Our objective was to compare, over a time-course, markers of oxidative stress, the REDOX environment, and the antioxidant enzymatic system in the liver of rats with methimazole-or thyroidectomy-caused hypothyroidism.
Methods. We used 60 male Wistar rats divided into four groups: 1) the euthyroid, which received only tap water, 2) false thyroidectomy, which received the surgery and postoperative treatment, 3) thyroidectomy-caused hypothyroidism, which had the thyroid gland removed and a parathyroid reimplant, and 4) methimazole-caused hypothyroidism in rats that received 60 mg/kg/d of the antithyroid drug in drinking water. Five rats of the euthyroid and methimazole-caused hypothyroidism groups were killed at the end of the first, second, third, and fourth week after treatment, and five rats of false thyroidectomy and thyroidectomy-caused hypothyroidism groups were killed at the end of the second and eighth week after the surgical procedure. Each liver was removed and stored at-70 °C until oxidative stress, REDOX environment, and antioxidant enzymatic system markers were tested. We also made a histological study at the end of the treatment.
Results. The histological study revealed that only the methimazole-caused hypothyroidism caused cell damage. This damage is associated with an increase of oxidative stress markers that were not compensated for by the antioxidant system. The catalase activity is reduced and this allows H2O2-caused damage. In conclusion methimazole causes cell damage in the liver, whereas hypothyroidism per se does not cause hepatic-cell damage.
Methimazole is an antithyroid drug used in the treatment of hyperthyroidism in United States, Europe, and Asia. Methimazole is actively concentrated by the thyroid gland against a concentration gradient. The primary effect is to inhibit thyroidhormone synthesis by interfering with thyroid-peroxidase-mediated thyroglobulin organification,1 However, there are methimazole extrathyroidal effects reported; agranulocytosis, cholestasis, urticaria, loss of taste and smell, nephrotoxicity, and liver damage.2-4 The thyroid hormone 3, 5, 3’-triiodothyronine (T3) has a fundamental role in the development, differentiation, and physiology of all cells in the organism.5 One of the most studied effects of thyroid hormone is the control of the basal metabolic rate. The hypermetabolic state caused by a hyperthyroidism uncoupled-respiratory mitochondrial chain generates free radicals causing oxidative stress and cellular damage.6 However, the hypothyroidism-caused hypometabolic state protects against oxidative damage caused by toxicants.7 Some reports have noted that antithyroid-caused hypothyroidism can cause cellular damage.8,9 Thus, other extrathyroidal effects of antithyroid drugs as methimazole could contribute to the oxidative stress and cellular damage in liver. In general, the cellular damage occurs when the balance between oxidants and antioxidants is disturbed and the antioxidant system does not neutralize the oxidants. An enhancement of the oxidant system helps cause lipid peroxidation, enhancement of reactive oxygen species, and nitration, carbonylation or glutathionylation of proteins, and fragmentation of DNA.10,11 Our objective was to determine if methimazole or hypothyroidism causes an alteration of the REDOX environment, oxidative stress, and cellular damage in the liver.
MethodsAnimals and housingSixty male Wistar rats from our animal care facilities were used. Animals were singly housed (metallic cages, 20 x 30 x 18 cm, with food and water ad libitum). The cages were located together in racks (so that auditory and olfactory contact was maintained) in a light (0800 to 2000 lights on) and temperature (21 ± 1 oC) controlled room. Food and water were always available. The rats were allowed to acclimatize to the colony-room conditions for at least 1 week before the start of the experiments. All experimental procedures described in this study are in accordance with the guidelines of the Laws and Codes of Mexico in The Seventh Title of the Regulations of the General Law of Health Regarding Health Research and Mexican Official Standard NOM-082-ZOO-1999 detailing the technical specifications for production, care, and use of laboratory animals. We used the minimum number of animals required to attain the goals of this study.
Experimental designThe rats were randomly divided into four groups: 1) the euthyroid (n = 20), which received only tap water, 2) false thyroidectomy (n = 10), which received the surgery and postoperative treatment, 3) thyroidectomy-caused hypothyroidism (n = 10), which had the thyroid gland removed, the parathyroid reimplanted, and postoperative treatment, 4) methimazole-caused hypothyroidism (n = 20), in which the rats received 60 mg/kg/day of the antithyroid drug (Sigma Chemical Co.) in drinking water daily during treatment. The dose was adjusted according to water intake and body weight every three days throughout the experiment, as described.12
The thyroidectomy was done in rats anesthetized with ketamine (Pisa-Mexico) (10 mg/kg, im) and xylazine (Pisa-Mexico) (5 mg/kg, im) as described.9 Briefly, using a stereomicroscope (Zeiss, Germany) for better observation, the stenothyroid muscle was cut and the trachea was exposed. The parathyroid gland was found, dissected from the thyroid gland, and reimplanted into the surrounding neck muscle. The thyroid gland was carefully dissected to avoid injury to the laryngeal nerve and completely excised. After surgery, we injected ketorolac (Syntex-Mexico) (50 mg/kg IM) and gentamicin (Schering Plough-Mexico) (10 mg/kg IM) over 5 days to alleviate pain and prevent infection.
During treatment the body weight and rectal temperature were evaluated to indirectly determine the thyroid state.
Five rats of the euthyroid and methimazole-caused hypothyroidism groups were killed by decapitation at the end of first, second, third, and fourth week after treatment. Also, five rats of the false thyroidectomy and thyroidectomy-caused hypothyroidism groups were killed by decapitation at the end of the second and eighth week after the surgical procedure. The liver of each rat was removed and stored at-70 °C until biochemical assay tests were made.
