Universal vaccination at birth and in infancy is key to the elimination of chronic hepatitis B infection. We aimed to assess hepatitis B immune-prophylaxis and perinatal transmission knowledge, in a large and ethnically diverse cohort of previously pregnant North American women, chronically infected with hepatitis B.
Materials and methodsThe Hepatitis B Research Network (HBRN) is comprised of 28 Clinical Centers in the United States and Canada. Female cohort participants were administered a questionnaire to assess: (1) their assertion of knowledge regarding HBV prophylaxis at birth, testing, and diagnosis of hepatitis B in their children, and (2) the percentage of affirmative to negative responses for each of the HBV-related interventions her child may have received. The relationship between asserted knowledge, actions taken and maternal demographics were assessed.
ResultsA total of 351 mothers with 627 children born in or after 1992 were included. Median age at enrollment was 39.8 years. Mothers were mostly foreign-born with the largest percentage from Asia (73.4%) and Africa (11.7%). Of the 627 children, 94.5% had mothers who asserted that they knew whether their child had received HBIG or HBV vaccine at birth, for 88.8% of the children, their mothers indicated that they knew if their child was tested for HBV and for 84.5% of children, their mothers knew if the child was diagnosed with HBV infection. Among children whose mothers asserted knowledge of their HBV management, 95.3% were reported to have received HBIG or HBV vaccine, 83.4% of children were said to have been tested for HBV, and 4.8% of children were said to have been diagnosed with HBV. Younger maternal age was the only factor significantly associated with higher percentage of children for whom mothers reported knowledge of testing (p=0.02) or diagnosis of HBV (p=0.02).
ConclusionsWhile high percentages of North American children had mothers asserting knowledge of HBV prophylaxis and testing, knowledge gaps remain, with mothers of 5.5–15.5% of children lacking knowledge of key components of the HBV prevention and diagnosis in the perinatal setting. Targeted education of HBsAg-positive mothers may aid in closing this gap and reducing vertical transmission.
Hepatitis B virus (HBV) infects over 240 million individuals worldwide [1] and chronic hepatitis B (CHB) is a significant cause of end stage liver disease and liver cancer. Prevention of vertical transmission of HBV is crucial for the elimination of chronic hepatitis B and its associated morbidity and mortality [2]. In 2014, the global coverage of HBV vaccination at birth was estimated at 38%, and up to 30 million newborns were still unvaccinated in countries that provided universal birth dose vaccine as part of their national immunization programs [3].
In the U.S. [4] and Canada [5], universal childhood HBV vaccination has been recommended since 1991. However, the current estimated universal birth dose coverage in the U.S. remains suboptimal at about 71% [6,7].
Specific recommendations and implementation strategies vary by country. Some countries recommend that the combination of hepatitis B vaccine and hepatitis B immunoglobulin (HBIG) be administered to the infants of mothers positive for HBsAg (65): the Advisory Committee on Immunization Practices (ACIP) recommends both within 12h of delivery [8]. The American Association for the Study of Liver Disease and the ACIP recommend HBsAg and anti-HBs testing for children born to HBsAg-positive women when they are at 9–12 months of age [9,10], and the ACIP also recommends counseling HBsAg positive mothers regarding the need for postnatal vaccination and HBIG, completing vaccination series, and post-vaccination serologic testing [7].
Limited studies have shown that a proportion of expectant mothers from mostly endemic countries have suboptimal knowledge related to HBV infection and its transmission [9–11]. For example, 76% of pregnant women in Nigeria had inadequate HBV knowledge [8] and in a recent study from China, 20% of pregnant women were not aware that HBV can be transmitted from mother to infant; however, over 80% were willing to be screened for HBV and have their babies receive HBV vaccine and HBIG [11]. Moreover, certain factors such as maternal age, education, occupation and family set-up may be associated with knowledge and understanding of HBV and the maternal-to-child transmission risk [10]. There is a scarcity of data on HBV-infected mothers’ knowledge of the use of immune prophylaxis and perinatal transmission in a low endemic area like North America.
We aimed to assess the overall knowledge and factors associated with knowledge of hepatitis B immune prophylaxis and perinatal transmission in a large and ethnically diverse North American cohort of previously pregnant women with CHB who were enrolled in the Hepatitis B Research Network (HBRN) Cohort Study. Evaluating gaps in maternal knowledge is critical to implement effective prevention strategies and to enhance the recommended immune prophylaxis programs and limit maternal transmission of HBV in high-risk populations.
2Material and methodsThe Hepatitis B Research Network (HBRN) is comprised of 28 clinical centers (21 adult and 7 pediatric centers) in the United States and Canada and a Data Coordinating Center (DCC). The HBRN includes a cohort of adults and children who were HBsAg positive at enrollment [12]. Women included in this analysis were enrolled between January 19, 2011 and April 11, 2016. The institutional review board (IRB) of each participating institution approved the protocol of this research study, and each subject gave written informed consent for their participation.
