 : 375 |
| Stage | |
| 0 | 95 (12%) |
| 1 | 193 (24%) |
| 2 | 158 (20%) |
| 3 | 127 (16%) |
| 4 | 225 (28%) |
Values expressed as n (%) or mean ± standard deviation as appropriate. F: female. M: male. AST: aspartate aminotransferase. ALT: alanine aminotransferase.
Y: yes. N: not. Genotyping was indeterminate in 50 patients (0.6%).
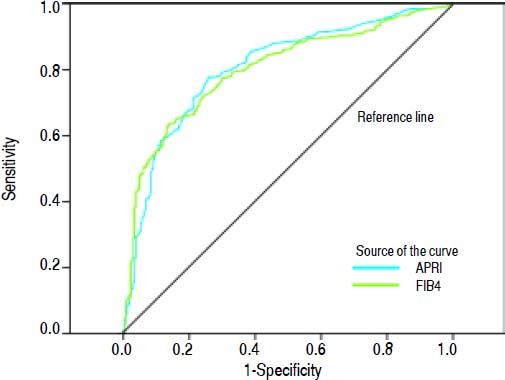
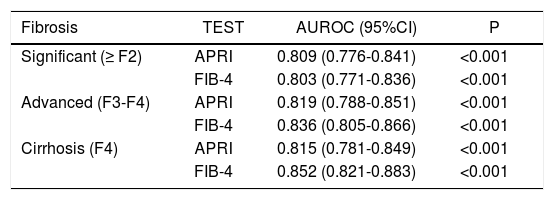
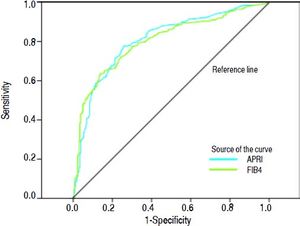
Areas under the ROC (AUROCs) for the evaluated tests at each degree of fibrosis (significant, advanced, and cirrhosis) are shown in table 2 and figure 1.
Diagnostic accuracy of the APRI and FIB-4 tests for different stages of hepatic fibrosis.
| Fibrosis | TEST | AUROC (95%CI) | P |
|---|---|---|---|
| Significant (≥ F2) | APRI | 0.809 (0.776-0.841) | <0.001 |
| FIB-4 | 0.803 (0.771-0.836) | <0.001 | |
| Advanced (F3-F4) | APRI | 0.819 (0.788-0.851) | <0.001 |
| FIB-4 | 0.836 (0.805-0.866) | <0.001 | |
| Cirrhosis (F4) | APRI | 0.815 (0.781-0.849) | <0.001 |
| FIB-4 | 0.852 (0.821-0.883) | <0.001 |
AUC: area under the curve. 95% CI: 95% confidence interval.
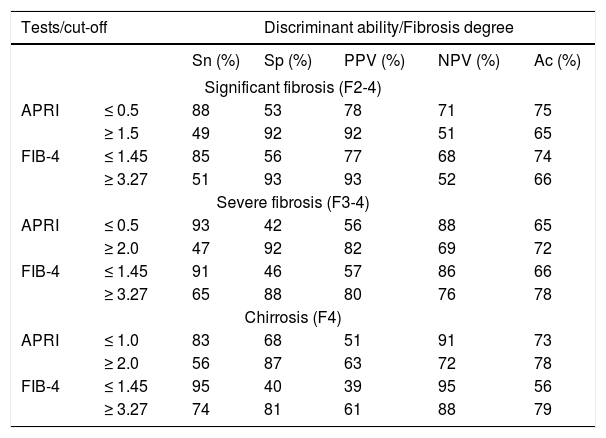
Measures of discriminant ability for the APRI and FIB4 tests for each stage of hepatic fibrosis, with optimal cutoff points, are shown in table 3.
