Chronic hepatitis due to hepatitis C (HCV) viral infection is one of the main causes of chronic liver disease in the world. In the near future, the number of cases developing complications is expected to rise and include the following: liver cirrhosis, liver failure (ascites, encephalopathy, spontaneous bacterial peritonitis, variceal hemorrhage), hepatocellular carcinoma, death or the need for liver transplantation. However, research in the field of hepatitis C diagnosis and treatment is one of the most active specially on the development of new therapeutic strategies potentially offering better viral eradication rates and fewer adverse events.
Although this disease is a frequent cause of consultation and hospitalization, data published in our country are insufficient. The last guidelines proposed by a medical association in Mexico were published in 20071–5 and those suggested by the General Council of Health were published in 2009.6 The aim of this study group was to analyze the available evidence on the diagnosis and treatment of hepatitis C in the Mexican population, in the context of published international clinical and therapeutic guidelines, in order to issue recommendations that are applicable in our midst. The Mexican Association of Hepatology convened a work group in Mexico City, on April 25th and 26th, 2014. Twenty specialists with particular interest and experience.
MethodologyWe conducted an electronic database search in English and Spanish to identify all published documents since the year 2000 that included the terms epidemiology, hepatitis C, diagnosis, treatment, therapy, liver cirrhosis, liver transplantation and Mexico. Previously dated documents were included if they were of particular relevance, as were abstracts presented in national meetings and international guidelines published by the World Health Organization or various medical associations. The bibliography was provided to all panelists before the meeting and was complemented by references suggested by each member of the consensus group.
The group was divided into five working subgroups:
- 1.
Disease impact and at-risk groups.
- 2.
Diagnostic and therapeutic assessment.
- 3.
Treatment of subjects with no previous therapy.
- 4.
Management of subjects with treatment failure.
- 5.
Management of special situations
A document was generated from each discussion subject and practical recommendations were proposed; each was assigned a level of evidence following the GRADE system (Grading of Recommendations, Assessment, Development and Evaluation System).3 The quality of the evidence is thus classified in three possible levels: high [A], moderate [B] or low [C] and either strong [1] or weak [2] (see Table 1). The proposed recommendations were presented to all panelists to obtain their comments and observations. Finally, a second work meeting was conducted in Mexico City on July 19th, 2014, in order to present the final document to the members of the consensus group for their review and approval.
GRADE system (Grading of Recommendations, Assessment, Development and Evaluation System).7
| Quality of the evidence | Description | Grade |
|---|---|---|
| High | Further research is very unlikely to change the confidence in the estimation of effect. | A |
| Moderate | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | B |
| Low | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Any change in the estimate is uncertain. | C |
| Recommendation | Description | Grade |
| Strong | Factors influencing the strength of recommendations include the quality of the evidence, relevant patient outcomes and costs. | 1 |
| Weak | There is variability in preferences and values or more uncertainty. The recommendation is less certain, more costly or uses more resources. | 2 |
The following are the documents presented by each working team with their proposed recommendations and grades of evidence.
This document will be updated periodically as medical advances and regulatory aspects permit the use of new treatments in our country
KeywordsConsensus. Management. Hepatology. Hepatitis C Virus. Therapeutic Agents.
1. Disease Impact and At-Risk GroupsWorldwide, it is estimated that over 185 million individuals are infected with the hepatitis C virus (HCV).8 In Latin America, there is an increased prevalence of the disease in certain age groups, that peaked in 55-64 year-olds between 1990-2005.8–10 In the United States, one of the groups at highest risk of being HCV carriers, includes people born between 1945 and 1965; however, we lack information in our country confirming this observation.11 In 2007, the Mexican Ministry of Health reported that liver cirrhosis is the 5th cause of general mortality,12 and approximately 50% of cases are due to chronic hepatitis B or C.13
The average prevalence of hepatitis C viral infection in the general population is 1.4-1.5%.9,11 However, this may vary according to the country’s geographical area: 2% in the north, 1.5% in the south and 1.1% in the country’s center.11,14 With a prevalence of 1.4 % and a population of 119 million individuals in Mexico, the estimate is that 1,652,000 individuals may be infected with HCV15 – with an estimated incidence of 19,300 new cases per year.16 Among all hepatitis C seropositive individuals, 85%-1,404,200 Mexicans–have chronic hepatitis9 and half of them are unaware of the disease.
The most frequent genotype in our population is genotype 1 –in 70% of cases– and predominantly, sub-type b.9,10,17–19 The prevalence of the interleukin- 28B (IL28B) C/C polymorphism in Mexican patients with HCV infection is 21 to 24%. Requesting this genetic marker is important in our population due to its highly predictive value in terms of sustained virological responses (SVR) to dual therapy with pegylated interferon alpha 2 (PegIFN) and ribavarin (RBV).20,21 The Q80K viral mutation-conferring resistance to simeprevir (SMV)-has a reported prevalence between 9 and 48% in patients with HCV genotype 1a. However to date, there are no studies on this subject in the Mexican population.22,23
Fifty to 75% percent of patients with HCV in our country were infected by transfusion of blood or blood products before 1995.9 Invasive procedures before 1995 are also considered a mechanism of transmission but we have no hard data to sustain it.
A form of transmission that deserves special attention is intravenous drug use (IDU). This activity has increased in recent years among 18 to 34 yearolds according to reports from the Consejo Nacional Contra las Adicciones (CONADIC) (Mexican National Council against Addictions)24 and as recognized in most countries. Other transmission routes documented in other countries remain to be studied in Mexico and include:
- •
Organ transplantation before 1995.
- •
Dental extraction with inadequate hygiene practices.
- •
In-hospital procedures – i.e. hemodialysis.
- •
Use of multiple dose vials.
- •
Endoscopy and biopsy sampling.
- •
Accidental puncture wound in health professionals.
- •
Use of inhaled drugs.
- •
Unsafe sexual practices-number of partners, men having sex with men, HIV co-infected partners, etc.
- •
Tattoos and body piercings.
- •
Sharing razors or toothbrushes with infected persons.
- •
Vertical mother-offspring transmission in HCV infected women.
At risk groups are shown in table 2.
At risk groups for hepatitis C infection.
| • Recipients of blood products or having undergone invasive procedures before 1995. |
| • Intravenous drug users.* |
| • Children of HCV carrier mothers.** |
| • Men having sex with men. |
| • Individuals with multiple sexual partners and unprotected sexual activity. |
| • HIV infection carriers. |
| • Inhaled drug and other illicit substance users. |
| • HBV infection carriers. |
| • Persons with artistic or cosmetic tattoos, or piercings. |
| • Prison or correctional facility interns. |
| • Hemophiliac patients. |
| • Patients on hemodialysis |
| • Health professionals, police and firefighters. |
| • Prostitutes. |
| • Psychiatric hospital and retirement home populations. |
In carriers of the human immunodeficiency virus (HIV), the prevalence of HCV is higher than in the general population, approximately 25%, so screening is a must.27 Further, HCV infection in coinfected patients increases mortality due to earlier development of end stage liver disease.28,29 It is important to emphasize that the infection is not transmitted by breastfeeding.11 Among monogamous, heterosexual, stable couples, the risk of transmission is minimal or practically nil and usual sexual practices may be continued.25
Infection by hepatitis C virus is asymptomatic and follows a variable course ranging from minimal histological injury to extensive fibrosis and liver cirrhosis with or without hepatocellular carcinoma.30 Individuals infected before age 40 have a 5% risk of developing cirrhosis while those infected after age 40, have a 20% risk. Annually, 4% of patients with cirrhosis develop decompensation and 1.6% develops hepatocellular carcinoma.31
There are factors –host and viral– that modify disease progression. The time period to progression to severe liver disease is about 20 years after acquiring the virus.