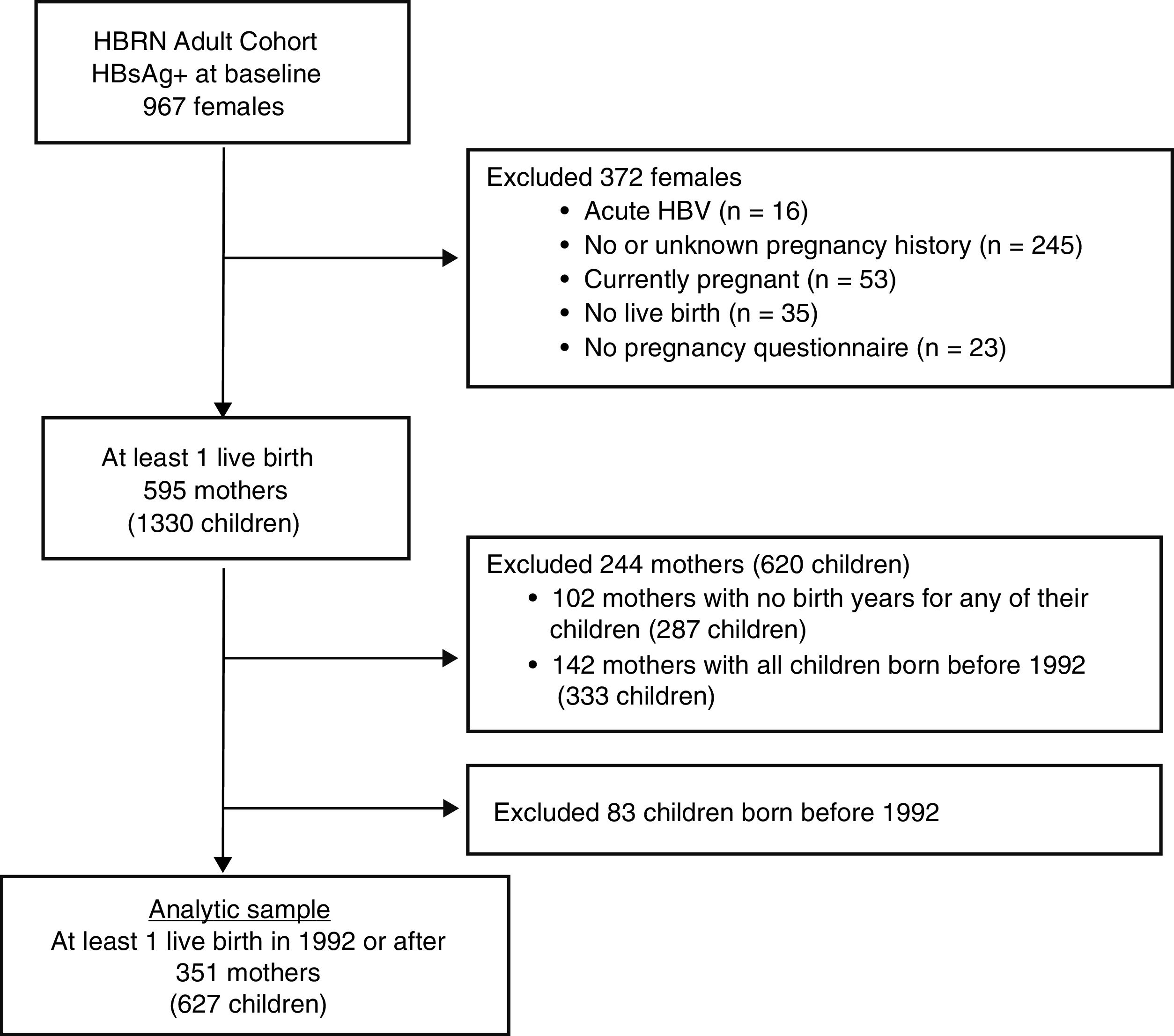
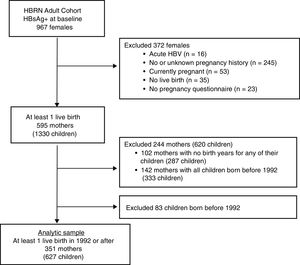
Only women with a completed pregnancy in or after 1992 (the year after the ACIP recommendation for HBV vaccination in the perinatal period was initiated [4] and who completed the 3 item questionnaire as part of an extensive data collection regarding the natural history of HBV infection in pregnancy (see Appendix for the questionnaire) were included (see Fig. 1). The questionnaire, which was administered by a study coordinator with help from an interpreter if needed, inquired about mothers’ knowledge regarding her children.
Specifically, for each of their children, mothers were asked 3 questions: whether the child: 1. received HBIG or HBV vaccine at birth, 2. was tested for hepatitis B, and 3. was diagnosed with hepatitis B. For each question, mothers could respond “Yes,” “No,” or “Do not know.” Thus, the questionnaire measured, two points: A) Assertion of knowledge (“Yes” or “No” response) versus lack of knowledge (“Do not know”) by the mother for each child born in 1992 or after, and B) For mothers that asserted knowledge (“Yes” or “No” response), the percentage of affirmative to negative responses for each of the HBV-related interventions her child may have received (Fig. 2).
Equations utilized for “Assertion of Knowledge” and “Reported Affirmative”, as seen in Tables 2A, 2B, 2C.
As the children of these mothers were not included in the HBRN cohort, the accuracy of the mother's information was not assessed. Our questionnaire focused on the mother's knowledge of her child's treatment as the source of truth for future health care providers seeing the child. So, for example, the questionnaire inquires of the mother whether each child received immunoprophylaxis, but it is not known whether or not each child actually did since the children are not included in the HBRN cohort.
The mother's demographics were examined to gain insight into potential factors associated with the survey responses. The factors assessed were age of the mother at survey completion, race, income level, education level, birth continent, child's birthplace, mother's HBV status the year of the child's birth, and the knowledge of the source of the mother's HBV infection.
2.1Statistical analysisDistributions of maternal characteristics are reported as frequencies and percentages for categorical data or as minimum, maximum, and quartiles (25th, median, 75th) for continuous data. Results of knowledge and action are presented as predicted percentages of children with 95% confidence intervals obtained from generalized estimating equations (GEE) to account for correlation of outcomes among children within mothers. p-Values are reported from unadjusted models. A p-value less than 0.05 was considered to be statistically significant. All analyses were performed using statistical analysis software (SAS).
