Background. Endothelial dysfunction has been previously described in metabolic syndrome patients. The levels of circulating endothelial progenitor cells (EPCs) inversely correlates with the incidence of cardiovascular disease. The aim of this study was to investigate the association between NAFLD, metabolic syndrome and EPC levels.
Material and methods. A cross-sectional pilot study was performed at a university hospital in Mexico. Two groups of patients without previously known chronic diseases were studied and classified according to the presence of NAFLD. Anthropometric, dietary, and biochemical variables, and circulating EPC number were measured and compared between the groups.
Results. Forty subjects were included and classified into two groups: patients with NAFLD (n = 20) and a control group (n = 20). The overall prevalence of insulin resistance and metabolic syndrome was 25% and 17.5%, respectively. EPC levels were found to be higher in the NAFLD group (p < 0.05) as in the patients with insulin resistance (p < 0.01) and metabolic syndrome (p < 0.01). These levels showed correlation with the severity of steatosis.
Conclusions. Patients with NAFLD have increased levels of EPC, such levels are associated with the severity of NAFLD. These findings may suggest that these cells may play a role in the early natural history of NAFLD. EPC might be increased in an attempt to repair the endothelial damage resulting from metabolic alterations accompanying NAFLD. Further studies are needed to establish the dynamics of these cells in NAFLD.
NAFLD comprises a wide spectrum of liver disorders such as simple steatosis, steatohepatitis, fibrosis and cirrhosis. The incidence of nonalcoholic fatty liver disease (NAFLD) has reached epidemic proportions, and the disease is the most frequent cause of chronic liver disease in Western countries. The prevalence reported for the general population is 20–30%, and this percentage is higher in patients with cardiovascular disease (CVD) risk factors such as diabetes mellitus, for which the prevalence rises to 70–90%.1 The mechanisms by which diabetes can cause NAFLD are complex and have been extensively studied, however mainly in isolated biological systems. Probably the principal mechanism involved in such pathway is that insulin resistance promotes release of free fatty acids from adipose tissue that accumulate in the liver cells causing steatosis.2
Hepatocyte damage in NAFLD is accompanied by the release of a variety of proinflammatory molecules also implicated in CVD and metabolic syndrome (MetS). Because both conditions share common risk factors, the relationship between MetS and its consequences and NAFLD has been studied extensively.3 Adams, et al. reported that ischemic heart dis-ease was the most common cause of death in a cohort of 420 NAFLD patients.4 More recently, Haring, et al. found that, in a population of 4,160 patients with NAFLD, CVD was the most frequent independent risk factor for NAFLD among other factors such as physical inactivity, waist circumference, blood pressure, and diabetes.5
Despite the limitations of such studies, all concluded that the presence of NAFLD predisposes a patient to CVD. The association between CVD and NAFLD aforementioned suggests that NAFLD plays an important role in the pathogenesis of CVD and endothelial damage.6 The relationship between NA-FLD and MetsS has been demonstrated in several studies, and it has been proven that cardiovascular outcomes are the leading cause of death in patients with NAFLD regardless of the histological severity.7
Atherosclerosis-related cardiovascular events represent a major cause of morbidity and mortality in Western countries.8,9 Strong evidence suggests that an altered endothelium contributes to the propagation of early lesions in atherogenesis.10 Such alterations appear to be related to a proinflammatory state induced by the MetS components such as insulin resistance, dyslipidemia, and obesity. Currently, it is thought that a failure in the repair of endothe-lial damage is a key factor in atherogenesis.11 The Jackson Heart Study demonstrated that fatty liver is an independent risk factor for cardiometabolic risk factors and atherosclerosis, and that the elevated risk could be explained by alterations in protein and adipokine pathways associated with visceral fat.12
On the other hand, Asahara, et al.13 reported the discovery of a new subclass of vascular stem cells and named them endothelial progenitor cells (EPCs). These cells are circulating bone marrow-derived cells that can be divided into two subpopulations according to phenotype: early EPCs, characterized by the expression of vascular endothelial growth factor receptor 2 (KDR), CD34, and CD133 surface markers, and late EPCs, which do not display CD133.14 EPCs play an important role in maintaining the integrity of the endothelium, angiogenesis, and neovascularization, probably through a paracrine pathway yet to be fully eluci-dated.15 The number of EPCs correlates inversely with the incidence of CVD.16 Several investigations have shown that a number of factors such as smoking, family history of coronary artery disease, older age, high concentrations of low-density lipoprotein and total cholesterol, hypertension, Framingham risk score, flow-mediated brachial reactivity, and diabetes are associated with lower levels of EPC.15 The aim of this study was to investigate the role of EPCs in the pathophysiology of NAFLD and to measure the number of EPCs in patients with this disease.