Forty percent (40%) of worldwide liver transplants are performed in patients with cirrhosis due to hepatitis C.32
Hepatitis C impacts the patients’ quality of life as well as health costs. The quality of life of HCV patients is impaired by cirrhosis complications. However, it is also compromised in the absence of clinically advanced liver disease and does not correlate with the stage of histological injury or aminotransferase values.33
Their quality of life is impaired by somatic extrahepatic manifestations: arthralgias, myalgias, sicca syndrome, cryoglobulinemia, glomerulonephritis and depression. Treated patients that have reached a SVR improve in terms of their physical quality of life scores.33
In terms of the disease’s economic impact, patients with HCV have more absenteeism than controls as well as decreased productivity.34 In the United States; the health system annually spends the equivalent to 8,352 dollars more per HCV carrier.34 Treatment costs hinge on the stage of infection and increase in proportion to the degree of fibrosis. The cost of treating severe liver disease ranges between 4,300 and 30,000 dollars per year.35 Some studies have shown that standard of care treatment with PegIFN/RBV or triple therapy regimens (adding a protease inhibitor such as boceprevir [BOC] or simeprevir [SMV]) is cost effective in previously untreated patients and in those with previous treatment failure.36,37
In our country, the cost of liver transplantation is equivalent to 150,000 dollars.32 Hence, treating patients in early disease stages is pivotal, before they develop complications and/or the need for a liver transplant.
Conclusions- •
The prevalence of HCV infection in the Mexican population is 1.4%. It may however, vary according to the geographical region. [A1]
- •
The most frequent genotype in the HCV infected population in Mexico is 1, present in 70% of cases; sub-type 1b predominates. [A1]
- •
In our country, blood or blood product transfu- sion before 1995, currently accounts for 50 to 75% of all HCV infected patients. [A1]
- •
Screening for HCV should be performed in all at-risk individuals. [A1]
- •
In monogamous, heterosexual, stable couples, the risk of transmission is minimal or practically nil; they may continue with their usual sexual practices. [B1]
- •
Hepatitis C impacts the patients’ quality of life as well as the associated health costs. [A1]
- •
Treatment of chronic hepatitis due to HCV is cost-effective. [B1]
Hepatitis C viral (HCV) infection is usually asymptomatic in its early stages and the diagnosis is obtained after incidentally finding of positive anti-HCV antibodies in blood banks, abnormal liver function tests (LFT) or advanced liver disease symptoms. Unfortunately the disease is mostly underdiagnosed, whereby only 30-50% of HCV infected individuals are aware of their disease and may be treatment candidates; effective treatment may prevent progression to cirrhosis and decrease the risk of viral propagation.38
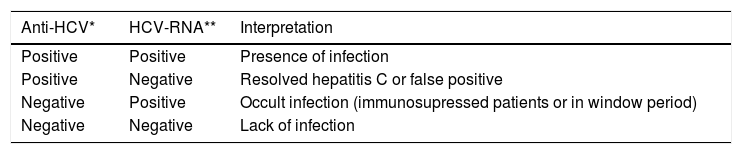
Screening refers to the application of a test that allows an early diagnosis. In the case of HCV infection, screening tests are serological assays that detect anti-HCV antibodies by enzymatic immunoassay (EIA). Confirmatory tests includes: qualitative and quantitative detection of HCV ribonucleic acid (RNA) by polymerase chain reaction (PCR) that determines whether the HCV-RNA is present or not in blood as well as its quantity.39
The 2nd generation EIA detects antibodies against epitopes from the nuclear region (C-22), region NS3 (C-33) and region NS4 (C-100), which increases its sensitivity to approximately 95% and lowers the rate of false positive results.39–42
Compared to the 1st and 2nd generation EIAs, the 3rd generation EIA is the currently recommended test. It has been complemented by the ability to detect antibodies against an antigen in region NS5 and/or the substitution of an epitope in region NS3 that is highly immunogenic. This innovation allows the detection of anti-HCV antibodies four to six weeks after infection with a sensitivity of 99% or greater.41 The immunoblot technique is no longer recommended.
- •
False positive results are more common in patients with positive rheumatoid factor and in populations with a low prevalence of hepatitis C, such as healthy blood or organ donors.41
- •
False negative results may occur in patients on hemodialysis or if severely immunosuppressed, as in HIV infection or in association with hematological malignancies.41
Where available, the quick capillary test may be used since it has the same sensitivity.
In any individual in whom anti-HCV is detected, a confirmatory qualitative test must be obtained for HCV-RNA; this is highly sensitive, it reports the presence or lack of HCV and is used to:
- •
Confirm the diagnosis of HCV infection.
- •
Screen blood or organ donors with positive anti-HCV.
- •
Confirm a sustained virological response (SVR) at several intervals after the end of treatment.
The quantitative test measures the amount of virus in blood at any given time; its values range between 15 IU/mL. and 10 million IU/mL. It currently has a very important role in treatment response monitoring.43
In the diagnosis of acute HCV infection or in immunosuppressed patients, the HCV-RNA determination is initially recommended; its minimum detection cutoff point is 15 IU/mL.39,40
The diagnosis of chronic infection is based on a positive anti-HCV and HCV-RNA in patients with clinical, biochemical and histological changes of chronic hepatitis.39,40 The interpretation of serological and molecular markers of HCV in different scenarios is detailed in table 3.
In individuals with a positive or reactive Anti-HCV and a negative or undetectable HCV-RNA, the molecular test should be repeated after 3 months to confirm or exclude the infection.42,43
In patients with an Anti-HCV positive and detectable HCV RNA, the genotype and sub-type must be obtained.42,43
The viral load and HCV genotypification are indispensable in patients considered potential therapeutic candidates.42,43 The aims of treating HCV are: to stop disease progression and avoid complications from cirrhosis, decrease the rates of hepatocellular carcinoma and finally, limit the dissemination of the infection.44,45
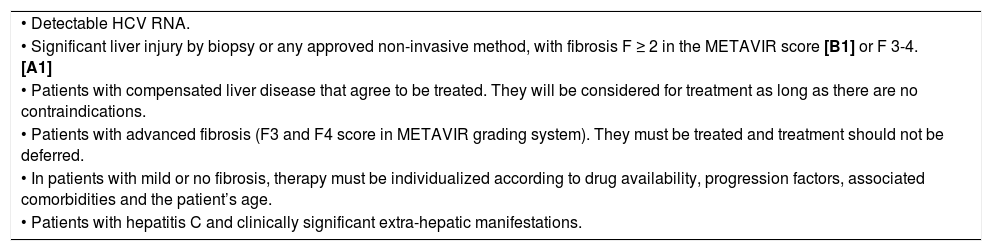
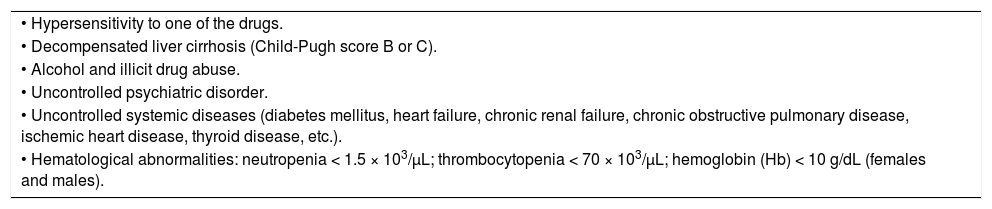
The indications and contraindications of antiviral therapy with IFN-based regimens are shown in tables 4 and 5.
Indications of antiviral therapy.
| • Detectable HCV RNA. |
| • Significant liver injury by biopsy or any approved non-invasive method, with fibrosis F ≥ 2 in the METAVIR score [B1] or F 3-4. [A1] |
| • Patients with compensated liver disease that agree to be treated. They will be considered for treatment as long as there are no contraindications. |
| • Patients with advanced fibrosis (F3 and F4 score in METAVIR grading system). They must be treated and treatment should not be deferred. |
| • In patients with mild or no fibrosis, therapy must be individualized according to drug availability, progression factors, associated comorbidities and the patient’s age. |
| • Patients with hepatitis C and clinically significant extra-hepatic manifestations. |
Interferon-based antiviral treatment contraindications.
| • Hypersensitivity to one of the drugs. |
| • Decompensated liver cirrhosis (Child-Pugh score B or C). |
| • Alcohol and illicit drug abuse. |
| • Uncontrolled psychiatric disorder. |
| • Uncontrolled systemic diseases (diabetes mellitus, heart failure, chronic renal failure, chronic obstructive pulmonary disease, ischemic heart disease, thyroid disease, etc.). |
| • Hematological abnormalities: neutropenia < 1.5 × 103/µL; thrombocytopenia < 70 × 103/µL; hemoglobin (Hb) < 10 g/dL (females and males). |
All HCV infected patients must undergo testing that will evaluate the stage of liver injury and associated conditions.