Discriminant ability of the APRI and FIB-4 tests for identification of significant fibrosis (F2-F4), severe fibrosis (F3-4) and cirrhosis (F4).
| Tests/cut-off | Discriminant ability/Fibrosis degree | |||||
|---|---|---|---|---|---|---|
| Sn (%) | Sp (%) | PPV (%) | NPV (%) | Ac (%) | ||
| Significant fibrosis (F2-4) | ||||||
| APRI | ≤ 0.5 | 88 | 53 | 78 | 71 | 75 |
| ≥ 1.5 | 49 | 92 | 92 | 51 | 65 | |
| FIB-4 | ≤ 1.45 | 85 | 56 | 77 | 68 | 74 |
| ≥ 3.27 | 51 | 93 | 93 | 52 | 66 | |
| Severe fibrosis (F3-4) | ||||||
| APRI | ≤ 0.5 | 93 | 42 | 56 | 88 | 65 |
| ≥ 2.0 | 47 | 92 | 82 | 69 | 72 | |
| FIB-4 | ≤ 1.45 | 91 | 46 | 57 | 86 | 66 |
| ≥ 3.27 | 65 | 88 | 80 | 76 | 78 | |
| Chirrosis (F4) | ||||||
| APRI | ≤ 1.0 | 83 | 68 | 51 | 91 | 73 |
| ≥ 2.0 | 56 | 87 | 63 | 72 | 78 | |
| FIB-4 | ≤ 1.45 | 95 | 40 | 39 | 95 | 56 |
| ≥ 3.27 | 74 | 81 | 61 | 88 | 79 | |
Sn: sensitivity. Sp: specificity. PPV: positive predictive value. NPV: negative predictive value. Ac: accuracy.
With these data, and using the combined cutoff values to rule out (negative predictive value, NPV) or confirm (positive predictive value, PPV) the degree of fibrosis of interest, we were able to assess the diagnostic accuracy of the tests employed and the percentage of patients not evaluated within the defined cutoff points for the different degrees of fibrosis.
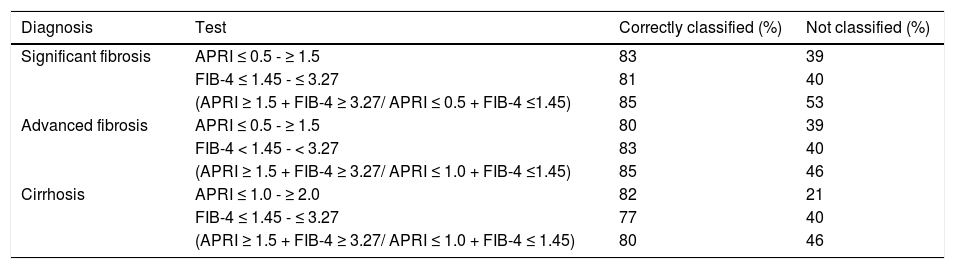
On average, approximately one-third of patients would require another method for assessment of hepatic fibrosis (Table 3), as their values were not located between the given cutoff points. After exclusion of these unclassified patients, on average, 80% of diagnoses were correct, with equivalence of the evaluated tests for significant fibrosis. Whereas FIB4 performed better for advanced fibrosis, APRI lower and upper values performed better for diagnosis of cirrhosis, despite the better AUROC obtained for the FIB4 test.
In an attempt to increase the reliability of these tests, we evaluated their use sequentially, as in the SAFE biopsy algorithm,15 and in combination, as in the Fibropaca algorithm.16
Sequential use of the APRI and FIB4 tests did not provide any advantage. For significant fibrosis, for instance, we used an APRI cutoff of ≥ 1.5, which had a PPV of 92%; however, approximately 50% of patients with grade F2 or higher had values below this cutoff point. An attempt at sequential “rescue” use of the FIB4 in these patients produced a modest increment in the number of correctly classified cases (to 55 from 51%) and modestly reduced the rate of unclassified patients (to 34 from 38%).
Combined application of the tests improved their discriminant ability for the different degrees of hepatic fibrosis and reduced the number of incorrectly diagnosed cases. However, a greater number of cases were excluded from analysis, as the obtained values fell outside the cutoff range (Table 4).