Material and MethodsSubjectsThis was a cross-sectional study undertaken in the check-up unit of the Diagnostic Clinic at the Medica Sur Clinic & Foundation between November 2010 and February 2011. Our sample population comprised a series of consecutive asymptomatic subjects (n = 260) who were referred to the check-up unit as an annual employment requirement, but not for symptomatic disease. The exclusion criteria were an alcohol intake of > 20 g/day, known liver disease, diabetes mellitus, or current use of medication. Participants who tested positive for hepatitis B antigen or hepatitis C antibody and those who reported a history of known liver disease, including viral, genetic, autoimmune, or drug-induced liver disease, were excluded. The study included 40 patients, 20 with NAFLD (categorized according to the degree of steatosis measured by ultrasonography) and 20 individuals without NAFLD (ramdomly selected from the control group n = 96).
This pilot study was approved by the Human Subjects Committee at the Medica Sur Clinic & Foundation and conformed to the ethical guidelines of the 1983 Declaration of Helsinki. Written informed consent was obtained from all participants before entry into the study.
Liver ultrasonographyThe diagnosis of NAFLD was based on the presence of a hyperechoic ‘bright’ liver on ultrasound scanning (Elegra; Siemens Medical Systems, Mountain Grove, CA). Real-time ultrasonographic studies were performed, by a blinded investigator, with the subject in the fasted state. A 3.5 MHz transducer was used to obtain a sagittal view of the right lobe of the liver and right kidney, transverse view of the left lateral segment of the liver and spleen, transverse view of the liver and pancreas, and any focal areas of altered echotexture.
Hepatic steatosis was classified as mild, moderate or severe according to Hamaguchi’s ultrasonographic score.17
SamplesDuring the check-up, 3 mL of whole blood was collected by venipuncture into 10.0 mL BD Vacutai-ner® plastic EDTA tube.
- •
Preparation of blood samples. One milliliter of the sample was incubated with FcR-blocking solution (Miltenyi Biotec, Gladbach Germany) (normal human IgG, 2 mg/mL final concentration) for 10 min. A 200 aliquot was incubated for 1 h with 20 of anti CD133–Fluorescein isothiocyanate (FITC), 40 of anti-CD45– allolphycocyanin (APC), 10 of KDR PerCP/ Cy55, and 40 of anti-CD34–Phycoerithrin (PE). Erythrocytes were lysed before flow cytometry analysis.
- •
Reagents. The antibodies used were: anti-CD133–FITC (Biorbyt, Riverside UK); anti-CD45–APC, anti-CD34–PE, and anti-PerCP-Cy55 (KDR; BioLegend, San Diego, CA, USA); and IgG2 (Sigma, St Louis, MO, USA).