An integral diagnostic evaluation must include: a complete clinical history ruling out other causes of liver disease, complete blood count (CBC), blood chemistry, liver function tests (LFT), thyroid-stimulating hormone (TSH), prothrombin time (PT), international normalized ratio (INR), hepatitis B virus (HBV) and HIV serologies, antinuclear antibodies (ANA), immunoglobulins, liver ultrasound (US) and evaluation of the grade of fibrosis by liver biopsy or other non-invasive methods such as elas- tography. All tests are performed to rule out conditions that could accelerate the progression of hepatic fibrosis.46,47
Liver biopsy is not essential to confirm the diagnosis. Its main use based on its ability to measure the severity of necroinflammatory activity, the grade of hepatic fibrosis and to identify other causes of liver disease. The degree of hepatic fibrosis may also be evaluated with non-invasive procedures such as elastography or serum biomarkers.46,47
IL28B determination is not a prerequisite to initiate treatment. It can be obtained if available, since it is a predictor of antiviral therapy response.48 The main predictors of a poor response to treatment are: genotype 1, high viral load (above 800,000 IU/mL in patients on double therapy), alcohol abuse, advanced fibrosis or cirrhosis, CT/TT IL28B genotype, HBV and HIV coinfection, metabolic syndrome and/or insulin resistance. Finally but no less important: lack of compliance to therapy.
DIAGNOSTIC RECOMMENDATIONS
- •
Anti-HCV antibody determination is the first diagnostic test to detect infection. [A1]
- •
In any individual with a positive Anti-HCV, a HCV-RNA by PCR must be obtained with a minimum detection cutoff point of 15 IU/mL. [A1]
- •
Individuals in whom acute HCV infection is suspected or in immunocompromised hosts, a HCV RNA should be initially obtained. [A1]
- •
In individuals with a positive Anti-HCV and undetectable HCV-RNA, RNA testing should be repeated after 3 months to confirm o exclude the infection. [A1]
- •
In patients with a positive HCV-RNA, HCV genotype and sub-type must be determined. [A1]
TREATMENT RECOMMENDATIONS
- •
The aims of HCV treatment are: to stop disease progression and avoid the complications of cirrhosis, decrease the rates of hepatocellular carcinoma and finally, limit dissemination of the infection. [A1]
- •
Patients with compensated liver disease and willing to be treated should be considered for therapy as long as there are no contraindications. [A1]
- •
Patients with advanced fibrosis (F3 and F4 in METAVIR score) should be treated and therapy should not be deferred. [A1]
- •
In patients with mild or no fibrosis, therapy will be individualized according to drug availability, progression risk factors, the presence of comorbidities and the patient’s age. [B1]
- •
In patients with hepatitis C and clinically significant extra-hepatic manifestations, treatment must be considered. [B2]
- •
Conditions or comorbidities that may accelerate hepatic fibrosis, must be investigated, evaluated and if need be, treated in any patient with HCV infection. [A1]
- •
Liver injury severity must be evaluated before initiating treatment. [A1]
- •
Fibrosis stage may be evaluated by liver biopsy or by non-invasive methods. [B1]
- •
Determining IL28B is not a prerequisite to initiate treatment. If available, it can be obtained since it is a predictor of response to dual antiviral therapy. [B2]
The degree of progression in patients with chronic HCV is variable since it depends on the presence of factors that increase the fibrotic process, including alcohol ingestion, male gender, acquiring the infection at an adult age and immunosuppression49–55 – and importantly, in our population, obesity, insulin resistance and diabetes mellitus type 2.56–60 According to the data obtained in the Encuesta Nacional de Salud (ENSANUT) (National Health Survey) of 2012, in Mexico 69.4 to 73% of adults above the age of 20 are overweight or obese and 9.2% of adults are diabetic.61
RECOMMENDATIONS
- •
Investigate other concomitant liver diseases that could affect the progression of chronic hepatitis due to HCV and initiate appropriate management. [A1]
- •
Treat concomitant pathologies: metabolic syndrome - insulin resistance, overweight, dyslipidemia, hypertension. [B2]
- •
Control psychiatric disease. [A1]
- •
Discontinue the use of alcohol [B2] and other addictions. [A1]
- •
All patients with advanced fibrosis/liver cirrhosis (F3-F4) should undergo screening and surveillance by ultrasound every 6 months for HCC in spite of a sustained virological response. [A1]
- •
All patients with advanced fibrosis/liver cirrhosis (F3-F4) should go endoscopic screening to detect esophageal varices. [A1]
- •
Other drugs or therapeutic alternatives including anti-fibrotic and immumodulating agents (i.e. silymarin, pirfenidone, transfer factor, stem cell transplant, etc.) have shown no efficacy and their use is not recommended. [C1]
In our country, access to new generation direct-acting antiviral (DAA) agents is limited. Currently, only boceprevir (BOC) and recently, (June 19, 2014) simeprevir (SMV) have been approved as triple therapy in combination with Pegylated Interferon (PegIFN)/ Ribavirin (RBV) in patients with chronic hepatitis and genotype 1. At present, in our country, PegIFN/RBV is the treatment of choice in patients with a genotype other than 1.
In patients with genotype 1, PegIFN/RBV may be very effective in those with a rapid virological response (RVR: undetectable HCV-RNA at 4 weeks of treatment) and although not ideal since the SVR is limited (approximately 40-50% of patients with genotype 1), response-guided treatment is a valid option and more accessible in our midst.62–68
The monitoring of the “on-treatment viral response” should be performed with quantitative HCV-RNA with the most sensitive technique available –Limit of Detection (LOD) 15 UI/mL– at the following timepoints:
- •
Therapy with PegIFN/RBV: At baseline, at weeks 4, 12, 24, at the end of treatment and 12 and 24 weeks post-treatment.
- •
Triple therapy (PegIFN/RBV+BOC): At baseline, at weeks 4, 8, 12, 24, at the end of treatment and 12 and 24 weeks post-treatment.
- •
Triple therapy (PegIFN/RBV+SMV): At baseline, at weeks 4, 12 and 24, at the end of treatment and 12 and 24 weeks post-treatment.
According to the obtained results, the response pattern is determined following the parameters shown in table 6.
