At present, cardiac metastasis of hepatocellular carcinoma is rarely mentioned in the literature. We report a hepatocellular carcinoma patient with cardiac metastasis misdiagnosed as hypertrophic cardiomyopathy in 2011. Two years later, on presentation of syncope, an abnormal ventricular septal size was recorded by ultrasound scan, and was subsequently shown by magnetic resonance imaging to be a tumour lesion. A myocardial biopsy confirmed infiltration of hepatocellular carcinoma. This observation underlines the risk of hepatocellular carcinoma cardiac metastasis, manifested in its infiltrative form as hypertrophic cardiomyopathy. In conclusion, we suggest that the ultrasound appearance of hypertrophic cardiomyopathy in hepatocellular carcinoma patients should be seen as a “red flag” and recommend the introduction of magnetic resonance imaging assessment of transplant candidates.
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and the second most common cause of cancer-related death. Symptoms attributable to HCC are usually absent. Consequently, the majority of patients are diagnosed with advanced disease and when symptomatic, HCC is often associated with nonspecific complaints.1 This has resulted, in part, in a 5-year overall survival rate of 12% and a median survival following diagnosis ranging from 6 to 20 months.2,3
Without specific treatment, HCC has a very poor prognosis: the median survival for patients with early and advanced tumors is 6-9 months and 1-2 months, respectively.4In accordance to this evidence, the treatment and the survival time of HCC depends entirely on the tumour stage. About that, the main classification method is the Barcelona Clinic Liver Cancer (BCLC) staging system.5,6 In particular, in an extensive cohort, the survival imes were 13.4, 9.5, 3.4, and 1.6 months for patients of BCLC stages 0/A, B, C, and D, respectively.7
Potentially curative partial hepatic resection or ortho-topic liver transplantation should be reserved for patients with a stage 0 or A tumour. Advanced tumour stages (C and D) with poor hepatocellular reserves are not sensitive to these surgical treatments, which are suitable only as palliative therapy. Verification of extrahepatic metastatic disease is therefore crucial when planning potential therapy for patients with HCC, and should be adopted in order to avoid unnecessary surgical intervention. The most frequent site of metastasis is the lung, followed by the regional lymphon-odes (periceliac nodes, portahepatic nodes and lymphaden-opathy mediastinum), musculoskeletal structures, peritoneal and omental sites, the gastrointestinal tract, the splenic tract and the pancreas. Cardiac metastases are