An initial gate (R1) was set on CD45 vs. side scatter (SSC) to eliminate all debris and nonleuko-cytes; the dot plot contained all CD45+ events including CD45dim and CD45bright cells. The lower limit of CD45 expression was adapted from a histogram of CD34 vs. CD45 performed on ungated data. R1 also contained a gate to define lymphocytes as CD45brightSSClow cells. The events in gate R1 were then displayed on a CD34 vs. SSC dot plot, and a second gate (R2) was used to include the cluster of CD34 + events. The third plot was obtained by plotting the events that fulfilled the criteria of gates R1 and R2 (i.e., sequential gating). Cells forming a cluster of blasts with characteristic SSClow and CD45dim fluorescence (SSClowCD45dim cells) were then gated on this third plot to produce a third region (R3). To differentiate between CD45dim and CD45bright cells, the right margin of the CD34+ cells in plot 5 served as the cutoff. At this step, the gating strategy defined CD34+CD45dim cells. The events fulfilling the criteria of all three gates (R1, R2, and R3) were then displayed on a forward light scatter (FSC) vs. SSC dot plot to confirm that the selected blasts fell into the lymphocyte region (R5). The lymphocyte region was adjusted from a SSC vs. FSC plot gated on lymphocytes from R4 using only small lymphocytes (FSClow, R5). CD45dimCD34+KDR+ from R5 + R2 + R3 were used to generate a new plot that included CD133+ cells.
The subpopulation expressing CD45dim, CD34, KDR, and CD133 was defined as early EPCs, and the subpopulation that had lost the CD133 marker was defined as late EPCs.
Statistical analysisContinuous variables are expressed as mean and standard deviation, and their distributions were summarized to compare patients with and without NAFLD. The number of EPCs did not have a normal distribution and was transformed as a logarithmic variable. All analyses were performed using SPSS/ PC version 16.0 (IBM Corp., Armonk, NY, USA). Differences were considered significant with P values < 0.05.
ResultsWe included 40 subjects > 18 years of age, 20 patients with NAFLD and 20 individuals without NAFLD. None of the participants had a history of CVD, liver disease, Diabetes Mellitus or dyslipidemia, and their alcohol consumption was < 20 g/day.
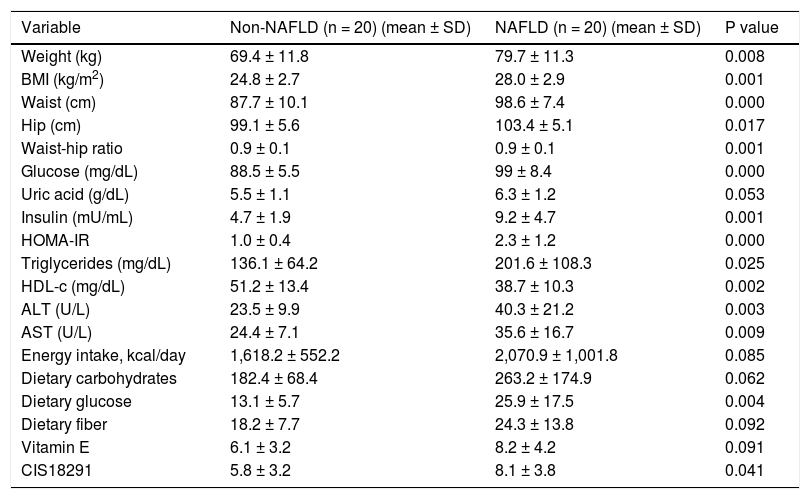
The baseline anthropometric characteristics are summarized in table 1. The average age was 46 years. As expected, the anthropometric variables differed significantly between the two groups (P < 0.001 for the homeostasis model assessment index, insulin level, glucose level, and body mass index). The levels of alanine aminotransferase and aspartate aminotransferase were significantly higher (P < 0.05), and the HDL cholesterol level was significantly lower (P < 0.05) in the NAFLD group than in the control group.
Clinical and biochemical characteristics of the groups.