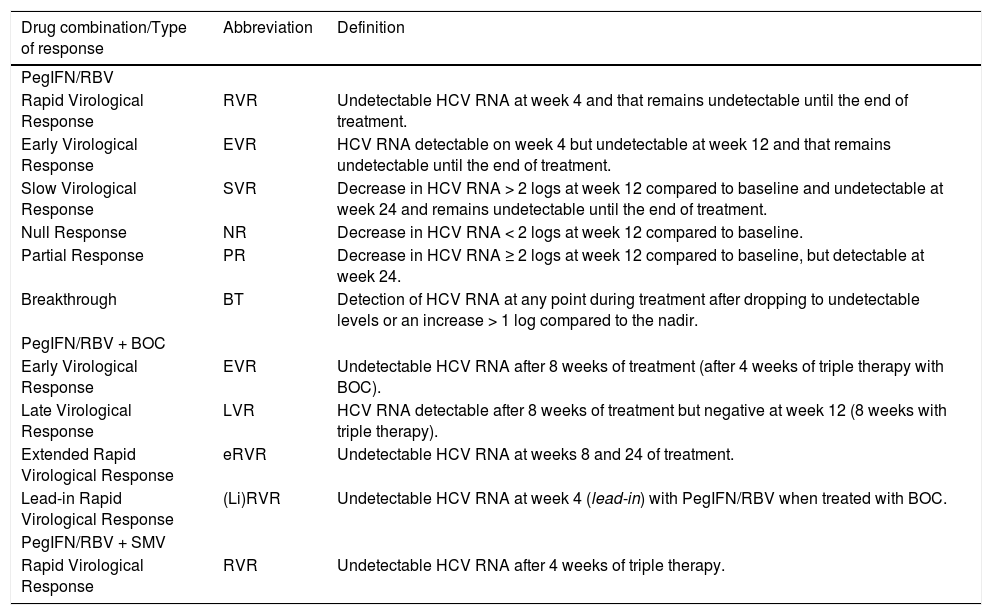
Definition of the type of response during antiviral therapy in patients with HCV infection.63
| Drug combination/Type of response | Abbreviation | Definition |
|---|---|---|
| PegIFN/RBV | ||
| Rapid Virological Response | RVR | Undetectable HCV RNA at week 4 and that remains undetectable until the end of treatment. |
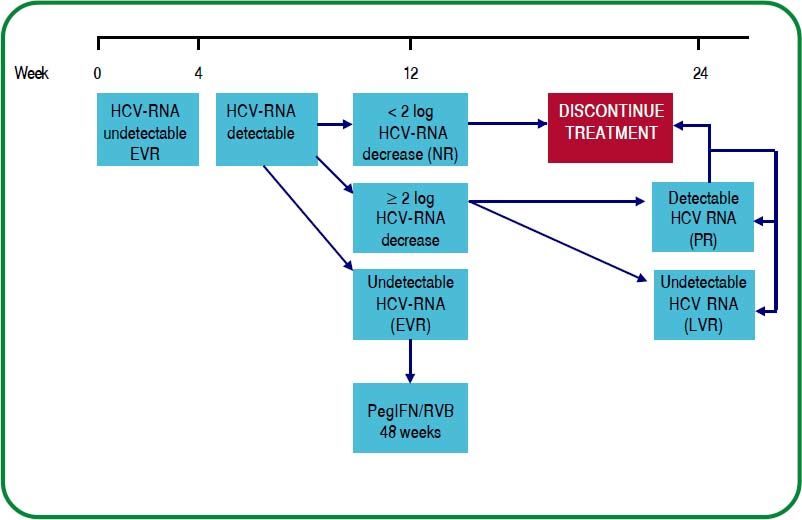
| Early Virological Response | EVR | HCV RNA detectable on week 4 but undetectable at week 12 and that remains undetectable until the end of treatment. |
| Slow Virological Response | SVR | Decrease in HCV RNA > 2 logs at week 12 compared to baseline and undetectable at week 24 and remains undetectable until the end of treatment. |
| Null Response | NR | Decrease in HCV RNA < 2 logs at week 12 compared to baseline. |
| Partial Response | PR | Decrease in HCV RNA ≥ 2 logs at week 12 compared to baseline, but detectable at week 24. |
| Breakthrough | BT | Detection of HCV RNA at any point during treatment after dropping to undetectable levels or an increase > 1 log compared to the nadir. |
| PegIFN/RBV + BOC | ||
| Early Virological Response | EVR | Undetectable HCV RNA after 8 weeks of treatment (after 4 weeks of triple therapy with BOC). |
| Late Virological Response | LVR | HCV RNA detectable after 8 weeks of treatment but negative at week 12 (8 weeks with triple therapy). |
| Extended Rapid Virological Response | eRVR | Undetectable HCV RNA at weeks 8 and 24 of treatment. |
| Lead-in Rapid Virological Response | (Li)RVR | Undetectable HCV RNA at week 4 (lead-in) with PegIFN/RBV when treated with BOC. |
| PegIFN/RBV + SMV | ||
| Rapid Virological Response | RVR | Undetectable HCV RNA after 4 weeks of triple therapy. |
* PCR-based techniques are recommended with LoQ of 25 IU/mL and LoD of 15 UI/mL. Adapted from: EASL Journal of Hepatology 2014; 60: 392-420.
In our country, the treatment of choice in patients with F ≥ 2-F4 fibrosis, is triple therapy with PegIFN/RBV + BOC, or triple therapy with PegIFN/RBV + SMV.
- a)
Triple therapy with PegIFN/RBV + boceprevir (BOC). The global rate of SVR in registry studies of previously untreated patients, was 63 to 66%.69,70[A1] with the following dosages:
- •
PegIFN alpha 2a: 180 mcg. SC./per week or PegIFN alpha 2b: 1.5 mcg/kg SC./per week.
- •
Ribavirin based on weight:
- °
< 75 kg. 1.0 g. PO/day.
- °
≥ 75 kg. 1.2 g. PO/day.
- °
- •
Boceprevir (BOC): 800 mg. PO q 8 hrs.
- •
After 4 weeks of therapy with PegIFN/RBV (Lead in phase), BOC is added. In special cases of rapid virological response (undetectable HCV RNA) after 4 weeks of double therapy (PegIFN/RBV), consider continuing the same therapy as long as the viral load remains undetectable by weeks 12 and 24. [B2]
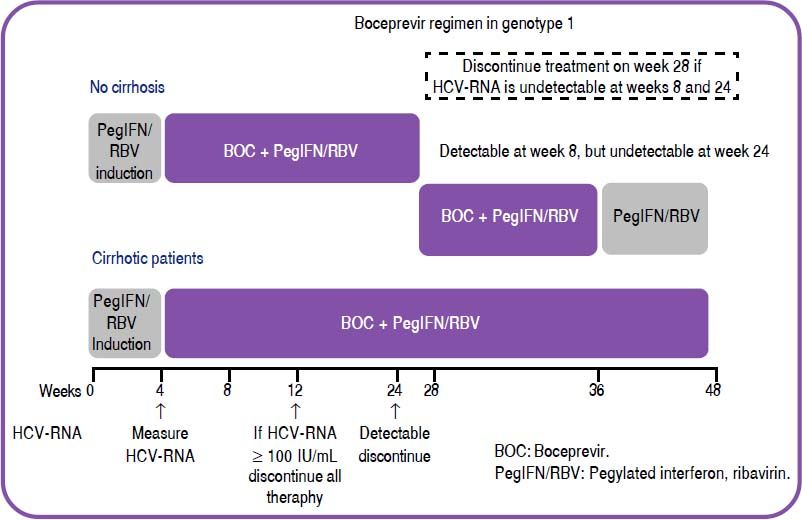
In cases in which there is no rapid virological response or triple therapy with PegIFN/RBV+BOC is available, treatment may be guided by the response according the following three options:
- 1)
Treatment for 28 weeks: Induction (Lead in phase) with PegIFN/RBV for 4 weeks, followed by 24 weeks of triple therapy in patients with undetectable HCV RNA from week 8 to 24. [B1]
- 2)
Treatment for 36 weeks: Induction (Lead in phase) with PegIFN/RBV for 4 weeks, followed by 32 weeks of triple therapy in individuals with detectable HCV RNA at week 8 but undetectable at week 24. [B1]
- 3)
Treatment for 48 weeks: Induction with PegIFN/RBV for 4 weeks, followed by 32 weeks of triple therapy and then 12 weeks of PegIFN/RBV in patients in whom, between weeks 8 and 12, the measurement is below 100 IU/ml. and undetectable by week 24. [B1]
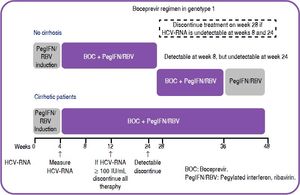
In patients with cirrhosis and those with decreases in HCV-RNA below 1 log10 during the four weeks of induction (lead in phase): treatment for 48 weeks (PegIFN/RBV for 4 weeks, followed by 44 weeks of triple therapy (see figure 1). [B1]
Based on results from real-life studies, a group of patients has been detected in whom the use of triple therapy with PegIFN/RBV+BOC is associated with a high rate of complications and serious/severe adverse events. This regimen should NOT be used in these patients. Of particular relevance, are patients with cirrhosis and signs of portal hypertension (esophageal varices, ascites), hypoalbuminemia (albumin < 3.5 mg/dL) and thrombocytopenia < 90,000/mL (see table 7).71,72[B1]
Contraindications to the use of triple regimen with PegIFN/RBV and boceprevir.
| • Decompensated liver cirrhosis. |
| • Liver cirrhosis with portal hypertension (esophageal varices, ascites), hypoalbuminemia (alb < 3.5 mg/dL) and thrombocytopenia < 90,000/mL. |
| • Decompensated comorbidities (i.e.: diabetes mellitus, systemic arterial hypertension, heart failure, renal failure). |
| • Uncontrolled psychiatric diseases. |
| • Solid organ transplant (except liver). |
| • Uncontrolled autoimmune diseases. |
| • Pregnancy or inability to use two birth control methods. |
| • Hypersensitivity to any of the drugs. |
| • Genotypes other than 1. |
| • Concomitant use of drugs with significant drug-to-drug interactions with boceprevir. |
| • Active alcohol and/or drug addiction. |
| • Poorly compliant patients. |
RECOMMENDATIONS FOR TREATMENT WITHDRAWAL (STOPPING RULES) FOR PATIENTS TREATED WITH TRIPLE THERAPY PEGIFN/RBV AND BOCEPREVIR
- •
Virologic treatment failure:
- °
HCV-RNA > 100 IU/mL after 12 weeks of treatment (8 weeks if on triple therapy). [B1]
- °
Decrease in HCV-RNA below 3 logs when compared to baseline on week 8 of treatment (4 weeks if on triple therapy). [B2]
- °
Detectable HCV-RNA at any point after week 24 of treatment. [A1]
- °
- •
Other conditions during treatment:
- °
Lack of compliance to the regimen. [A1]
- °
Severe adverse events relating to therapy: [A1]
- ¤
Anemia refractory to medical treatment (decrease RBV dosage and/or use of erythropoietin).