We evaluated the serum concentration of thyroid hormones (T3 and T4) in all groups when the rats were killed. In the surgical groups we measured the serum concentration of calcium and phosphorus.
We also made a histological study at the end of the treatment to demonstrate hepatic damage.
Biochemical assaysAll livers were individually homogenized in 7 mL of 10 mM phosphate buffer, pH 7.4, and the homogenate used to determine oxidative stress, the REDOX environment, and the antioxidant enzymatic markers.
The oxidative stress markers evaluated were lipid peroxidation (LP) and the quantification of reactive oxygen species (ROS) as described.13
The REDOX environmental markers evaluated were reduced glutathione (GSH), oxidized glutathione (GSSG), and the GSH2/GSSG ratio, as described.13
The activity of catalase, glutathione peroxidase (GPX), and the isoforms of superoxide dismutase (SOD) were determined as the antioxidant enzymatic-system markers, as described.13
Histological studyParts of the livers, not homogenized, were fixed with Buoin fixer for 16 h and then were embedded in paraffin. Coronal cuts of 7 μm were obtained with a standard microtome. Each section was stained with hematoxylin-eosin, dehydrated, and mounted with resin. The microscopic description of the section was made by a person blind to the experiment by checking about 10 sections for each animal.
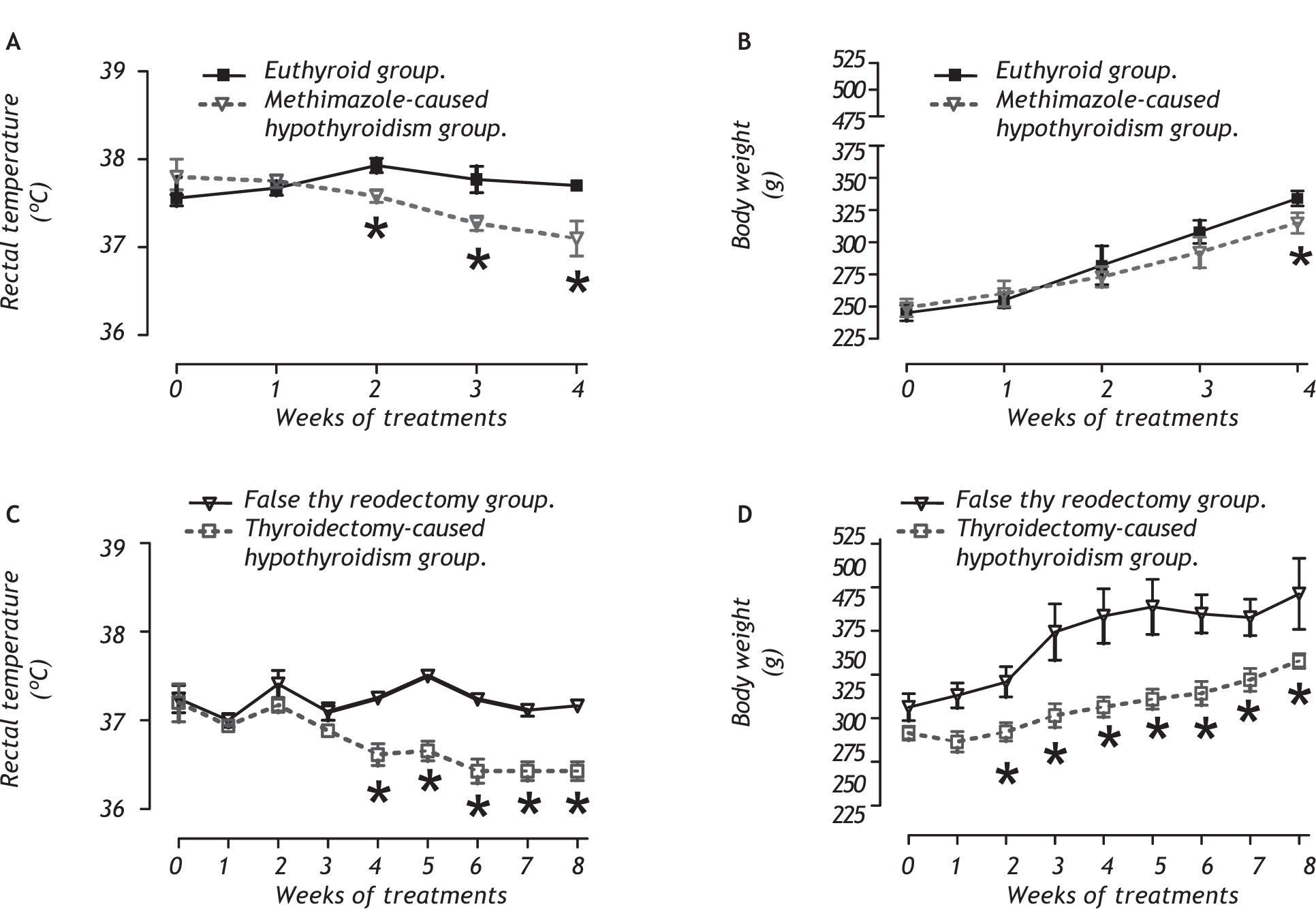
ResultsPhysical measures and hormone determination related to the hypothyroid stateFigure 1 shows the physical variables evaluated; rectal temperature (panels A and C) and body weight (panels B and D) during treatment. We noted that both hypothyroidism models had reduced rectal temperatures and a decreased body weight during the antithyroid treatment.