3Results3.1Study populationBetween January 2011 and April 2016, 967 HBsAg-positive women were enrolled in the HBRN adult cohort study (Fig. 1). After excluding women with acute HBV infection and those who had no live births in 1992 or later, the final study group consisted of 351 mothers who completed the pregnancy questionnaire with 627 children born in 1992 or later. Among the 351 mothers were 124 who had only one live birth in or after 1992 and 227 women with multiple live births, at least one of which was in or after 1992.
The median age of mothers when they completed the pregnancy questionnaire was 39.8 years (51% between 18 and 40 years, 49% older than 40 years). Mothers were mostly Asian (79.2%) or Black/African American (13.4%). The majority were foreign-born with the mother's country of birth Asia in 73.4% and Africa in 11.7%. The presumed route of maternal HBV infection was vertical transmission for 65.1% of the 261 mothers for whom a mode was recorded. The education level was reported in 343 mothers with 49.6% having a bachelor's degree education or higher (Table 1).
Characteristics of the mothers (n=351).
| Maternal characteristics | n (% of mothers) |
|---|---|
| Age at visit (years) | |
| 18–40 | 178 (50.7) |
| >40 | 173 (49.3) |
| Age at visit (years) | |
| Median | 39.8 |
| (25th, 75th percentile) | (34.1, 46.1) |
| (minimum, maximum) | (19.4, 67.6) |
| Race | |
| White | 14 (4.0) |
| Black/African-American | 47 (13.4) |
| Asian | 278 (79.2) |
| American Indian | 1 (0.3) |
| Hawaiian | 7 (2.0) |
| Mixed | 4 (1.1) |
| Continent of birth | n=350 |
| Africa | 41 (11.7) |
| Asiaa | 257 (73.4) |
| Europe | 2 (0.6) |
| North America | 43 (12.3) |