uncommon in the literature. They are usually associated with an echocardiographic mass-like shape and infiltrative right atrium and vena cava lesions.8,9 In the case of early diagnosis, the opportunity for surgical intervention may be greater. The results of liver transplantation back to the 1980s for HCC have so far been poor, with the 5-year survival rates of less than 40%, mainly due to tumour recurrence. After Milan criteria were introduced, the results of liver transplantation improved notably, with 5-years survival rates of more than 70%.10 It is well established that patients with single lesions of up to 5 cm in diameter, or up to three lesions of less than 3 cm in the absence of vascular invasion,11,12 have an almost zero recurrence rate for HCC and the prognosis after transplantation is the same as for transplantation to treat liver disease without HCC.13-15 Although after resection the residual liver continues to have malignant potential, resection of HCC is a viable option, with short-term survival very similar to transplantation, the majority of early mortality due to liver failure. Recurrence rates of 50-60% five years after resection are usual,16,17 the majority of this recurrence being intrahepatic, representing either satellite nodules or de novo tumour development. A number of non-surgical therapies are in clinical use for treating HCC, including percutaneous ablative therapies, most commonly using ethanol injection, and radiofrequency ablation, where high-frequency ultrasound probes are placed in the liver mass, usually under ultrasound control. Chemo-embolisa-tion has also been widely used as a primary therapy for inoperable HCC. There is evidence from non-controlled data series that small HCCs are more likely to respond to this technique.18,19
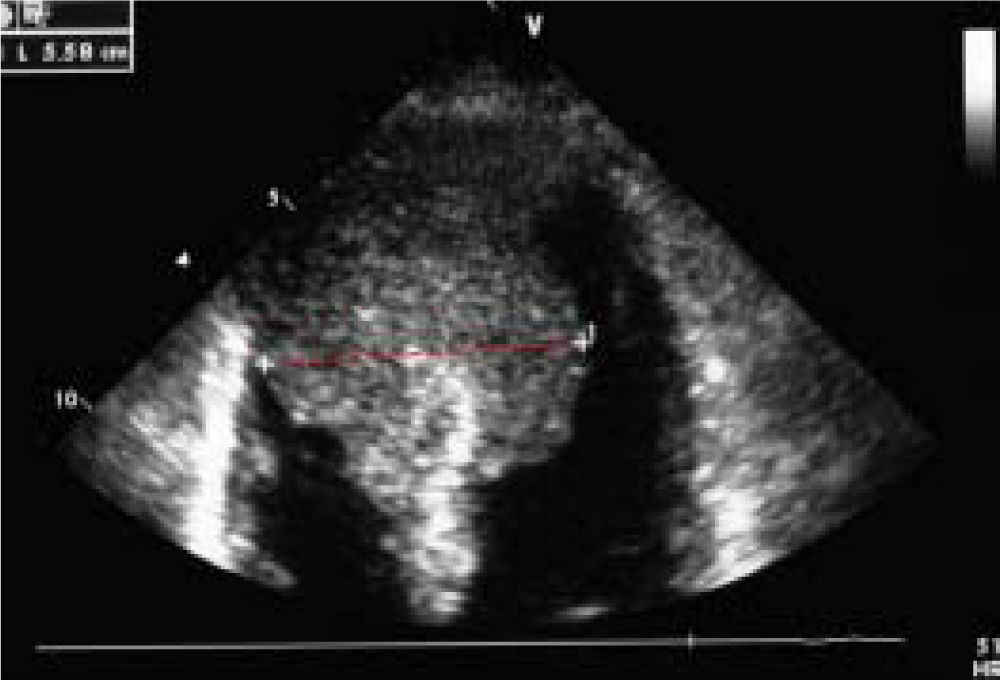
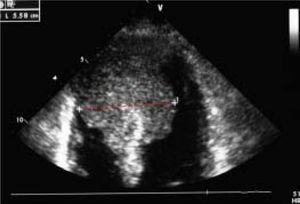
Case ReportWe report on a 61 year-old HCC patient without cardiovascular risk factors but with anamnestic hepatitis C virus-related liver cirrhosis, unresponsive to interferon therapy. The occurrence of HCC was diagnosed in 2011, when the patient underwent the first radiofrequency ablation session on the VII segment. In March 2013, a second round of radiofrequency ablation was performed on the II-III segments. In October 2013, the patient was admitted to the emergency room following the occurrence of a syncope-like episode. The electrocardiogram showed a Q wave in the inferior leads and inverted T waves in the front and side leads. Echocardiography performed during cardiological consulting demonstrated a large and iso-echogenic ventricular septal size (5.58 cm), extending to the cardiac apex and septum (Figure 1).