Methimazole-caused hypothyroidism reduced the rectal temperature from week 2 to the end of the treatment (between group: F1,40 = 7.98, P < 0.009; between week: F4,40= 43.97, P < 0.001; interaction group x week: F4,40 = 33.97, P < 0.001). The treatment decreased the body weight at week 4 (between group: F1,40 = 2.98, P > 0.05; between week: F4,40 = 4.50, P < 0.05; interaction group x week: F4,40 = 0.33, P = 0.88).
Thyroidectomy-caused hypothyroidism decreased the rectal temperature from week 4 to the end of the treatment (between group: F1,20 = 47.98, P < 0.001; between week: F8,20 = 53.97, P < 0.001; interaction group x week: F8,20 = 49.80, P < 0.001). The body weight decreased in the treated group from the second week to the end of the treatment (between group F1,20 = 17.98, P < 0.01; between week: F8,20 = 33.17, P < 0.001; interaction group x week: F8,20 = 19.80, P < 0.01).
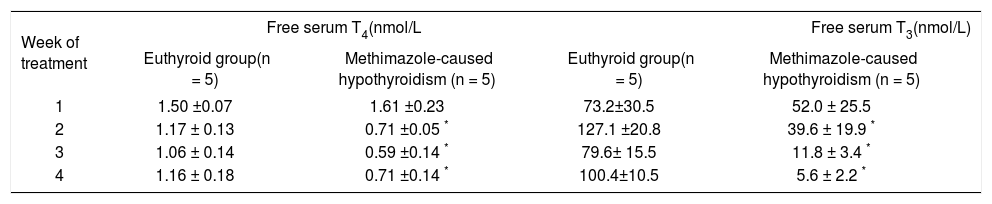
The thyroid state was determined by means of a free thyroid-hormone determination. In table 1, it shows that the methimazole-caused hypothyroidism reduced free-serum T4 (between group: F1,40 = 10.12, P < 0.001; between week: F3,40 = 30.00, P < 0.001; interaction group x week: F3,40 = 27.97, P < 0.001) and T3 (between group: F1, 40 = 11.10, P < 0.001; between week: F3,40 = 29.85, P < 0.001; interaction group x week: F3,40 = 22.15, P < 0.001) from week 2 to the of the end treatment. Thyroidectomy-caused hypothyroidism caused a reduction of free T4 in both weeks evaluated (between group: F1,19 = 78.98, P < 0.001 (Table 2); between week: F2,31 = 60.01, P < 0.001; interaction group x week: F2,31 = 59.82, P < 0.001). The free T3 was decreased at the end of the treatment (between group: F1,l9 = 43.12, P < 0.001; between week: F2,31 = 50.00, P < 0.001; interaction group x week: F2,31 = 48.23, P < 0.001).
Determination of free serum T3 and T4 in methimazole-caused hypothyroidism group.
| Week of treatment | Free serum T4(nmol/L | Free serum T3(nmol/L) | ||
|---|---|---|---|---|
| Euthyroid group(n = 5) | Methimazole-caused hypothyroidism (n = 5) | Euthyroid group(n = 5) | Methimazole-caused hypothyroidism (n = 5) | |
| 1 | 1.50 ±0.07 | 1.61 ±0.23 | 73.2±30.5 | 52.0 ± 25.5 |
| 2 | 1.17 ± 0.13 | 0.71 ±0.05 * | 127.1 ±20.8 | 39.6 ± 19.9 * |
| 3 | 1.06 ± 0.14 | 0.59 ±0.14 * | 79.6± 15.5 | 11.8 ± 3.4 * |
| 4 | 1.16 ± 0.18 | 0.71 ±0.14 * | 100.4±10.5 | 5.6 ± 2.2 * |
Determination of free serum T3 and T4 in thyroidectomy-caused hypothyroidism group.
| Week of treatment | Free serum T3 (nmol/L) | Free serum T4 (nmol/L) | ||
|---|---|---|---|---|
| False thyroidectomy group (n = 5) | Thyroidectomy-caused hypothyroidism group (n = 5) | False thyroidectomy group (n = 5) | Thyroidectomy-caused hypothyroidism group (n = 5) | |
| 2 | 1.55 ±0.27 | 1.94 ±0.14 | 115.7±18.0 | 3.4 ±1.2 * |
| 8 | 1.73 ± 0.12 | 101.2±11.6 | 1.21 ±0.13 * | 3.8 ± 0.4 * |
The thyroidectomy-caused hypothyroidism did not modify the serum Ca2+ and P5+ (Table 3).
Determination of serum Ca2+ and P5+ in thyroidectomy-caused hypothyroidism group.
| Week of treatment | Serum Ca2+ (mg/dL) | Serum P5+ (mg/dL) | ||
|---|---|---|---|---|
| False thyroidectomy group (n = 5) | Thyroidectomy-caused hypothyroidism group (n = 5) | False thyroidectomy group (n = 5) | Thyroidectomy-caused hypothyroidism group (n = 5) | |
| 2 | 7.37 ± 0.55 | 8.10 ± 0.50 | 3.52 ± 0.24 | 3.71 ± 0.31 |
| 8 | 8.00 ± 0.54 | 7.88 ± 0.61 | 2.91 ± 0.33 | 3.26 ± 0.33 |
Data values are mean ± SE.