- ¤
Decompensated comorbidities. [A1]
- ¤
Neutropenia 500/µL. [B1]
- ¤
Thrombocytopenia 50,000/µL. [B1]
- ¤
De novo severe and uncontrollable psychiatric disease. [A1]
- ¤
- °
- •
In case the protease inhibitor causes an adverse event warranting its discontinuation, double therapy may be considered if there has been a virological response. [B2]
- •
The boceprevir dose should never be modified. [A1]
- •
Boceprevir should never be used as monotherapy. [A1]
- •
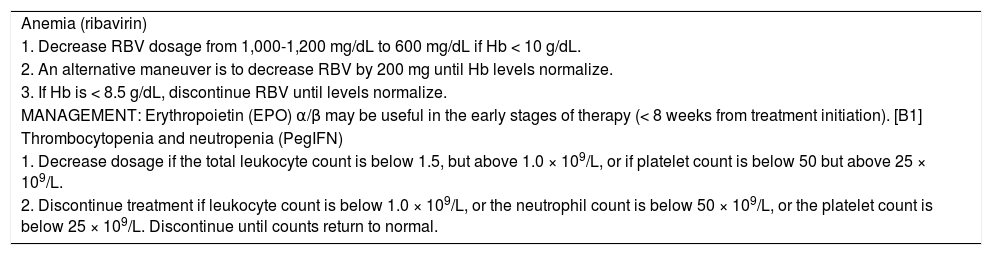
Dose modification/discontinuation of PegIFN and RBV are the same as in double therapy (SeeTable 8).73–84[A1]
Table 8.Management of secondary effects.
Anemia (ribavirin) 1. Decrease RBV dosage from 1,000-1,200 mg/dL to 600 mg/dL if Hb < 10 g/dL. 2. An alternative maneuver is to decrease RBV by 200 mg until Hb levels normalize. 3. If Hb is < 8.5 g/dL, discontinue RBV until levels normalize. MANAGEMENT: Erythropoietin (EPO) α/β may be useful in the early stages of therapy (< 8 weeks from treatment initiation). [B1] Thrombocytopenia and neutropenia (PegIFN) 1. Decrease dosage if the total leukocyte count is below 1.5, but above 1.0 × 109/L, or if platelet count is below 50 but above 25 × 109/L. 2. Discontinue treatment if leukocyte count is below 1.0 × 109/L, or the neutrophil count is below 50 × 109/L, or the platelet count is below 25 × 109/L. Discontinue until counts return to normal. MANAGEMENT: There is no clear evidence supporting the use of growth factors such as filgrastim or eltrombopag. [B2]
- •
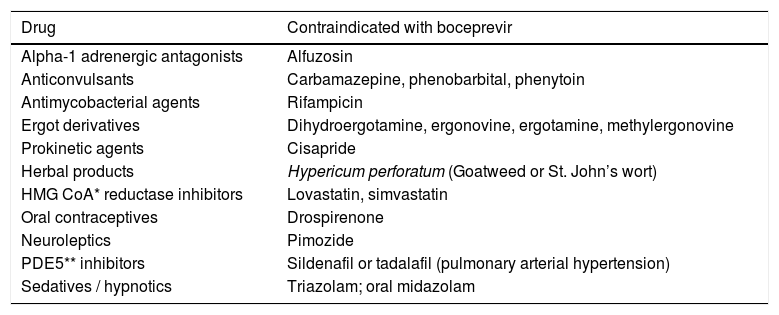
Since boceprevir is a CYP 450 inhibitor, many pharmacological interactions may occur and should be considered when prescribed (SeeTable 9).[A1]
Table 9.Drug interactions with boceprevir.
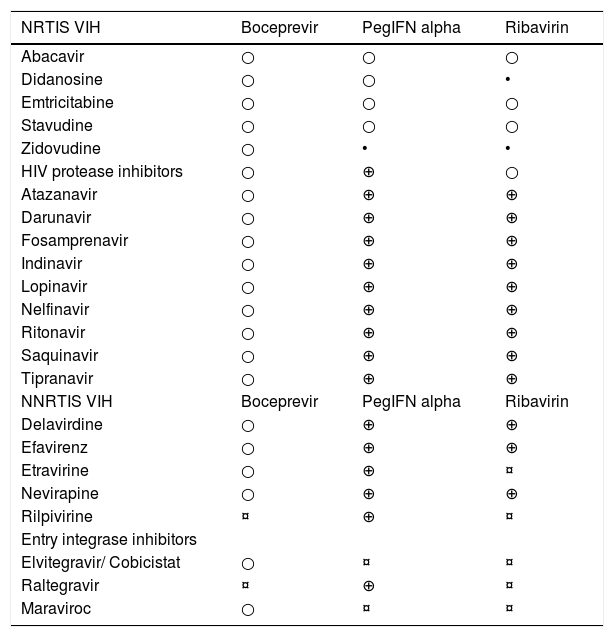
Drug Contraindicated with boceprevir Alpha-1 adrenergic antagonists Alfuzosin Anticonvulsants Carbamazepine, phenobarbital, phenytoin Antimycobacterial agents Rifampicin Ergot derivatives Dihydroergotamine, ergonovine, ergotamine, methylergonovine Prokinetic agents Cisapride Herbal products Hypericum perforatum (Goatweed or St. John’s wort) HMG CoA* reductase inhibitors Lovastatin, simvastatin Oral contraceptives Drospirenone Neuroleptics Pimozide PDE5** inhibitors Sildenafil or tadalafil (pulmonary arterial hypertension) Sedatives / hypnotics Triazolam; oral midazolam A useful tool when deciding what drug to use for treatment is the www.hep-druginteractions.org website, also available for mobile devices.
A useful tool when deciding what drug to use for treatment is the www.hep-druginteractions.org website, also available for mobile devices.
Telaprevir is unavailable and has never been submitted for approval in Mexico, so no recommendations will be provided (Tables 8 and 9).
- b)
Triple therapy with PegIFN/RBV + simeprevir (SMV). This triple therapy combination leads to a SVR of 80-81%, based on approval studies.85,86[A1]
Patients infected with genotype 1b have a SVR of 85% vs. 84% in patients with genotype 1a. In cases infected with genotype 1a and a baseline Q80K variant, the SVR decreases to 58%.85,86 The liver fibrosis stage also affects the possibilities of obtaining a SVR; it is 84% in patients with an F0-F1 score (according to the METAVIR scale), 73% in F3 and 60% in patients with cirrhosis.64,66,85,86 However, it may reach 93% in individuals with a rapid virologic response (undetectable HCV RNA by week 4) but decreases to 63% in those without a rapid response.64,66,85,86
The recommended doses are:
- •
Simeprevir: 150 mg. PO qd.
- •
PegIFN alpha 2a: 180 mcg. SC/week or PegIFN alpha 2b: 1.5 mcg/kg SC/week.
- •
Ribavirin based on weight:
- °
< 75 kg. 1.0 g. PO/day.
- °
> 75 kg. 1.2 g. PO/day.