| South America | 1 (0.3) |
| Australia | 6 (1.7) |
| Mode of HBV transmission | n=261 |
| Vertical transmission | 170 (65.1) |
| Horizontal transmission | 69 (26.4) |
| Adult household contact | 5 (1.9) |
| Sexually transmitted | 5 (1.9) |
| Occupational | 1 (0.4) |
| Medical/surgical | 5 (1.9) |
| Transfusion | 4 (1.5) |
| Injection drug use | 0 (0.0) |
| Cosmetic Procedure | 2 (0.8) |
| Education level | n=343 |
| Less than high school | 62 (18.1) |
| High school or equivalent (GED) | 58 (16.9) |
| Some college or equivalent | 53 (15.5) |
| Bachelor's degree or higher | 170 (49.6) |
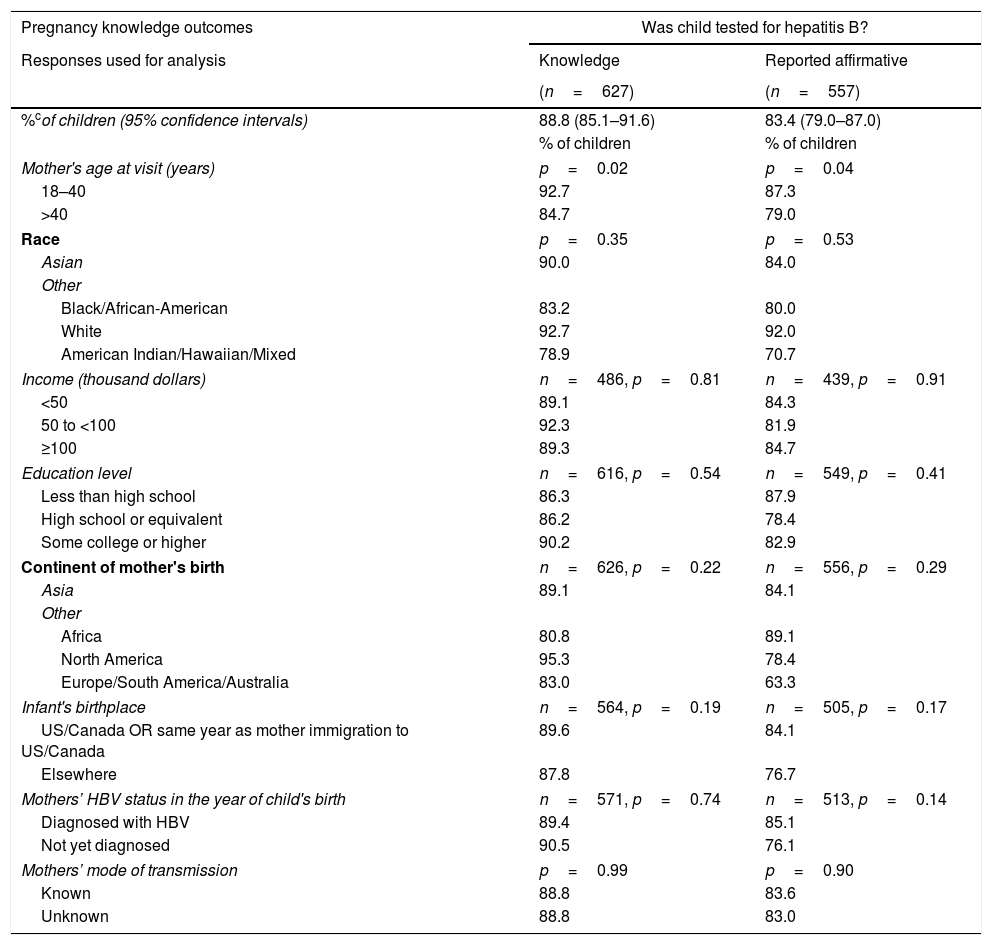
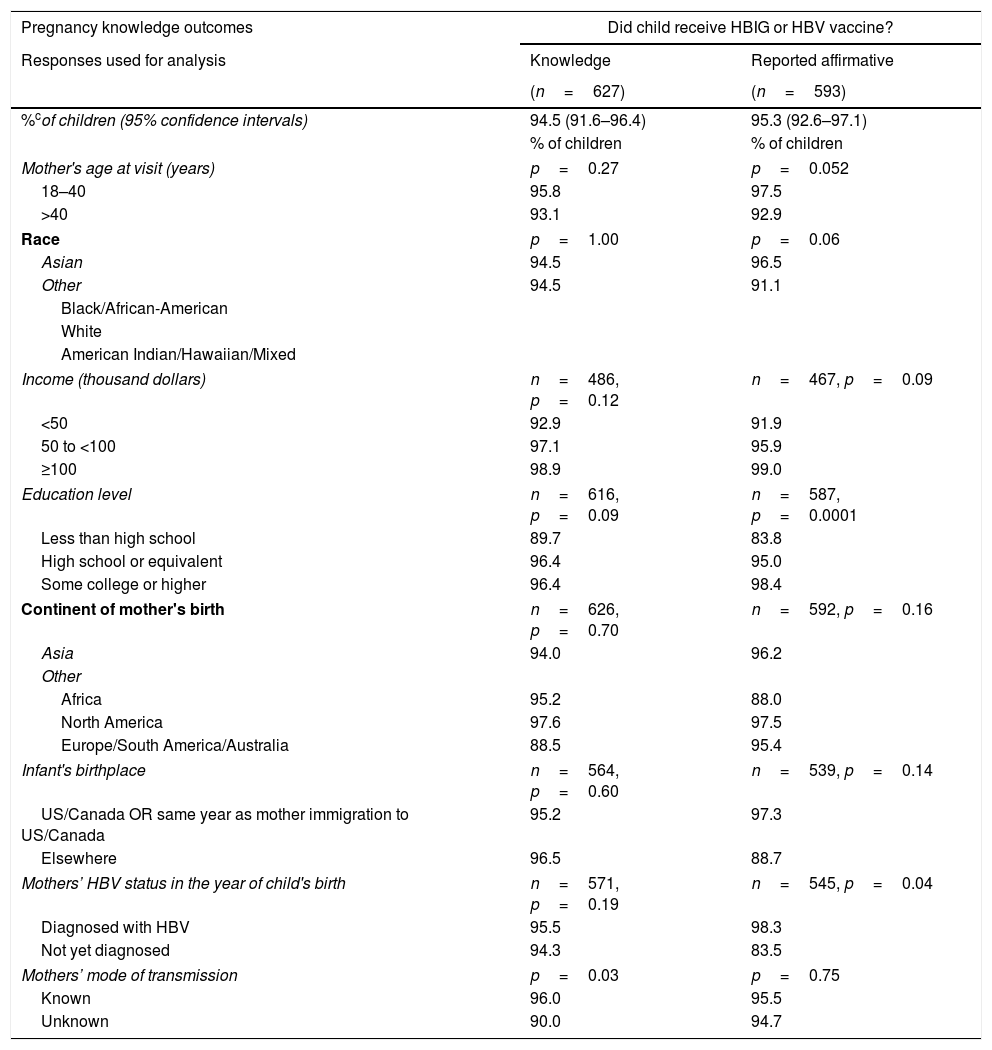
Initially (A) the questionnaire asked the mothers whether they had knowledge (“Yes” or “No” versus “Do not know”) of their child's post-natal prophylaxis, HBV testing, and HBV diagnosis. Mothers of 94.5% (95% CI: 91.6–96.4%) of the 627 children stated they knew whether or not their children received HBV vaccine or HBIG. A somewhat lower percentage of children had mothers who knew their child was tested for HBV: 88.8% (95% CI: 85.1–91.6%). Finally, 84.5% (95% CI: 80.5–87.9%) of children had mothers who asserted knowledge of whether or not their child was diagnosed with HBV.
Children of mothers who were younger (18–40 years of age vs older) when they completed the survey were more likely to have had their mothers report knowledge of whether or not they were tested for HBV (p=0.02) or were diagnosed with HBV (p=0.02) (Table 2A). Percentages of children whose mothers asserted knowledge related to testing, diagnosis or immunization were not significantly different by other variables examined (Tables 2B and 2C).