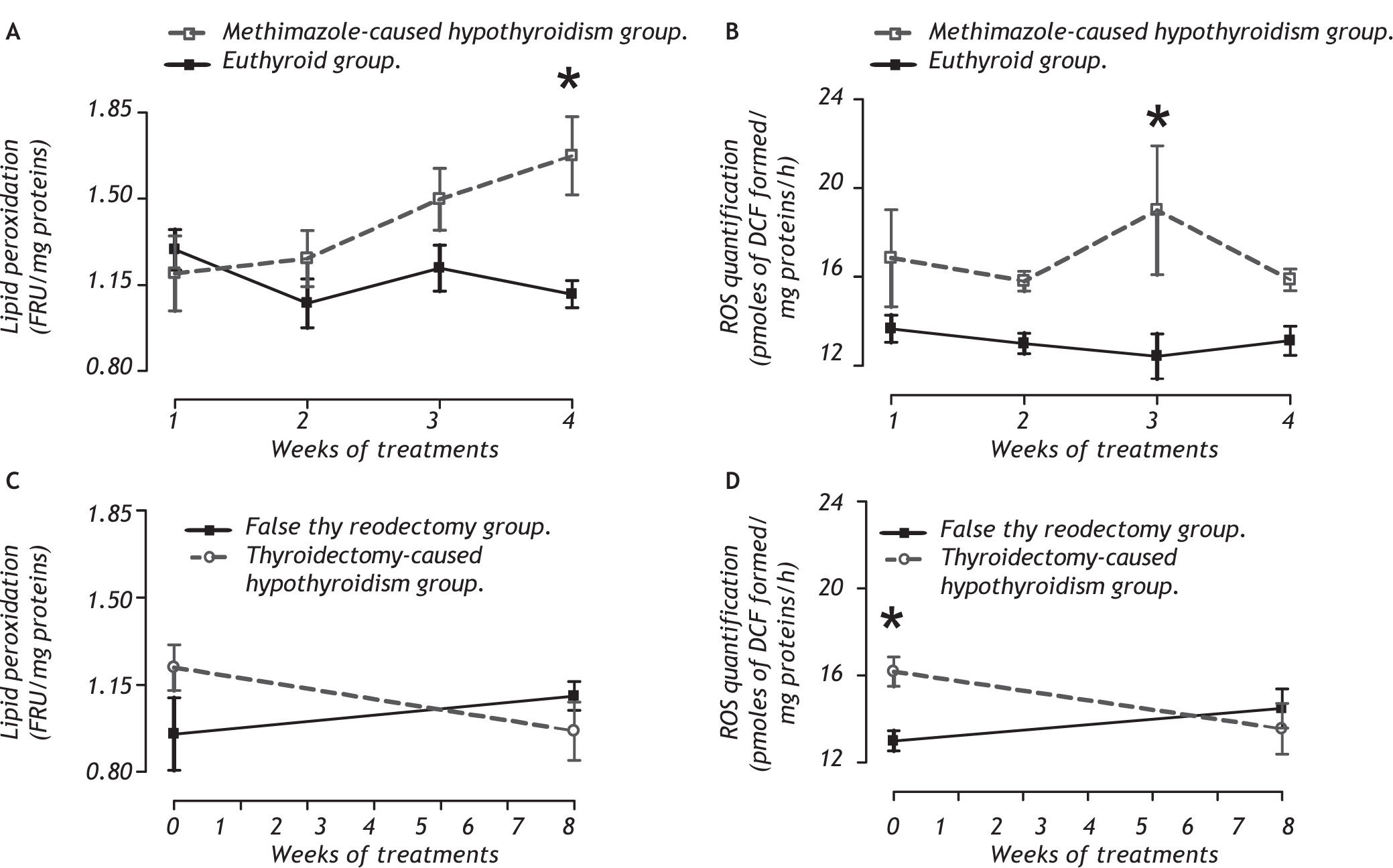
Panel A of figure 2 shows that the methimazolecaused hypothyroidism causes an increase of lipid peroxidation at the fourth week (between group F1,32 = 7.98, P < 0.009; between week F3,32 = 1.597, P = 0.215; and interaction groups x week F 1,32 = 2.68, P < 0.059) because it enhances the ROS concentration on the third week of treatment (panel B, between group F 1,32 = 14.68, P < 0.001; between week F 1,32 = 0.66, P = 0.618; and interaction groups X week F3,32 = 1.09, P = 0.370). Panel C shows thyroidectomy-caused hypothyroidism does not cause lipid peroxidation, but it enhances the ROS concentration in the second week postsurgery (between group F1,16 = 1.43, P = 0.250; between week F1,16= 0.994, P = 0.334; and interaction groups x week F1,16 = 5.17, P < 0.05).