- °
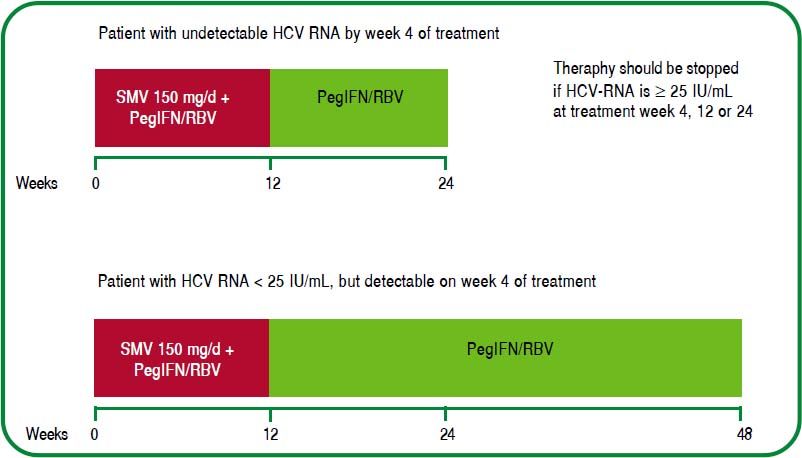
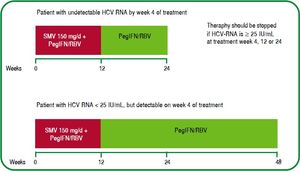
The regimens approved in Mexico are: triple therapy with PegIFN/RBV+SMV for 12 weeks, followed by 12 or 36 weeks of double therapy with PegIFN/RBV depending on the on-treatment viral response: [A1]
- 1.
Previously untreated patients with undetectable HCV RNA after 4 weeks of triple therapy with PegIFN/RBV+SMV, should be treated for another 12 weeks with PegIFN/RBV double therapy (total treatment duration: 24 weeks). [A1]
- 2.
Individuals with HCV RNA < 25 IU/mL, but detectable by week 4, should receive additional treatment for 36 weeks with PegIFN/RBV after completing 12 weeks of triple therapy with PegIFN/RBV+SMV (total duration of treatment: 48 weeks). [A1]
The prevalence of the Q80K mutation is unknown in our country, and reports vary in different countries. In multicenter approval studies, they have been reported as: Australia/New Zealand 7%, Eu- rope 19%, North America 48% and South America (including Mexico) 9%.86,87
The most frequently reported adverse events in approval clinical trials are: skin rash (7.6%), pruritus (3.1%) and photosensitivity (0.8%); since SMV inhibits OATP1B1 and MRP2 transporters in hepatocytes, isolated increases in the serum bilirubin levels may be present in 7.4% of cases. This adverse events may vary from mild to moderate and has led to treatment discontinuation in 0.1% of cases.86,87 (Figure 2).
RECOMMENDATIONS FOR TREATMENT DISCONTINUATION (STOPPING RULES) FOR PATIENTS TREATED WITH TRIPLE THERAPY PEGIFN/RBV AND SIMEPREVIR
- •
Virologie response failure: [A1]
- 1.
HCV-RNA ≥ 25 IU/mL at week 4 of treatment (discontinue PegIFN/RBV and SMV).
- 2.
Detectable HCV-RNA at week 12 (discontinue PegIFN/RBV). Treatment with SMV ends on week 12.
- 3.
Detectable HCV-RNA at week 24 (discontinue PegIFN/RBV). Treatment with SMV ends on week 12.
- 1.
In scenarios 2 and 3, reevaluate HCV-RNA, to confirm the HCV-RNA levels before discontinuing treatment. Other previously mentioned recommendations on the use of boceprevir are also applicable to triple therapy with simeprevir.
- •
The dosage of simeprevir must never be modified. [A1]
- •
Simeprevir should never be used as monotherapy. [A1]
- •
Dose modifications/discontinuation of PegIFN and RBV are the same as in double therapy (Seetable 8).73–84[A1]
- •
If the individual misses a dose but remembers within 12 hours, he can take the missed dose (with meals) and continue with the regimen. In case the missed dose is beyond 12 hours, he should NOT take the dose but rather, continue with the next programmed dose.85
- •
In accordance with other international management guidelines, triple therapy with PegIFN/ RBV+SMV is NOT recommended in patients with genotype 1a and the Q80K mutation.64,66
- •
Since SMV is a CYP 450 inhibitor, many pharmacological interactions may develop and should be considered when prescribing the drug (Seetable 10). [A1] We must emphasize the fact that the dosage should not be modified and it can be used in combination with immunosuppressants such as cyclosporine and tacrolimus.88
Table 10.Contraindicated drugs in patients on simeprevir.
Type of drug Agent Anticonvulsants Carbamazepine, oxcarbazepine, phenobarbital, phenytoin. Antibiotics Erytromycin, clarithromycin, telithromycin, rifampicin, rifabutine, rifapentine. Systemic antifungals Itraconazole, ketoconazole, posaconazole, fluconazole, voriconazole. Systemic corticosteroids Dexamethasone. Gastrointestinal agents Cisapride. Herbal products Hypericum perforatum (goatweed and St. John’s wort), silymarin. Anti-retrovirals Cobicistat, efavirenz, delavirdine, etravirine, neviparine, ritonavir and any anti-HIV regimen with a protease inhibitor, boosted or not with ritonavir.
A useful tool when deciding what drug to use for treatment is the www.hep-druginteractions.org website, also available for mobile devices.
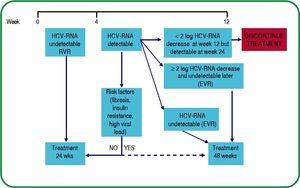
Double therapy (pegIFN/RBV) in patients with genotype 1The main reason for recommending therapy with PegIFN/RBV, is the low availability of protease in- hibitors in our country.89–92 However, double therapy may be effective in patients with good response prognostic factors, particularly: a rapid virologic response, a low viral load and a C/C IL28B polymorphism.93,94
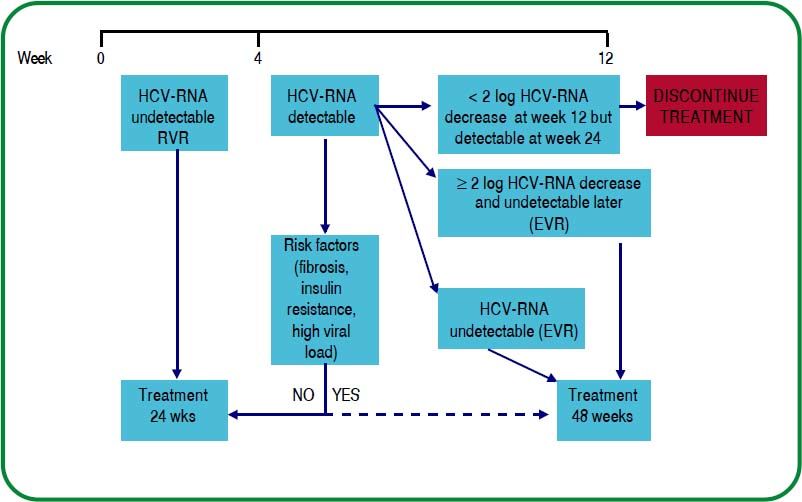
DUAL THERAPY RECOMMENDATIONS:
- •
SVR with double therapy is 42-46%, but lower in Hispanics (34%).95[A1]
- •
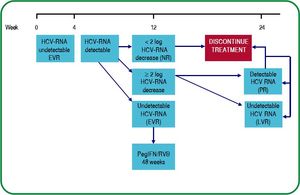
Double therapy should be administered according to the following recommendations:
- °
Rapid virologic response:Undetectable HCV-RNA by week 4 of treatment.
- °
Early virologic response:≥ 2 log decrease in HCV-RNA or undetectable at week 12.
- °
Undetectable HCV-RNA at 24 weeks.
- °
In case these points are fulfilled, continue until week 48. [A1]
There are studies suggesting that in patients with genotype 1 and good response predictors, treatment may be shortened. However, there are no such studies in the Mexican population that can allow us to make this recommendation.96–100[C2]
Extended therapy over 72 weeks has not shown superior efficacy in patients with a slow virologic response (> 2 log decrease by week 12 and undetectable by week 24) when compared with treatment for 48 weeks.101–106[C2]
The response-guided treatment regimen is shown in figure 3.
TREATMENT IN PATIENTS WITH > GENOTYPES 2 AND 3
- •
Standard treatment is the combination of PegIFN/RBV leading to a SVR of 76 to 82%.[A1]
- •
The PegIFN dosage is the same as that used in genotype 1. The RBV dose in patients with genotypes 2 and 3 is 800 mg/day. Patients with baseline unfavorable prognostic factors should be treated with RBV doses adjusted to the patient’s weight (15 mg/kg/day).90[A2]
- •
If HCV-RNA is undetectable by week 4, the recommended duration of therapy is 24 weeks.[A1]
- •
In selected patients with genotype 2/3 and good response prognostic factors, some authors suggest the use of a shorter course of therapy, between 12 and 16 weeks. Currently, there is no such confirmatory evidence in the Mexican population.107–114[C2]
- •
In subjects without a RVR (undetectable HCV-RNA by week 4), consider treatment for up to 48 weeks as long as there is a ≥ 2 log decrease in HCV-RNA by week 12 and it is undetectable by week 24.114,115[A1]
The response-guided treatment algorithm for patients with HCV infection by genotype 2 and 3 is shown in figure 4.