Percentage of cases with significant fibrosis (F2-F4), advanced fibrosis (F3-F4), and cirrhosis (F4) classified with the APRI and FIB-4 tests.
| Diagnosis | Test | Correctly classified (%) | Not classified (%) |
|---|---|---|---|
| Significant fibrosis | APRI ≤ 0.5 - ≥ 1.5 | 83 | 39 |
| FIB-4 ≤ 1.45 - ≤ 3.27 | 81 | 40 | |
| (APRI ≥ 1.5 + FIB-4 ≥ 3.27/ APRI ≤ 0.5 + FIB-4 ≤1.45) | 85 | 53 | |
| Advanced fibrosis | APRI ≤ 0.5 - ≥ 1.5 | 80 | 39 |
| FIB-4 < 1.45 - < 3.27 | 83 | 40 | |
| (APRI ≥ 1.5 + FIB-4 ≥ 3.27/ APRI ≤ 1.0 + FIB-4 ≤1.45) | 85 | 46 | |
| Cirrhosis | APRI ≤ 1.0 - ≥ 2.0 | 82 | 21 |
| FIB-4 ≤ 1.45 - ≤ 3.27 | 77 | 40 | |
| (APRI ≥ 1.5 + FIB-4 ≥ 3.27/ APRI ≤ 1.0 + FIB-4 ≤ 1.45) | 80 | 46 |
Chronic hepatitis C virus infection is characterized by high rates of progression to chronic liver disease, with progressive hepatic fibrosis, potentially leading to cirrhosis and its complications.4,5 For many years, liver biopsy has been regarded as the gold standard for structural evaluation of hepatic tissue, despite several studies that have demonstrated its “imperfect” status due to its limitations. The accuracy of liver biopsy is directly related, among other factors, to the size of the tissue fragment obtained and to the experience of the examiner.7–9 Furthermore, it is invasive, costly, and not entirely risk-free. Consequently, its performance is refused by many patients and impossible in others.
Within this context, the identification of noninvasive markers of liver fibrosis has been the object of extensive research, not in an attempt to replace liver biopsy altogether but in the hope of restricting its use to specific cases. The use of noninvasive markers for assessment of liver fibrosis is considered preferable to invasive testing in the recent World Health Organization Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection.10 The APRI, FIB4, Fibrotest®, and transient elastography were all recommended in this guideline; however, for low- and middle-income countries, the APRI and FIB4 were specifically recommended for their very low cost, their ease of access, their use of tests routinely ordered in clinical practice (AST, ALT, and platelet count), and their substantial accuracy for identification of fibrosis and cirrhosis.
In its original report, the APRI11 had an AUROC of 0.80 and 0.89 for identification of significant fibrosis (Ishak 3-6) and 0.88 and 0.94 for identification of cirrhosis (Ishak 5-6). Using the high (APRI > 2) and low (APRI < 0.5) cutoff points, this test had a discriminant ability of 81% for presence and absence of cirrhosis. Likewise, it was able to predict significant fibrosis in 51% of patients. In a previous study by our group analyzing a cohort of selected patients, we found similar results for identification of significant fibrosis (F2-F4) and cirrhosis (F4).17 Later studies assessing the accuracy of this method for prediction of fibrosis reported less robust results. In two recent meta-analyses including over 8,000 patients, the APRI exhibited very similar diagnostic performance for identification of significant fibrosis (AUROC = 0.77 and 0.76) and cirrhosis (AUROC = 0.83 and 0.82).18,19
The FIB4 was originally assessed in a cohort of patients with HIV/HCV coinfection,12 with an AUROC of 0.74 and 0.71 to predict fibrosis (F4-6 and Ishak F2-6), and later validated in individuals with HCV monoinfection,13 with an AUROC of 0.85 for METAVIR F3-F4 fibrosis. In a large U.S. cohort, the FIB4 had an AUROC of 0.83 for identification of advanced fibrosis (F3-4). Of the 981 patients with a FIB4 score >2.0, 87.9% had grade >F2.20
The objective of the present series was to assess the diagnostic value of these noninvasive tests in a real-world setting, namely, in the daily practice of the outpatient liver disease clinic of a tertiary referral center. Toward this end, liver enzyme measurements and platelet counts were collected retrospectively from patient records. There was no specific research protocol for determination of the parameters of interest.
Under these conditions, the AUROCs obtained for the APRI and FIB4 tests for detection of significant fibrosis, advanced fibrosis, and cirrhosis were approximately 0.80 (Table 2, Figure 1), a value quite close to those previously reported in the literature, which demonstrates the good reproducibility of these tests even in real-world clinical practice.