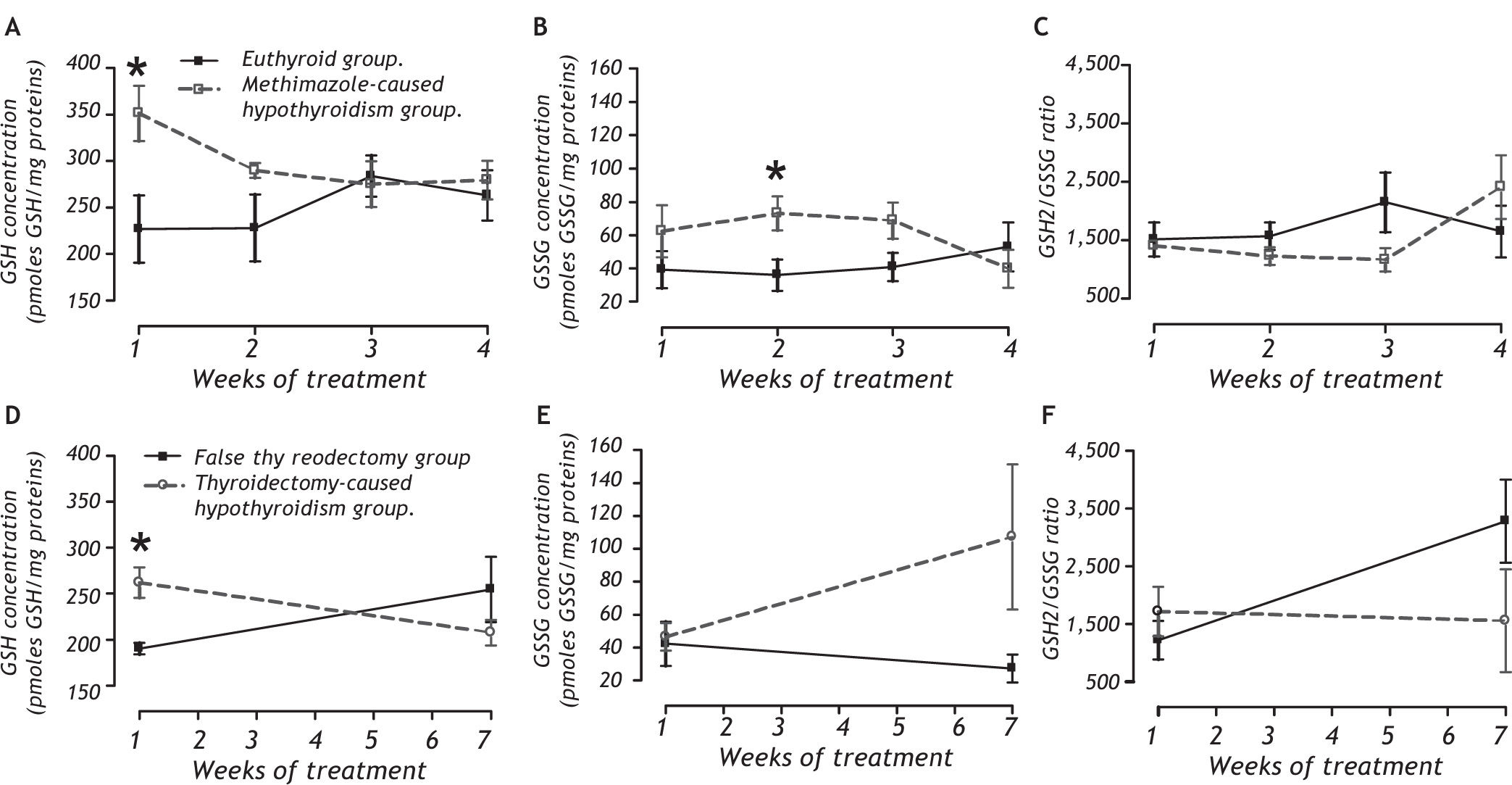
REDOX environment markersPanel A of figure 3 shows that the methimazolecaused hypothyroidism causes an increase of GSH during the first week of treatment (between group: F1,32 = 6.55, P < 0.05; between week: F3,32 = 0.45, P = 0.719; and interaction groups x week: F1,32 = 2.13, P = 0.12). This treatment causes an enhancement of GSSG during the second week (panel B; between group: F1,32 = 4.41, P < 0.05; between week: F3,32 = 0.20, P = 0.90; and interaction groups x week: F1,32 = 1.81, P = 0.17). Panel C shows that there are no changes in the GSH2/GSSG ratio.
Quantification of reduced glutathione (GSH; panels A and D), oxidized glutathione (GSSG; panels B and E), and the GSH2/GSSG ratio (C and F) as REDOX environmental markers in the liver of methimazole-caused hypothyroidism group (A-C) or thyroidectomy-caused hypothyroidism group (D-F).* P < 0.05 vs. respective control.
The thyroidectomy-caused hypothyroidism group did have an increase in GSH after the second week postsurgery (panel D). The GSSG concentration was not affected (panel E) and there was no change in the GSH2/GSSG ratio (panel F).