| Variable | Non-NAFLD (n = 20) (mean ± SD) | NAFLD (n = 20) (mean ± SD) | P value |
|---|---|---|---|
| Weight (kg) | 69.4 ± 11.8 | 79.7 ± 11.3 | 0.008 |
| BMI (kg/m2) | 24.8 ± 2.7 | 28.0 ± 2.9 | 0.001 |
| Waist (cm) | 87.7 ± 10.1 | 98.6 ± 7.4 | 0.000 |
| Hip (cm) | 99.1 ± 5.6 | 103.4 ± 5.1 | 0.017 |
| Waist-hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.001 |
| Glucose (mg/dL) | 88.5 ± 5.5 | 99 ± 8.4 | 0.000 |
| Uric acid (g/dL) | 5.5 ± 1.1 | 6.3 ± 1.2 | 0.053 |
| Insulin (mU/mL) | 4.7 ± 1.9 | 9.2 ± 4.7 | 0.001 |
| HOMA-IR | 1.0 ± 0.4 | 2.3 ± 1.2 | 0.000 |
| Triglycerides (mg/dL) | 136.1 ± 64.2 | 201.6 ± 108.3 | 0.025 |
| HDL-c (mg/dL) | 51.2 ± 13.4 | 38.7 ± 10.3 | 0.002 |
| ALT (U/L) | 23.5 ± 9.9 | 40.3 ± 21.2 | 0.003 |
| AST (U/L) | 24.4 ± 7.1 | 35.6 ± 16.7 | 0.009 |
| Energy intake, kcal/day | 1,618.2 ± 552.2 | 2,070.9 ± 1,001.8 | 0.085 |
| Dietary carbohydrates | 182.4 ± 68.4 | 263.2 ± 174.9 | 0.062 |
| Dietary glucose | 13.1 ± 5.7 | 25.9 ± 17.5 | 0.004 |
| Dietary fiber | 18.2 ± 7.7 | 24.3 ± 13.8 | 0.092 |
| Vitamin E | 6.1 ± 3.2 | 8.2 ± 4.2 | 0.091 |
| CIS18291 | 5.8 ± 3.2 | 8.1 ± 3.8 | 0.041 |
BMI: body mass index. HOMA-IR: homeostasis model assessment of insulin resistance. HDL-c: high-density lipoprotein cholesterol. ALT: alanine-aminotransferase. AST: aspartate-aminotransferase.
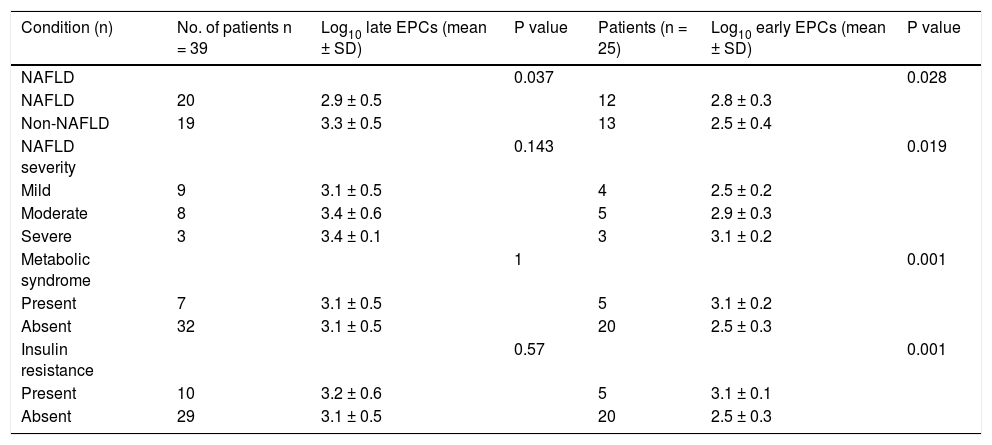
Early EPCs were detected in 25 subjects, 12 individual diagnoses with NAFLD. Interestingly, the number of early EPCs was higher in the NAFLD group (P < 0.05). An analysis combining the degree of hepatic steatosis and the number of EPCs showed that the number of EPCs was directly related to the degree of steatosis. The number of early EPCs was higher in patients with severe steatosis (n = 3) compared with patients with mild (n = 4) or moderate steatosis (n = 5) (P < 0.05). In these patients, who had no history of CVD, liver disease, or dyslipidemia, the number of early EPCs was higher even after considering the presence of the metabolic syndrome and insulin resistance (P < 0.01) (Table 2).