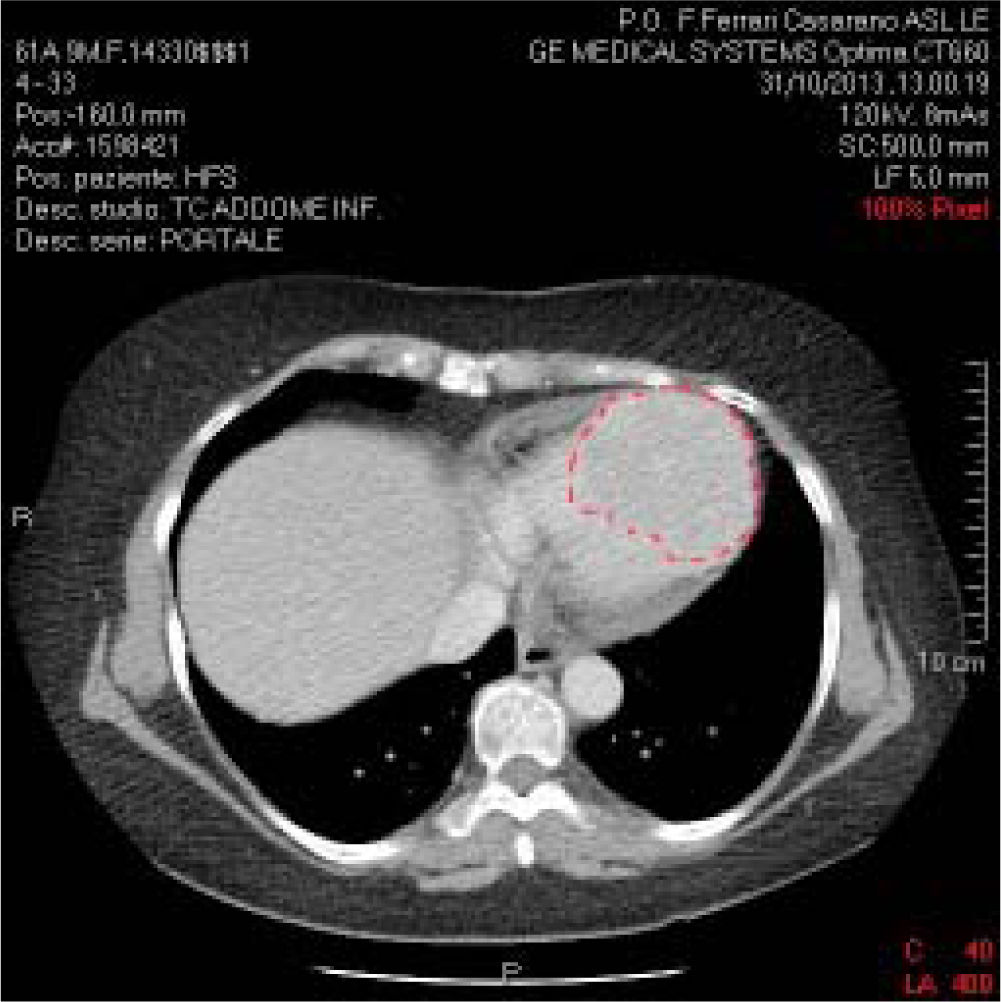
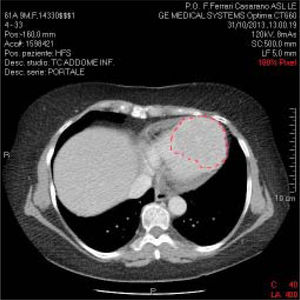
The patient presented documentation of echocardio-graphic and cardiac magnetic resonance imaging (MRI) performed previously, with a diagnosis of hypertrophic cardiomyopathy. We recommended hospitalization in order to further clinical evaluation. However, although clinical examination and ECG both pointed to hypertrophic cardiomyopathy, the echocardiographic data were not in agreement. Indeed, the described lesion appeared to be vascularized, unlike hypertrophic cardiomyopathy. Moreover, associated polylobed formations were readily detectable, and there was also obliteration of the right and left heart chambers during the systole. Consistent with the diagnosis of hypertrophic cardiomyopathy, on admission the patient received beta-blocker therapy and the repeated echocardiogram confirmed several aspects associated with hypertrophic cardiomyopathy. Blood tests were normal except for liver transaminases, which were fairly high (GOT: 120 IU/L; GPT: 115 IU/L, compared with normal values ranging from 40 to 60 UI/L); aFP value was lower than 400 ng/mL. Serial ECG confirmed the above-mentioned electrocardiographic alterations. The holter-ECG examination showed neither hyper or hypokinetic arrhythmias worthy of note (only a triplet of ventricular ex-trasystoles). A body CT scan with contrast medium confirmed the large polylobate enhancement at the inter-ventricular septum and cardiac apex (Figure 2).