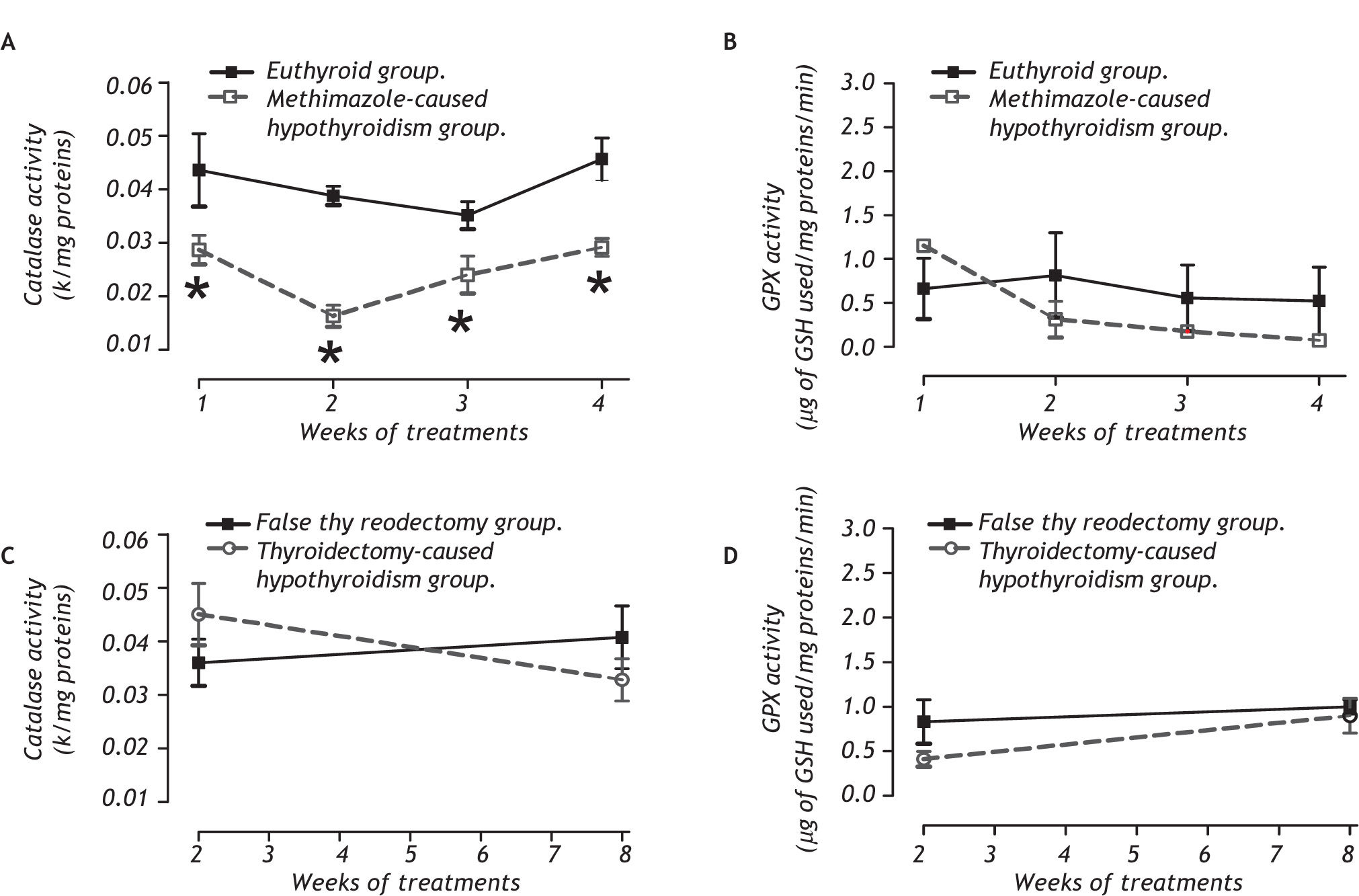
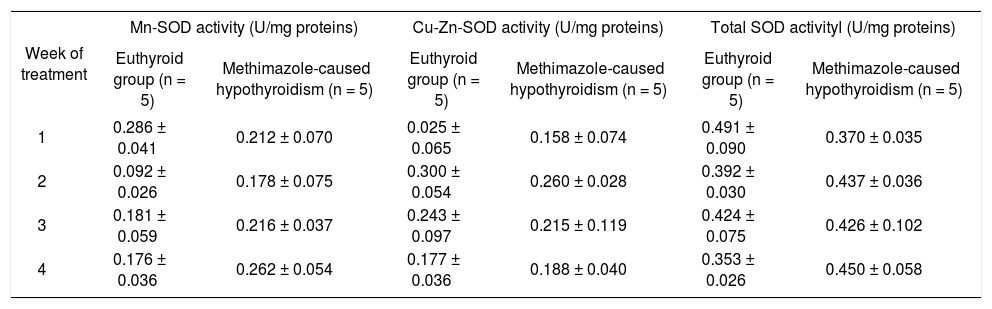
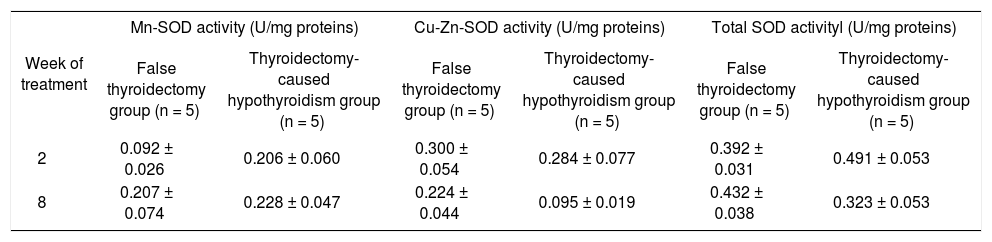
Antioxidant enzymatic systemFor peroxidases, panel A of figure 4 shows that only the methimazole treatment produces a decrease of the catalase activity (between group: F 1,32 = 44.93, P < 0.001; between week: F3,32 = 3.98, P < 0.05; and interaction groups x week: F 1,32 = 2.99, P < 0.05), without any effect on the glutathione peroxidase. The activities of the SOD isoforms of both hypothyroid groups are indicated in the tables 4 and 5. There were no changes of the SOD in these groups.