| Pregnancy knowledge outcomes | Was child tested for hepatitis B? | |
|---|---|---|
| Responses used for analysis | Knowledge | Reported affirmative |
| (n=627) | (n=557) | |
| %cof children (95% confidence intervals) | 88.8 (85.1–91.6) | 83.4 (79.0–87.0) |
| % of children | % of children | |
| Mother's age at visit (years) | p=0.02 | p=0.04 |
| 18–40 | 92.7 | 87.3 |
| >40 | 84.7 | 79.0 |
| Race | p=0.35 | p=0.53 |
| Asian | 90.0 | 84.0 |
| Other | ||
| Black/African-American | 83.2 | 80.0 |
| White | 92.7 | 92.0 |
| American Indian/Hawaiian/Mixed | 78.9 | 70.7 |
| Income (thousand dollars) | n=486, p=0.81 | n=439, p=0.91 |
| <50 | 89.1 | 84.3 |
| 50 to <100 | 92.3 | 81.9 |
| ≥100 | 89.3 | 84.7 |
| Education level | n=616, p=0.54 | n=549, p=0.41 |
| Less than high school | 86.3 | 87.9 |
| High school or equivalent | 86.2 | 78.4 |
| Some college or higher | 90.2 | 82.9 |
| Continent of mother's birth | n=626, p=0.22 | n=556, p=0.29 |
| Asia | 89.1 | 84.1 |
| Other | ||
| Africa | 80.8 | 89.1 |
| North America | 95.3 | 78.4 |
| Europe/South America/Australia | 83.0 | 63.3 |
| Infant's birthplace | n=564, p=0.19 | n=505, p=0.17 |
| US/Canada OR same year as mother immigration to US/Canada | 89.6 | 84.1 |
| Elsewhere | 87.8 | 76.7 |
| Mothers’ HBV status in the year of child's birth | n=571, p=0.74 | n=513, p=0.14 |
| Diagnosed with HBV | 89.4 | 85.1 |
| Not yet diagnosed | 90.5 | 76.1 |
| Mothers’ mode of transmission | p=0.99 | p=0.90 |
| Known | 88.8 | 83.6 |
| Unknown | 88.8 | 83.0 |
Knowledge: Estimated percentages of children whose mothers asserted that they knew whether or not child was tested, diagnosed, or received vaccine, respectively.
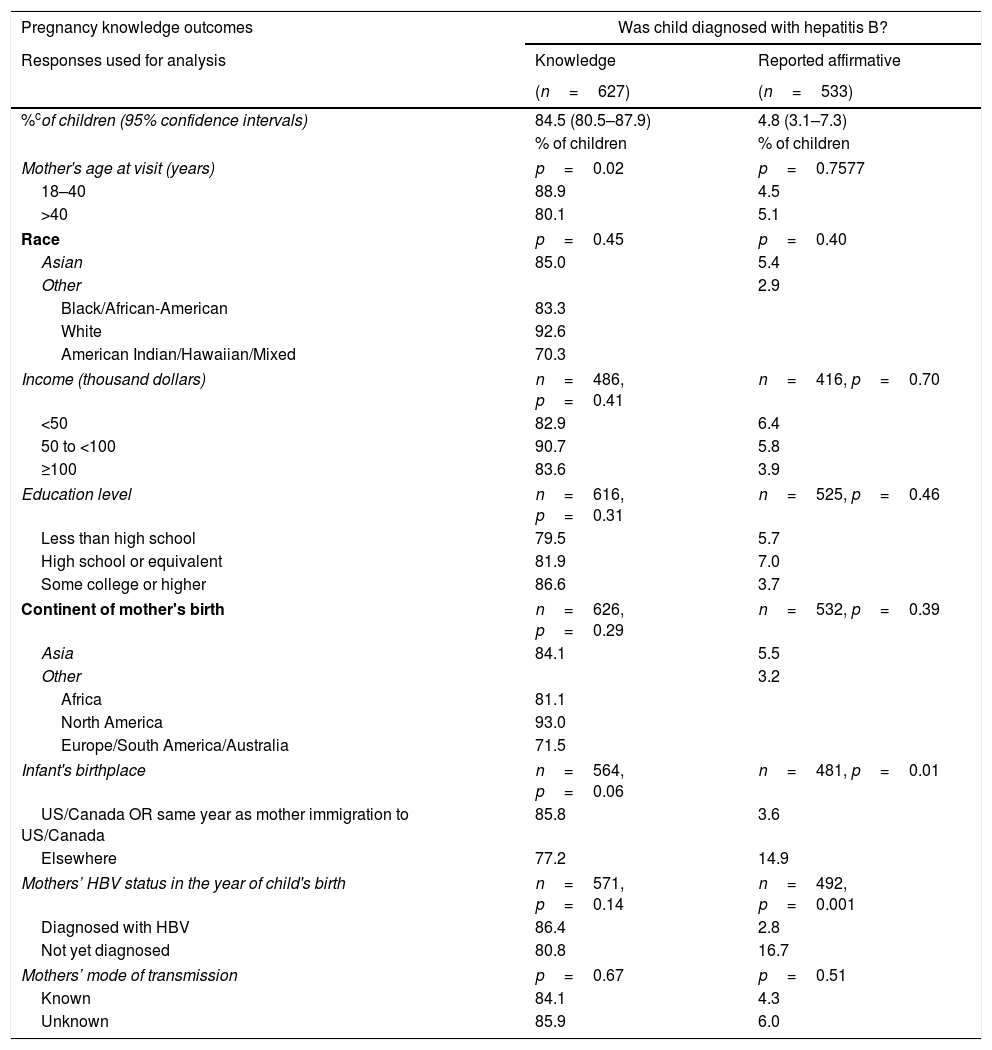
| Pregnancy knowledge outcomes | Was child diagnosed with hepatitis B? | |
|---|---|---|
| Responses used for analysis | Knowledge | Reported affirmative |
| (n=627) | (n=533) | |
| %cof children (95% confidence intervals) | 84.5 (80.5–87.9) | 4.8 (3.1–7.3) |
| % of children | % of children | |
| Mother's age at visit (years) | p=0.02 | p=0.7577 |
| 18–40 | 88.9 | 4.5 |
| >40 | 80.1 | 5.1 |
| Race | p=0.45 | p=0.40 |
| Asian | 85.0 | 5.4 |
| Other | 2.9 | |
| Black/African-American | 83.3 | |
| White | 92.6 | |
| American Indian/Hawaiian/Mixed | 70.3 | |
| Income (thousand dollars) | n=486, p=0.41 | n=416, p=0.70 |
| <50 | 82.9 | 6.4 |
| 50 to <100 | 90.7 | 5.8 |
| ≥100 | 83.6 | 3.9 |
| Education level | n=616, p=0.31 | n=525, p=0.46 |
| Less than high school | 79.5 | 5.7 |
| High school or equivalent | 81.9 | 7.0 |
| Some college or higher | 86.6 | 3.7 |
| Continent of mother's birth | n=626, p=0.29 | n=532, p=0.39 |
| Asia | 84.1 | 5.5 |
| Other | 3.2 | |
| Africa | 81.1 | |
| North America | 93.0 | |
| Europe/South America/Australia | 71.5 | |
| Infant's birthplace | n=564, p=0.06 | n=481, p=0.01 |
| US/Canada OR same year as mother immigration to US/Canada | 85.8 | 3.6 |
| Elsewhere | 77.2 | 14.9 |
| Mothers’ HBV status in the year of child's birth | n=571, p=0.14 | n=492, p=0.001 |
| Diagnosed with HBV | 86.4 | 2.8 |
| Not yet diagnosed | 80.8 | 16.7 |
| Mothers’ mode of transmission | p=0.67 | p=0.51 |
| Known | 84.1 | 4.3 |
| Unknown | 85.9 | 6.0 |
Knowledge: Estimated percentages of children whose mothers asserted that they knew whether or not child was tested, diagnosed, or received vaccine, respectively
| Pregnancy knowledge outcomes | Did child receive HBIG or HBV vaccine? | |
|---|---|---|
| Responses used for analysis | Knowledge | Reported affirmative |
| (n=627) | (n=593) | |