TREATMENT IN PATIENTS WITH OTHER GENOTYPES
- •
Due to their low prevalence in Mexico, there are no studies that allow the proposal of definitive recommendations. In other countries,64,66,68the recommended regimens are:
- •
Genotype 4:Genotype 1 regimen. [C1]
- •
Genotypes 5 and 6:Genotype 2 and 3 regimen. [C1]
- •
Upon the recent approval of SMV, one may consider its use (if available), as first-line therapy in patients infected with HCV genotype 4.64,66
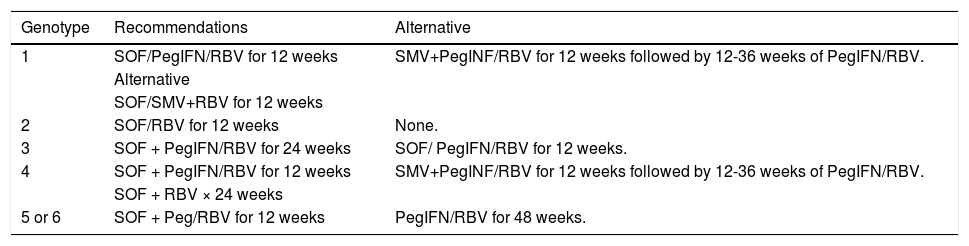
Other treatmentsDifferent medical associations and governments have approved the use of several drugs that are not available in Mexico.85,86,116–121 These are shown in table 11 only for information purposes. Moreover, other direct antiviral agents are undergoing phase III trials and will soon be submitted for evaluation by regulatory agencies-some are shown in table 12.122–126
Treatment regimens recommended in other countries.
| Genotype | Recommendations | Alternative |
|---|---|---|
| 1 | SOF/PegIFN/RBV for 12 weeks | SMV+PegINF/RBV for 12 weeks followed by 12-36 weeks of PegIFN/RBV. |
| Alternative | ||
| SOF/SMV+RBV for 12 weeks | ||
| 2 | SOF/RBV for 12 weeks | None. |
| 3 | SOF + PegIFN/RBV for 24 weeks | SOF/ PegIFN/RBV for 12 weeks. |
| 4 | SOF + PegIFN/RBV for 12 weeks | SMV+PegINF/RBV for 12 weeks followed by 12-36 weeks of PegIFN/RBV. |
| SOF + RBV × 24 weeks | ||
| 5 or 6 | SOF + Peg/RBV for 12 weeks | PegIFN/RBV for 48 weeks. |
SOF: sofosbuvir 400 mg/day. SMV: simeprevir 150 mg/day. PegIFN: pegylated interferon. RBV: ribavirin.
The rates of SVR with PegIFN/RBV in genotype 1 range between 40 and 50%, and are close to 80% in HCV genotypes 2 and 3; hence, the number of patients failing therapy is high67 but we have no clear statistics on this feature in our country.
There are different groups of patients with treatment failure. Their categorization is important because it establishes their possibility of access to new therapies and subsequent response:
- •
Non-responders are patients whose HCV-RNA levels did not decrease at least 2 logs IU/mL at week 12 of treatment.
- •
Partial responders, HCV-RNA decreased at least 2 logs at week 12, but was still detectable by week 24.
- •
Relapsers, HCV-RNA became undetectable during treatment but reappeared after treatment discontinuation.67
In order to approach this problem, we proposed the following clinical scenarios considering the type of response and the administered treatment.
- •
Treatment failure to standard interferon monotherapy or in association with ribavarin. Al- though treatment with IFN with/without RBV is not currently used, patients with this type of treatment failure can still be found.127
There are three classical studies suggesting that between 13-16% of non-responders to standard IFN, reach SVR with retreatment with PegIFN/ RBV double therapy regimen.128–130 The available evidence has shown that the use of PegIFN/RBV in patients with HCV chronic hepatitis is more effective in achieving a SVR than standard IFN and RBV (RR 0.81;95% CI 0.76, 0.86).131,132
RECOMMENDATIONS
- •
The combination of PegIFN/RBV is the recommended treatment of HCV chronic infection over standard interferon and RBV.68[A1]
- •
Treatment failure to PegIFN/RBV. Patients with treatment failure to PegIFN/RBV may be treated again with PegIFN/RBV if there had previously been poor compliance or the drugs’ dosing was inadequate. However if this option is chosen, one must keep in mind that response rates may be as low as 7-9%. Maintenance treatment with low PegIFN doses is not recommended 133-134
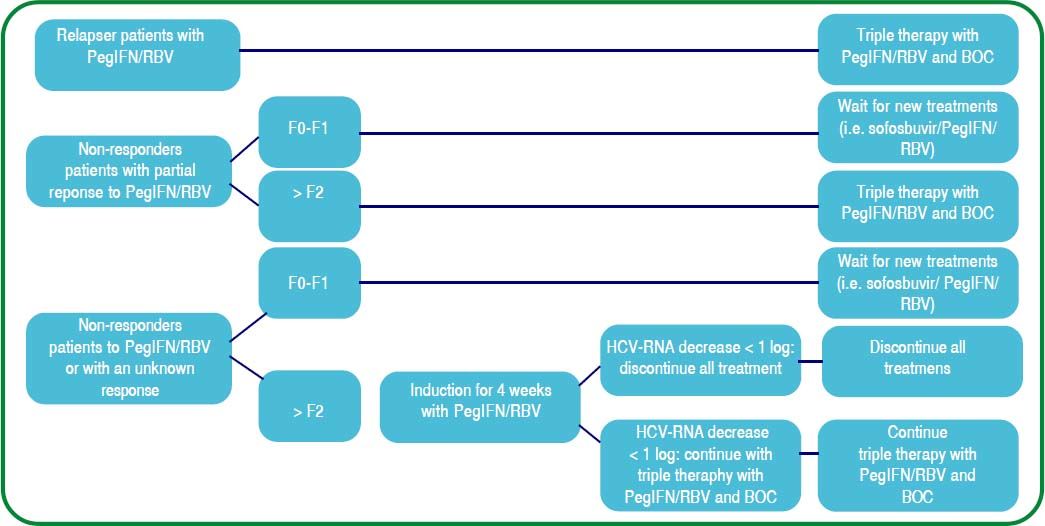
The available protease inhibitors (PI) in Mexico are boceprevir (BOC) (since August 2012) and simeprevir (SMV) (since June 2014) for the management of HCV chronic hepatitis genotype 1 and genotypes 1 and 4, respectively. Figure 5 shows the suggested treatment decision-making flowchart according to the virologic response and grade of hepatic fibrosis in case of treating with triple therapy PegIFN/RBV + BOC.
Treatment algorithm in previously treated patients when using triple therapy with PegIFN/RBV and boceprevir.135 PegIFN/RBV: Pegylated Interferon, ribavirin. BOC: boceprevir. F: fibrosis.
It is important to mention that in monoinfected patients, maximum effectiveness will be obtained by initiating triple therapy in those with chronic infection and advanced liver disease; patients with mild involvement should await future treatment modalities that will hopefully, be more effective and with less adverse effects. One must still keep in mind that patients with HCV chronic hepatitis have needs to be met and attempting to offer them the safety and efficiency of available health resources is paramount.135
RECOMMENDATIONS
- •
Retreatment with PegIFN/RBV is not recommended in patients that did not reach a SVR after a complete regimen, even if administering a different type of PegIFN.133,134 (In relapses [C2];in non-responders: [B2]).
- •
Maintenance treatment with low PegIFN doses is not recommended (A1).