Relationship between EPCs and the presence and severity of NAFLD, the metabolic syndrome, and insulin resistance.
| Condition (n) | No. of patients n = 39 | Log10 late EPCs (mean ± SD) | P value | Patients (n = 25) | Log10 early EPCs (mean ± SD) | P value |
|---|---|---|---|---|---|---|
| NAFLD | 0.037 | 0.028 | ||||
| NAFLD | 20 | 2.9 ± 0.5 | 12 | 2.8 ± 0.3 | ||
| Non-NAFLD | 19 | 3.3 ± 0.5 | 13 | 2.5 ± 0.4 | ||
| NAFLD severity | 0.143 | 0.019 | ||||
| Mild | 9 | 3.1 ± 0.5 | 4 | 2.5 ± 0.2 | ||
| Moderate | 8 | 3.4 ± 0.6 | 5 | 2.9 ± 0.3 | ||
| Severe | 3 | 3.4 ± 0.1 | 3 | 3.1 ± 0.2 | ||
| Metabolic syndrome | 1 | 0.001 | ||||
| Present | 7 | 3.1 ± 0.5 | 5 | 3.1 ± 0.2 | ||
| Absent | 32 | 3.1 ± 0.5 | 20 | 2.5 ± 0.3 | ||
| Insulin resistance | 0.57 | 0.001 | ||||
| Present | 10 | 3.2 ± 0.6 | 5 | 3.1 ± 0.1 | ||
| Absent | 29 | 3.1 ± 0.5 | 20 | 2.5 ± 0.3 |
Late EPCs were detected in 39 patients, including the 20 patients with NAFLD. Late EPC number increased with the degree of steatosis and the presence or absence of the metabolic syndrome and insulin resistance. In contrast to early EPCs, the number of late EPCs was not significantly related to the severity of steatosis or to the presence of the metabolic syndrome or insulin resistance.
DiscussionThe association between NAFLD and MetS has been demonstrated clearly using a range of approaches from epidemiological association studies to measurement of subclinical atherosclerosis markers in patients with NAFLD. However, the sequence of changes -whether the pathophysiology of NAFLD precedes the development of CVD or vice versa- is unclear. There is evidence suggesting that the outcomes in patients with NAFLD are related to CVD and metabolic consequences of MetS such as diabetes mellitus.9
Many hypotheses have been built around the event that links these two diseases. Some hypothesize the involvement of procoagulant factors, proinflam-matory molecules, and insulin resistance-related molecules, but no cellular or molecular component has showed to be directly related to NAFLD.9 Ahmed, et al. suggested that the link between NA-FLD and MetS involves the interactions between insulin resistance and acceleration of dyslipidemia, carotid atherosclerosis, the degree of endothelial dysfunction, oxidative stress, adipocytokines and postprandial hyperlipidemia. If so, specific treatment of NAFLD might modulate CVD risk factors.18
Noninvasive methods such as flow-mediated dilation and measurement of carotid intima-media thickness have been used to evaluate endothelial function, and abnormal results in these tests are considered early markers of endothelial damage.19 We defined cells displaying the surface marker CD133 as early EPCs and those that had lost this marker as late EPCs. The number of EPCs was greater in patients with NAFLD, MetS or insulin resistance compared with those without such conditions, and EPC number was directly proportional to the degree of steatosis. Our results are consistent with those of one study of adolescents showing increased cell number in overweight and obese adolescents compared with controls. Interestingly, in that study, the number of EPCs did not correlate with the presence of CVD risk factors.20 Satoh, et al. have reported increased EPC number, oxidative DNA damage, decreased telomerase activity and telomeres length in patients with coronary artery disease and MetS.21 Another recent study also reported increased EPCs in patients with erectile disfunction and MetS.22,23
Our findings differ from those of an earlier study2 of patients with established CVD (those with chronic endothelial damage) that reported fewer cells in individuals with this pathology. In a prospective study, Werner et al.10 found that low EPC number was associated with increased risk of major cardiovascular events, revascularization, and death from cardiovascular causes, and that this association was independent of other factors such as age, sex, and CVD risk factors. We note that, in their study, the highest EPC number was associated with a lower risk of death from cardiovascular causes. Recently Chiang et al reported decreased number of EPCs in patients with NAFLD compared in patients without NAFLD,24 however their sample population was obtained from a series of patients undergoing corona-riography for suspected CAD, contrary to our population: patients without known comorbidities, this may mean that patients in the study by Chiang had higher degrees of endothelial damage and this could explain the fewer number of EPCs.