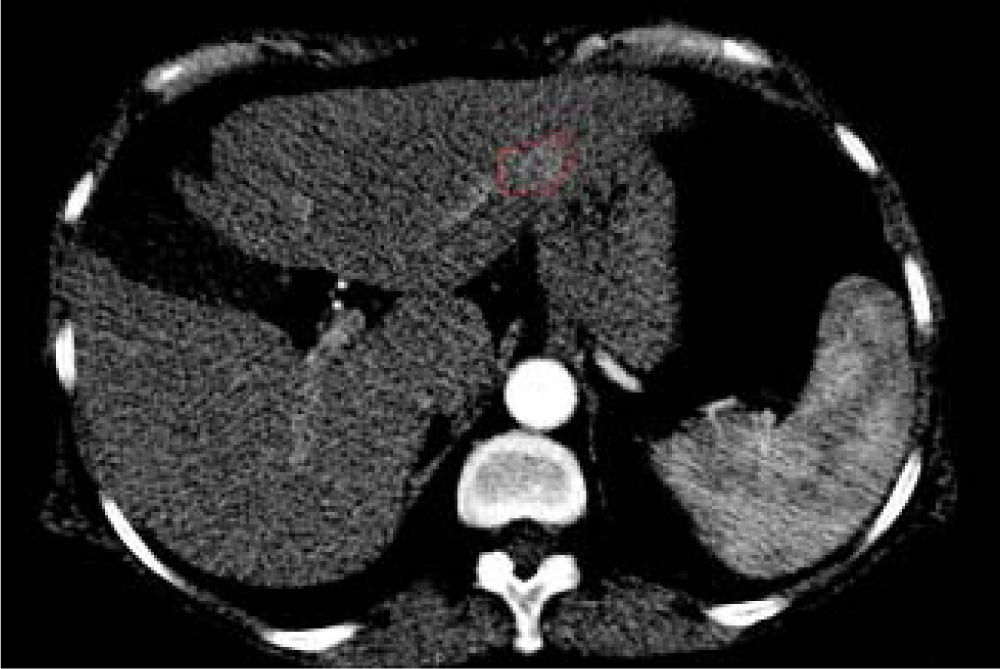
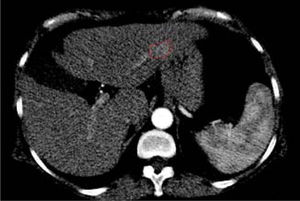
In addition, two brain lesions compatible with the diagnosis of meningioma were noted. No extrahepatic abdominal abnormalities were observed. As expected, we assessed an enhanced small area at the III hepatic segment characterized by a rapid washout in the portal phase. This area reflected a new nodule as a distant recurrence of previously treated nodular lesion in March 2013 (Figure 3).
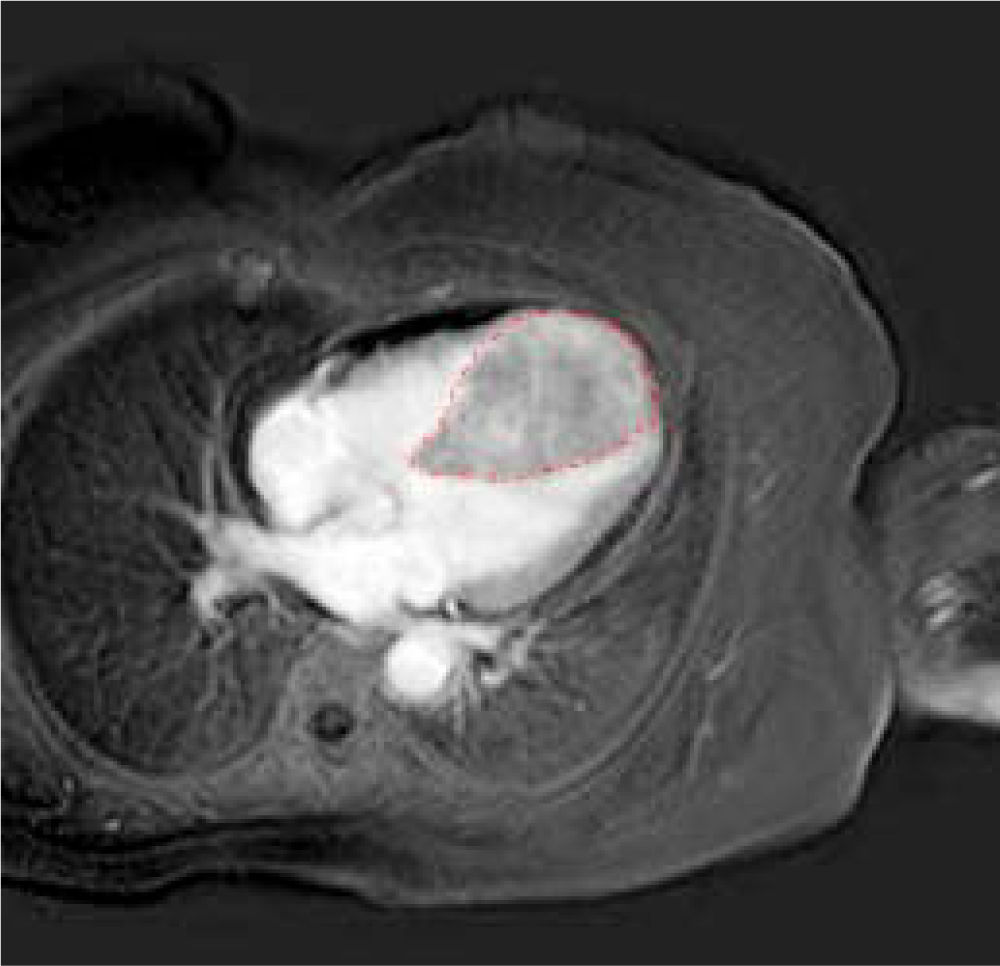
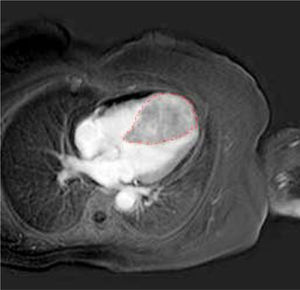
A further cardiac MRI documented the abnormal thickness of the interventricular septum extending to the entire apex and the anterior and lower segments of the left ventricle. This lesion had regular margins, a homogeneous structure and tenuous polylobated enhancement (Figure 4).
In addition, it induced obliteration of the left and right ventricles during systole, which had not been noted in the previous MRI examination. Thus, we performed a myo-cardial biopsy that confirmed infiltration of well-differentiated hepatocellular carcinoma reacting to HEPAR-1 and Alpha-fetoprotein. Finally, the patient was judged inoperable and was prescribed oncological palliation.
The described individual gave written informed consent and the study protocol conformed to the ethical guidelines of the Declaration of Helsinki (1975).