As recommended by WHO, our analyses used the established combination of low and high cutoff values for these biomarkers for detection of fibrosis, thus avoiding the creation of new indices that might hinder practical application of the evaluated tests. These cutoff values (0.5, 1.5, and 2.0 for the APRI and 1.45 and 3.27 for the FIB4) were thus used for detection of significant fibrosis (≥ F2), advanced fibrosis (F3-F4), and cirrhosis (F4). As shown in table 3, the APRI and FIB4 had similar diagnostic ability, except for detection of cirrhosis, in which the APRI proved superior. Strikingly, approximately 30% of patients could not be classified with either of these tests. Therefore, we chose to combine the two in an attempt to increase the number of correctly diagnosed cases. A similar strategy was employed by Sebastiani, et al., who used the APRI and Fibrotest sequentially in the SAFE biopsy study.15 In the present study, however, sequential use of the APRI and FIB4 tests did not result in a significant reduction in the number of undiagnosed cases, as also reported by Crisan, et al.,21 which shows that these biomarkers have very similar diagnostic profile. When using both tests concomitantly, we observed improvement in discriminant ability for the different stages of fibrosis, as well as a reduction in the number of misdiagnosed cases, as compared with either tests in isolation or both tests sequentially. However, this was offset by a significant loss in the number of patients covered; approximately 50% of patients in our sample remained unclassified with this strategy.
The combination of these serum markers and mechanical methods, particularly FibroScan® transient elastography, would probably increase their diagnostic efficacy and reduce the number of patients with an indeterminate result. This hypothesis is based on the fact that nearly half of all patients with significant fibrosis and an APRI <1.5 had METAVIR grade 3 or 4 fibrosis – precisely the patient population in which elastometry is most indicated.22,23 Such an increase in diagnostic accuracy when combining mechanical methods and biomarkers has already been reported in published studies.24–26
Some limitations of the present study must be stressed, including its retrospective design and its setting (specialty care centers for patients with chronic hepatitis), which justifies the high percentage of patients with cirrhosis and advanced fibrosis. The retrospective design might have an impact on the sensitivity and specificity of the evaluated tests, as well as on the selected cases. The attrition rate of our study due to missing data was only approximately 1%, as the blood tests used for calculation of the APRI and FIB4 scores are performed as part of these patients’ routine care. On the other hand, the present analysis sought to portray a real-world patient care environment, with no interference from especially designed protocols for collection of biochemical parameters. We believe referral centers are the most appropriate setting for application of these tests, as they are the venues where treatment-related decisions are made; however, their implementation in primary care settings, where a lower prevalence of advanced cases is to be expected, would also be of the utmost importance.
We conclude that use of the APRI or FIB-4 tests for detection of hepatic fibrosis may be a viable alternative for routine practice at referral centers for treatment of chronic hepatitis C in low- or middle-income settings. Their diagnostic accuracy of approximately 80% in cases amenable to classification would provide a more than reasonable rate of correct diagnosis. However, the foremost factor to be considered is the number of patients with advanced disease who might lie outside the detection rate of the two methods, as these are precisely the patients that require treatment most urgently. Combination of the serum markers with commercially available biomarkers or transient elastography could improve this scenario significantly, but cost would then become a significant factor in the equation. Therefore, we believe that, taking into account the limitations of these methods, isolated use of the APRI or FIB4 tests should be considered for low-and middleincome countries or in settings where access to referral centers is difficult.
Abbreviations- •
Ac: accuracy.
- •
ALT: alanine aminotransferase.
- •
APRI: AST-to-platelet ratio index.
- •
AST: aspartate aminotransferase.
- •
AUROC: area under the receiver operating curve.
- •
CHC: chronic hepatitis C.
- •
FIB4: fibrosis 4.
- •
H&E: hematoxylin and eosin.
- •
HCV: hepatitis C virus.
- •
HCV-RNA: HCV ribonucleic acid.
- •
HIV: human immunodeficiency ví+irus.
- •
NPV: negative predictive value.
- •
PCR: polymerase chain reaction.
- •
PPV: positive predictive value.
- •
Sn: sensitivity.
- •
Sp: specificity.
- •
WHO: World Health Organization.