Because cardiovascular outcomes frequently involve conditions such as insulin resistance, Diabetes mellitus, NAFLD, and the metabolic syndrome, we hypothesized initially that EPC number would be low in patients with these conditions. Our findings suggest that, because NAFLD is an early stage in the process of systemic endothelial damage, the increase in EPC number may reflect a compensatory mechanism against endothelial injury.
One of the limitations of our study was our limited sample size, therefore our findings must be confirmed by further studies. Another important limitation of our study is that fatty liver disease was diagnosed by hepatic ultrasound, whereas it has been demonstrated that liver biopsy is the gold standard for the diagnosis of NAFLD.
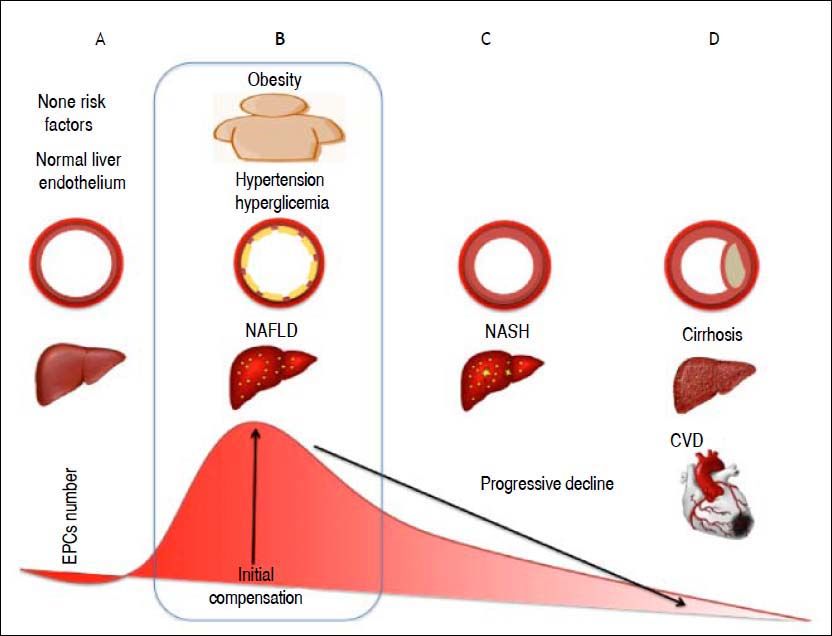
In conclusion, the results of our study suggest an important role of EPCs in the interaction between NAFLD and features of MetS. The increase in EPC number observed in these NAFLD patients may reflect repair mechanisms in response to endothelial damage (Figure 1). Damage to the endothelium associated with risk factors such as hyperglycemia, hypercholesterolemia, or the group of risks factors caused by NAFLD induces an inflammatory process in the endothelium. We propose that such damage stimulates EPCs to repair the injured endothelium, as reflected in an increased number of these cells. However, these results should be interpreted with caution because of the limitations previously described. Further studies are warranted to confirm these preliminary findings and to define the role of EPCs in the outcomes of NAFLD patients with metabolic syndrome.
This figure shows our theory to explain how the number of EPCs changes according to the natural history of liver steatosis. A. Normal liver histology and a normal endothelium. B. The first stage of NAFLD, in the presence of other CVD risk factors such as hypertension or obesity that alter the endothelium and cause a continuous state of inflammation. The number of EPCs increases in an attempt to repair the damage to the endothelial wall caused by inflammation. C. Progression of NAFLD to steatohepatitis and increase in the intima-media thickness. D. Plaque formation with cardiovascular outcomes and progression of liver disease.
None.
FundsMedica Sur Clinic and Foundation.
AcknowledgmentsThis study was supported by Medica Sur Clinic & Foundation.