ConclusionsUntil now, there have been few descriptions of cardiac metastasis of hepatocellular carcinoma. The particularity of this case is the metastatization to the heart misdiag-nosed as hypertrophic cardiomyopathy, a very singular development for this cancer type. Cardiac metastases appear in the course of recurrent intrahepatic neoplasm, with no other repetitive lesions. Cardiac metastases can appear during local recurrence, via blood dissemination. In this type of patient, who exhibited cardiac symptoms, health practitioners should investigate the presence of metastases in the cardiovascular system. Thus, in patients with a history of HCC, it is advisable to perform careful evaluation of cardiac structures to exclude early metastasis. HCC can metastasize to an “abnormal” place, like the heart, assuming, as in this case, a non-nodular, but diffuse and infiltra-tive shape, resulting in problems of differential diagnosis, given the potential for confusion with hypertrophic cardi-omyopathy. Any diagnosis of hypertrophic cardiomyopa-thy (or recent onset of related symptoms) in the course of HCC should be considered a “red flag” and investigated in order to exclude metastasis from infiltrative HCC. In accordance with these observations, we propose to introduce MRI in the study of liver transplant candidates with symptoms or signs of hypertrophic cardiomyopathy. In these cases, the clinical result may be the conversion of the prognosis and treatment protocols from curative to palliative.
Abbreviations- •
HCC: hepatocellular carcinoma.
- •
MRI: magnetic resonance imaging.
All the authors, Greco A, De Masi R, Orlando S, Me-trangolo A, Zecca V, Morciano G, De Donno A, Bagordo F and Piccini G declare no conflict of interest.
Financial SupportThe authors declare that they have no specific financial support.