| %cof children (95% confidence intervals) | 94.5 (91.6–96.4) | 95.3 (92.6–97.1) |
| % of children | % of children | |
| Mother's age at visit (years) | p=0.27 | p=0.052 |
| 18–40 | 95.8 | 97.5 |
| >40 | 93.1 | 92.9 |
| Race | p=1.00 | p=0.06 |
| Asian | 94.5 | 96.5 |
| Other | 94.5 | 91.1 |
| Black/African-American | ||
| White | ||
| American Indian/Hawaiian/Mixed | ||
| Income (thousand dollars) | n=486, p=0.12 | n=467, p=0.09 |
| <50 | 92.9 | 91.9 |
| 50 to <100 | 97.1 | 95.9 |
| ≥100 | 98.9 | 99.0 |
| Education level | n=616, p=0.09 | n=587, p=0.0001 |
| Less than high school | 89.7 | 83.8 |
| High school or equivalent | 96.4 | 95.0 |
| Some college or higher | 96.4 | 98.4 |
| Continent of mother's birth | n=626, p=0.70 | n=592, p=0.16 |
| Asia | 94.0 | 96.2 |
| Other | ||
| Africa | 95.2 | 88.0 |
| North America | 97.6 | 97.5 |
| Europe/South America/Australia | 88.5 | 95.4 |
| Infant's birthplace | n=564, p=0.60 | n=539, p=0.14 |
| US/Canada OR same year as mother immigration to US/Canada | 95.2 | 97.3 |
| Elsewhere | 96.5 | 88.7 |
| Mothers’ HBV status in the year of child's birth | n=571, p=0.19 | n=545, p=0.04 |
| Diagnosed with HBV | 95.5 | 98.3 |
| Not yet diagnosed | 94.3 | 83.5 |
| Mothers’ mode of transmission | p=0.03 | p=0.75 |
| Known | 96.0 | 95.5 |
| Unknown | 90.0 | 94.7 |
Knowledge: Estimated percentages of children whose mothers asserted that they knew whether or not child was tested, diagnosed, or received vaccine, respectively.
The second point (B) measured by the questionnaire used data from mothers who asserted knowledge (only those that answered “Yes” or “No”) to understand their children's management. The proportions of affirmative responses to each question, adjusting for intra-mother correlations of her children, were calculated. According to their mothers, 95.3% (95% CI: 92.6–97.1%) of children received HBV vaccine or HBIG, 83.4% (95% CI: 79.0–87.0%) of children were tested for HBV, and 4.8% (95% CI: 3.1–7.3%) were diagnosed with HBV.
More highly educated mothers and mothers who had been diagnosed with HBV before or in the same year as the child was born, were more likely to report in the affirmative that their child received HBIG or HBV vaccine (p=0.0001 and p=0.04, respectively; Table 2). Children of mothers≤40 years old at study visit were more likely to have been reported by their mothers that they underwent HBV testing (p=0.04). A larger percentage of children born outside the United States or Canada were reported to be diagnosed with HBV (14.9% vs 3.6%, p=0.01). Children were more likely to be reported to have been diagnosed with HBV if the mothers had not been diagnosed with HBV by the year of the child's birth (16.7% vs 2.8%, p=0.001).
4DiscussionThe goal of pre-and post-natal management of children born to women infected with HBV is to prevent vertical transmission of HBV. The current strategy is administration of both HBIG and the first dose of the hepatitis B vaccine within 12h of delivery of a child born to a woman HBsAg positive or a woman with unknown HBV status [7]. Versions of this strategy have been in effect since 1991 [4]. Coupled with universal screening of pregnant women and universal vaccination, this strategy has led to substantial reductions in both chronic HBV infection in children and has lowered the rate of hepatocellular carcinoma [13–15]. The addition of an HBV anti-viral treatment after 28–32 weeks of gestation when the pregnant woman has a high viral load (>200,000IU/mL or >6 log10copies/mL) further reduces maternal-child transmission [14].