Activy of SOD isoforms in the liver of methimazole-caused hypothyroidism group.
| Week of treatment | Mn-SOD activity (U/mg proteins) | Cu-Zn-SOD activity (U/mg proteins) | Total SOD activityl (U/mg proteins) | |||
|---|---|---|---|---|---|---|
| Euthyroid group (n = 5) | Methimazole-caused hypothyroidism (n = 5) | Euthyroid group (n = 5) | Methimazole-caused hypothyroidism (n = 5) | Euthyroid group (n = 5) | Methimazole-caused hypothyroidism (n = 5) | |
| 1 | 0.286 ± 0.041 | 0.212 ± 0.070 | 0.025 ± 0.065 | 0.158 ± 0.074 | 0.491 ± 0.090 | 0.370 ± 0.035 |
| 2 | 0.092 ± 0.026 | 0.178 ± 0.075 | 0.300 ± 0.054 | 0.260 ± 0.028 | 0.392 ± 0.030 | 0.437 ± 0.036 |
| 3 | 0.181 ± 0.059 | 0.216 ± 0.037 | 0.243 ± 0.097 | 0.215 ± 0.119 | 0.424 ± 0.075 | 0.426 ± 0.102 |
| 4 | 0.176 ± 0.036 | 0.262 ± 0.054 | 0.177 ± 0.036 | 0.188 ± 0.040 | 0.353 ± 0.026 | 0.450 ± 0.058 |
Data values are mean ± SE.
Activy of SOD isoforms in the liver of thyroidectomy-caused hypothyroidism group.
| Week of treatment | Mn-SOD activity (U/mg proteins) | Cu-Zn-SOD activity (U/mg proteins) | Total SOD activityl (U/mg proteins) | |||
|---|---|---|---|---|---|---|
| False thyroidectomy group (n = 5) | Thyroidectomy-caused hypothyroidism group (n = 5) | False thyroidectomy group (n = 5) | Thyroidectomy-caused hypothyroidism group (n = 5) | False thyroidectomy group (n = 5) | Thyroidectomy-caused hypothyroidism group (n = 5) | |
| 2 | 0.092 ± 0.026 | 0.206 ± 0.060 | 0.300 ± 0.054 | 0.284 ± 0.077 | 0.392 ± 0.031 | 0.491 ± 0.053 |
| 8 | 0.207 ± 0.074 | 0.228 ± 0.047 | 0.224 ± 0.044 | 0.095 ± 0.019 | 0.432 ± 0.038 | 0.323 ± 0.053 |
Data values are mean ± SE.
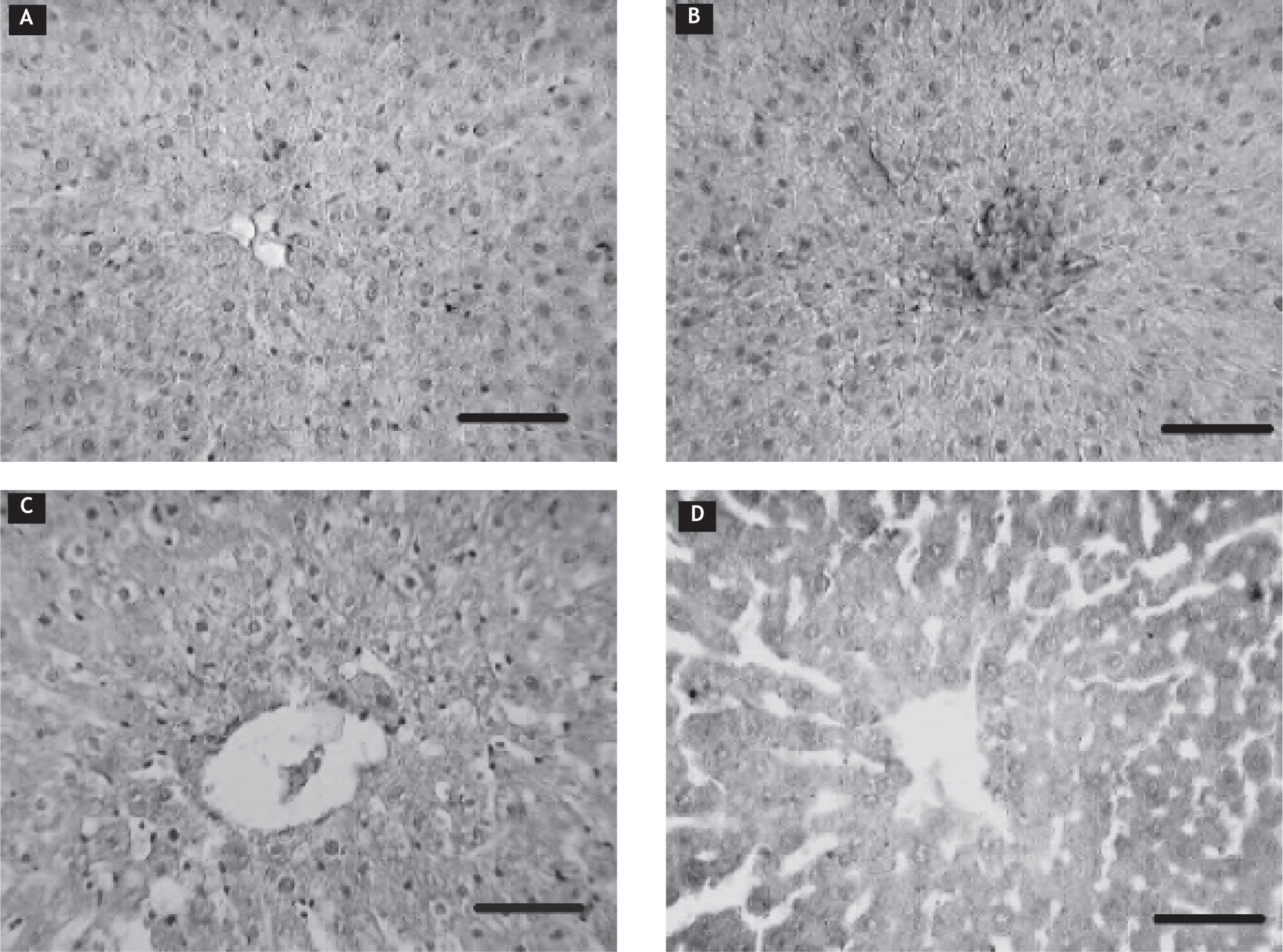
We found in the histological analysis of liver parenchyma (Figure 5) that the microscopic appearance of the euthyroid group (panel A), false thyroidectomy group (panel B), and thyroidectomy group (panel C) had similar characteristics with their hepatocytes in a radial distribution. The methimazole-caused hypothyroidism group showed cellular discontinuity, the hepatocyte radial distribution was lost, and there was hyperplasia of the hepatocytes and alteration of the nucleus-cytoplasm ratio (panel D).