The women in HBRN who had given birth to a child in, or after, 1992, report that, in the majority of cases, medical providers follow these requirements to limit vertical transmission of hepatitis B. However, for 5.5% of the children born in the years after the recommendation for universal infant vaccination in the United States, their mothers did not know if they received HBV immunization and/or HBIG in the neonatal period and for those children whose mother did know, 4.7% of their children did not receive prophylaxis. Other studies have demonstrated variable rates of prophylaxis implementation. Data from the United Kingdom showed that 99.4% of infants born to HBsAg positive women received the birth dose of the HBV vaccine [16], however a similar retrospective analysis in Denmark showed that only 93% of children born to HBsAg positive women were vaccinated within 48h of birth [17].
Of more concern is the percentage of children whose mothers lack knowledge of their child's health status with respect to HBV prevention. In primary care clinics, mothers are often the source of information regarding their child's past medical care. Further, providers may experience gaps in HBV knowledge [18–20] and management and mothers’ knowledge of disease is likely to influence provider recommendations. Providers with higher proportions of at-risk populations in practice are more likely to be aware of HBV testing, preventative strategies and management in their patients. For example, having a greater than 25% proportion of Asian population in practice has been associated with more than double the rates of HBV testing in practice by provider report [20]. Our data demonstrate that HBV positive mothers are often unaware of their child's HBV status and their child's perinatal care. Furthermore, when mothers are aware, the percentage of children who were diagnosed with HBV differed with respect to mothers’ knowledge of their own HBV status. These data show that if mothers had been diagnosed with HBV in the year that their child was born, their children had a lower rate of HBV infection than children of mothers who had not yet been diagnosed with HBV. This suggests that mothers who knew that they had chronic HBV took actions to prevent vertical transmission.
Thus, mothers’ lack of knowledge regarding their own HBV status and that of their child, or of their child's status regarding HBV preventive measures, can lead to poor recognition of key medical care issues in these children. In the USA, state immunization databases record infant immunizations and HBIG administration, but these data are not available unless the child remains in the state of their birth; moreover, not all providers participate in these databases. Thus, the child's future medical providers may not have the information they need to appropriately manage these children. In particular, completing the full HBV vaccine series is challenging [16,17,21], yet crucial to prevent acquisition of HBV by the child.
According to the mothers in our study, 4.8% of children whose mothers asserted knowledge of the diagnosis were diagnosed with HBV. This is similar to the documented effectiveness of immune prophylaxis in newborns of 90–95% [24], though the mothers of some of the children who asserted they were diagnosed with HBV did not assert that they were vaccinated. Improving this number may require strategies beyond current preventive programs. However, for 11.2% of the children, their mothers did not know if their child was tested for HBV and for 15.5% of children, their mothers did not know if they were diagnosed with HBV. Diagnosis requires testing, generally after the third dose of HBV vaccine [7]. As patient and provider factors are known to influence HBV care and preventative services [18–20], the fact that mothers are unsure of whether their children were tested, raises concerns about provider-parent communication, provider knowledge of the appropriate care of a child at HBV risk and whether the full HBV vaccination schedule was completed.
This study has some limitations. Maternal knowledge of neonatal HBV management is by self-report since direct data on the children's HBV prophylaxis, HBV status, and outcomes are not part of this study. Therefore, the accuracy of a mothers’ report cannot be confirmed and past events may not be remembered accurately. Additionally, as immune prophylaxis includes two possible injections (vaccine and HBIG), the data reported here do not distinguish between receipt of only vaccine, HBIG, or both. We also do not have data on the number of subsequent HBV vaccine doses that were completed. Nonetheless, no prior study has attempted to measure gaps in HBV-infected mothers’ knowledge with respect to mother-to-child transmission of the infection in a North American population.
The cause of any apparent missed prophylaxis in the HBRN sample could not be determined. Newborns may fail to receive prophylaxis because they are seriously ill in the newborn period, because they are below the weight threshold for vaccination or because the mother was unaware of the recommendations [21]. Another possible explanation may be that some of the children in this cohort were born outside North America where barriers to effective delivery of HBV prophylaxis may exist. Another possibility is that mothers were unaware of their child's treatment or forgot the events that occurred peripartum. Studies have demonstrated both false positive and false negative memories of vaccination among parents after a decade or more [22,23].
In conclusion, among HBV-infected women living in the United States or Canada, approximately one-in-ten lacked knowledge regarding the HBV testing status of their children. This knowledge gap can be improved by educating both parents and providers of the importance of both prevention and diagnostic algorithms. It also emphasizes the need for providers to confirm the HBV status of all family members of a person with HBV, but especially to insure testing of children in the family. Providing this knowledge can be expected to improve the care of children born to HBsAg positive mothers and ultimately contribute to the global efforts to eliminate HBV.AbbreviationsHBV Hepatitis B virus chronic hepatitis B Hepatitis B immunoglobulin Hepatitis B Research Network hepatitis B surface antigen Advisory Committee of Immunization Practices World Health Organization United States of America generalized estimated equations
The HBRN was funded as a Cooperative Agreement between the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to the following investigators Lewis R. Roberts, MB, ChB, PhD (U01-DK082843), Anna Suk-Fong Lok, MD (U01-DK082863), Steven H. Belle, PhD, MScHyg (U01-DK082864), Kyong-Mi Chang, MD (U01-DK082866), Michael W. Fried, MD (U01-DK082867), Adrian M. Di Bisceglie, MD (U01-DK082871), William M. Lee, MD (U01-DK082872), Harry L. A. Janssen, MD, PhD (U01-DK082874), Daryl T-Y Lau, MD, MPH (U01-DK082919), Richard K. Sterling, MD, MSc (U01-DK082923), Steven-Huy B. Han, MD (U01-DK082927), Robert C. Carithers, MD (U01-DK082943), Mandana Khalili, MD, MAS (U01-DK082944), an interagency agreement with NIDDK, also partially supported by NIH grant K24AA022523. Lilia M. Ganova-Raeva, PhD (A-DK-3002-001) and support from the intramural program, NIDDK, NIH: Marc G. Ghany, MD. Additional funding to support this study was provided to Kyong-Mi Chang, MD, the Immunology Center, (NIH/NIDDK Center of Molecular Studies in Digestive and Liver Diseases P30DK50306, NIH Public Health Service Research Grant M01-RR00040), Richard K. Sterling, MD, MSc (UL1TR000058, NCATS (National Center for Advancing Translational Sciences, NIH), Mandana Khalili, MD, MAS (CTSA Grant Number UL1TR000004) and in part supported by K24AA022523, Michael W. Fried, MD (CTSA Grant Number UL1TR001111), and Anna Suk-Fong Lok (CTSA Grant Number UL1RR024986, U54TR001959.) Additional support was provided by Gilead Sciences, Inc. and Roche Molecular Systems via a CRADA through the NIDDK.
Conflict of interestDr. Mauricio Lisker-Melman. Speaker Bureau Gilead, Abbvie, SimplySpeaking.
Dr. Mandana Khalili: Institutional Research Grants from Gilead Sciences, and Intercept Pharmaceuticals. Scientific advisory panel: Gilead.
Dr. Norah Terrault: Institutional Research Grants from Gilead Sciences; Speaker for SimplySpeaking, Clinical Care Options.
The remaining authors of this manuscript report no conflict of interests.
Harvard Consortium: Daryl T-Y Lau, MD, MPH (Beth Israel Deaconess Medical Center, Boston, MA). Minnesota Alliance for Research in Chronic Hepatitis B Consortium: Lewis R. Roberts, MB, ChB, PhD (Mayo Clinic Rochester, Rochester, MN), Mohamed A. Hassan, MD (University of Minnesota, Minneapolis, MN). Midwest Hepatitis B Consortium: Adrian M. Di Bisceglie, MD, (Saint Louis University School of Medicine, St Louis, MO). University of Toronto Consortium: Harry L. A. Janssen, MD, PhD (Toronto General Hospital, Toronto, Ontario), David K. Wong, MD (Toronto General Hospital, Toronto, Ontario), Joshua Juan, MD (Toronto General Hospital, Toronto, Ontario), Jordan Feld, MD, MPH (Toronto General Hospital, Toronto, Ontario), Colina Yim, NP, MN (Toronto General Hospital, Toronto, Ontario), Keyur Patel, MD (Toronto General Hospital, Toronto, Ontario). HBV CRN North Texas Consortium: William M. Lee, MD (Division of Digestive and Liver Diseases, University of Texas Southwestern Medical Center at Dallas, Dallas, TX), Carol S. Murakami, MD (Division of Digestive and Liver Diseases, University of Texas Southwestern Medical Center at Dallas, Dallas, TX), Robert Perrillo, MD, (Baylor University Medical Center, Dallas, TX), Son Do, MD (University of Texas Southwestern, Dallas, TX). Los Angeles Hepatitis B Consortium: Steven-Huy B. Han, MD (David Geffen School of Medicine, UCLA, Los Angeles, CA). San Francisco Hepatitis B Research Group Consortium: Stewart L. Cooper, MD (Division of General and Transplant Hepatology, California Pacific Medical Center, San Francisco, CA). Michigan Hawaii Consortium: Anna Suk-Fong Lok, MD (University of Michigan, Ann Arbor, MI), Robert J. Fontana, MD (University of Michigan, Ann Arbor, MI), Barak Younoszai, DO (The Queen's Medical Center, University of Hawaii, Honolulu, HI). Chapel Hill, NC Consortium: Michael W. Fried, MD, (University of North Carolina at Chapel Hill, Chapel Hill, NC), Andrew Muir, M.D. (Duke University Medical Center, Durham, NC), Donna Evon, Ph.D. (University of North Carolina at Chapel Hill, Chapel Hill, NC), Jama M. Darling, MD (University of North Carolina at Chapel Hill, NC). PNW/Alaska Clinical CenterConsortium: Robert C. Carithers, MD (University of Washington Medical Center, Seattle WA), Margaret Shuhart, MD (Harborview Medical Center, Seattle, WA), Kris V. Kowdley, MD (Virginia Mason Medical Center, Seattle WA), Chia C. Wang, MD (Virginia Mason Medical Center, Seattle WA). Virginia Commonwealth University Medical Center: Richard K. Sterling, MD, MSc (Virginia Commonwealth University Health System, Richmond, VA), Velimir A. Luketic, MD (Virginia Commonwealth University Health System, Richmond, VA). Liver Diseases Branch, NIDDK: Marc G. Ghany, MD, MHsc (National Institutes of Health, Bethesda, MD), T. Jake Liang, MD (National Institutes of Health, Bethesda, MD). Liver Disease Research Branch, NIDDK: Jay H. Hoofnagle, MD (National Institutes of Health, Bethesda, MD), Edward Doo, MD (National Institutes of Health, Bethesda, MD). Immunology Center: Kyong-Mi Chang, MD, (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA), Jang-June Park, PhD (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA). Data Coordinating Center: Abdus Wahed, PhD (Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA), Yona Cloonan, PhD (Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA). Central Pathology: David Kleiner, MD, PhD (Center for Cancer Research, National Cancer Institute, NIH, Bethesda, MD).