Photomicrography of hepatic tissue. Euthyroid group (A); false thyroidectomy (B); thyroidectomy-caused hypothyroidism (C); methimazole-caused hypothyroidism group (D). Histological alterations were present only in the methimazole-caused hypothyroidism group (panel D). Horizontal line represents 50 μm.
Methimazole is widely used in the treatment of hyperthyroidism because it inhibits thyroid peroxidase and avoids thyroglobulin organification. However, there are reported extrathyroidal effects related to cellular damage from methimazole. The methimazole-caused hypothyroidism has two effects on cellular damage. One effect is a protective mechanism against toxicant-caused oxidative stress.14,15 and the other is the mechanism of methimazole-caused oxi-dative stress and cellular damage.9,16,17 In our research we show that methimazole and not the hypothyroidism is responsible for the hepatic damage. We demonstrated in this study that methimazolecaused hypothyroidism caused oxidative stress, alteration of the GSH-GSSG couple, and reduction of catalase activity, without modifying the SOD actvity. All these were related to hepatic damage, as we observed. In addition, thyroidectomy-caused hypothyroidism enhanced both the ROS and GSH during the second week without developing hepatic damage.
The liver is an organ with a high metabolic activity that generates ROS. Under physiological conditions the presence of antioxidant enzymes, in particular peroxidases and dismutases, prevent oxidative stress and tissue damage.18,19 However, some drugs, like methimazole, disturb the physiological steady state. Methimazole alters the intracellular REDOX environment and causes cellular damage because of oxidant generation and ROS, and consequently lipid peroxidation is not completely neutralized by the antioxidant system.
The central mechanism of methimazole-caused liver damage is based on the reduction of catalase activity caused by a methimazole-inactivated catalytic center.20,21 Catalase is one of the most important pe-roxidases in the liver that prevents the metabolismenhanced H2O2 22 Although Glutathione peroxidase (GPX) did not modify its activity, the catalase inactivation causes high levels of H2O2 in the cells. This substance can diffuse through the plasma membrane and affect neighboring cells. High H2O2 concentration causes the increase of ROS and thus lipid peroxidation (LP).10
Most studies that relate hypothyroidism with liver damage are unacceptable because they used thionamide-caused hypothyroidism,23,24 and this drug itself causes liver damage, as described.9
Other methimazole-caused damage mechanisms are associated with its chemical structure and its biotransformation. Some investigators using methimazole-caused hepatoxicity have shown that the drug binds covalently in the hepatocytes, mainly those next to the hepatic triad.25,26 For biotransformation, methimazole may be oxidized by P450 enzymes to form the 4,5-epoxide. The enzymatic or nonenzymatic hydrolysis of the epoxide formed would produce an unstable hemiketal-like intermediate, which is expected to undergo spontaneous ring cleavage to form glyoxal and N-methylthiourea. The metabolism of N-methylthiourea is complex, but it is believed that sulfur oxidation, mediated mainly by flavin-monoxigenase (FMO, EC. EC 1.14.13.8), proceeds primarily to the sulfenic acids and then possibly to the sulfinic acids. This step is necessary in the bioactivation of thioureas resulting in protein binding, enzyme inactivation, and organ toxicity.27,28
The condition of hypothyroidism itself predisposes hepatocyte damage because of an increase in polyunsaturated fatty acid (PUFA) synthesis, which compounds are susceptible to peroxidation,29 and the reduction of liver proliferation.30,31 We did not see hepatic alterations in the thyroidectomy group. Besides, there are molecular mechanisms by which hypothyroidism itself may produce a protected state of the liver, such as reducing the enzyme activity associated with the mitochondrial respiratory chain,32 the decrease in adenine nucleotide translocase,33 reduced activity of cytochrome-c oxidase,34 and the resistance to forming the permeability transition-pore formation of the inner mitochon-drial membrane.35
Finally, we can agree that the sole hypothyroidism-caused thyroidectomy does not affect the intracellular REDOX environment. We agree with several groups that hypothyroidism does not cause liver damage per se and this state is protected against toxic agents.
AcknowledgementThanks to Dr. Ellis Glazier for editing this English-language text.
This study was partially supported by SIP-IPN 20070318 and 20080113. M.F.C. is fellow of EDI, CO-FAA and SNI. R.O-B is fellow of EDI and COFAA. V.B-V is fellow of CONACyT and PIFI-COFFA.