- •
Patients with HCV genotype 1 chronic hepatitis and previous antiviral treatment failure, should be considered for retreatment with PegIFN/RBV and protease inhibitor triple therapy.135[A1]
- •
Patients with cirrhosis and previous treatment failure to PegIFN/RBV. In patients with advanced fibrosis or cirrhosis, a SVR not only implies infection cure but also a good long-term prognosis. In the absence of contraindications, antiviral therapy is recommended in patients with compensated liver cirrhosis to prevent mid or long-term complications.67 Initiating treatment in this group of patients is recommended if the following contraindications are not present: decompensated cirrhosis with 7 or more points in the Child-Pugh score and albumin ≤ 3.5 g/dL, platelet count of 75,000/mm3, severe mental health issues or autoimmune disease that might be exacerbated by the use of PegIFN. Precautions must be taken in individuals over the age of 65.71[B2]
Triple therapy in this group of patients -evaluated in the CUPIC study- with BOC, offers the possibility of achieving an overall SVR of 41%. However when stratifying patients, the SVR was 54% in relapsers, 38% in partial responders and nil in non-responders. Other factors influencing the response were: the lack of an induction phase, previous response to treatment, subtype 1b and a total platelet count below 100,000/mm3.136
The usefulness of SMV in this group of patients has been evaluated in several studies. Results of the PROMISE clinical trial that evaluated the response to triple therapy with PegIFN/RBV and SMV in patients with genotype 1 and previous relapse after PegIFN/RBV obtained SVR in 74%. The ASPIRE study documented SVR of 82% in patients with partial response (PR) and 31% in non-responders to previous therapy with PegIFN/RBV and liver cirrhosis when treated with a triple regimen of PegIFN/RBV + SMV.137 Patients with cirrhosis are not candidates to response-guided triple therapy and should be treated for 48 weeks. These patients have a greater risk of developing treatment-associated complications and drug interactions should be closely monitored during therapy. If feasible, we recommended that these patients be managed by a multidisciplinary team with significant experience in the evaluation of these patients.67,138 The recommended BOC regimen includes an induction phase of 4 weeks with PegIFN/RBV and 44 weeks of triple therapy with PegIFN/RBV + BOC.67[B2] If using simeprevir in patients with cirrhosis, PegIFN /RBV + SMV is recommended for the first 12 weeks followed by 36 additional weeks of PegIFN/RBV.136
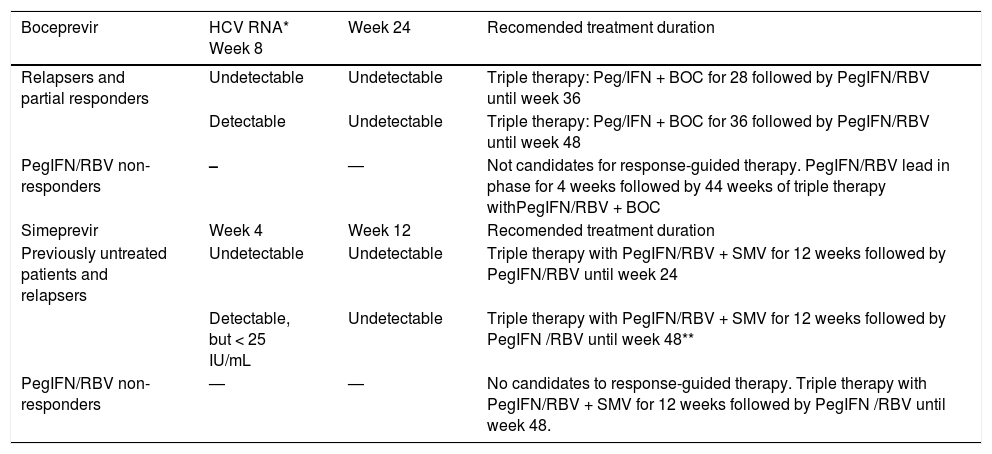
The management duration according to “on therapy” response to BOC or simeprevir are shown in table 13; indications for triple therapy discontinuation are mentioned in table 14.
Treatment duration with PegIFN alpha/RBV + boceprevir or simeprevir triple therapy in patients with previous treatment failure.138
| Boceprevir | HCV RNA* Week 8 | Week 24 | Recomended treatment duration |
|---|---|---|---|
| Relapsers and partial responders | Undetectable | Undetectable | Triple therapy: Peg/IFN + BOC for 28 followed by PegIFN/RBV until week 36 |
| Detectable | Undetectable | Triple therapy: Peg/IFN + BOC for 36 followed by PegIFN/RBV until week 48 | |
| PegIFN/RBV non-responders | – | — | Not candidates for response-guided therapy. PegIFN/RBV lead in phase for 4 weeks followed by 44 weeks of triple therapy withPegIFN/RBV + BOC |
| Simeprevir | Week 4 | Week 12 | Recomended treatment duration |
| Previously untreated patients and relapsers | Undetectable | Undetectable | Triple therapy with PegIFN/RBV + SMV for 12 weeks followed by PegIFN/RBV until week 24 |
| Detectable, but < 25 IU/mL | Undetectable | Triple therapy with PegIFN/RBV + SMV for 12 weeks followed by PegIFN /RBV until week 48** | |
| PegIFN/RBV non-responders | — | — | No candidates to response-guided therapy. Triple therapy with PegIFN/RBV + SMV for 12 weeks followed by PegIFN /RBV until week 48. |
Recommended PCR-based techniques are those with lower limit of Quantification (LLQ) of 25 IU/mL and lower limit of detection (LLD) of 15 UI/mL.
Patients with detectable but <25 UI/mL. HCV RNA on week 4 of treatment, should receive PegIFN/RBV for 48 weeks. A 24 week regimen may be considered in selected patients with no fibrosis or IL28B CC polymorphism. Dose: PegIFN alpha 2a 180 mcg. SC per week or PegIFN alpha 2 b 1.5 mcg. SC/week. RBV 1000 mg. (< 75 kg.) or 1,200 mg (> 75 kg.) PO/day divided in two doses, with meals; SMV 150 mg. PO/day, with meals; BOC 800 mg, PO q 8 h.
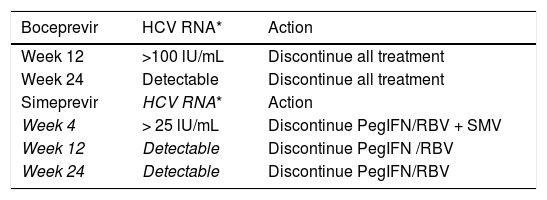
Stopping Rules for treatment due to lack of viral response to boceprevir and simeprevir.139
RECOMMENDATIONS
- •
Triple therapy with PegIFN/RBV + PI (BOC or SMV) is recommended in compensated patients with cirrhosis with treatment failure to PegIFN/RBV, if there are no contraindications; care must be taken in individuals above the age of 65. [B2]
- •
Patients with cirrhosis are not candidates for response-guided triple therapy and must be treated with regimens of 48 weeks. [B2]
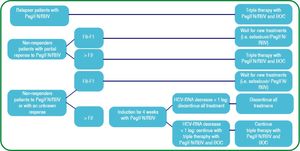
- •
Non-cirrhotic genotype 1 patients with previous treatment failure to PegIFN/RBV. In the RESPOND 2 study, BOC was proven effective in this group of patients with a SVR between 59 and 66% in those on triple therapy vs. 21% in the placebo arm. When analyzed by sub-groups, the rates of SVR were: 69-75% in relapsers and 40-52% in Partial responders. Non-responders were not included in that study.67
In the ASPIRE study, the efficacy of triple therapy with PegIFN/RBV + SMV was evaluated in patients who failed to dual therapy. Rates of SVR were: 77-89% in relapsing patients, 48-86% in partial responders and 38-59% in non-responders. These results correlate with those obtained in the PROMISE study in which patients reached a SVR of 79% (70% in genotype 1a and 86% in genotype 1b) vs. 37% in the placebo group; those with mild or no fibrosis had an 82% rate of SVR.137
Relapsers, non-cirrhotic patients and those with a partial response to previous treatment with PegIFN/RBV, are candidates to response-guided treatment with any of the available PIs. In the case of BOC, treatment for potentially 36 weeks is recommended in relapsing patients with PegIFN/RBV; non-responders should continue a fixed induction regimen with PegIFN/RBV for 4 weeks and triple therapy for 44 weeks since they are not candidates to response-guided therapy.67 In the case of SMV, relapsers must begin therapy with PegIFN/RBV + SMV and HCV RNA levels must be quantified on week 4 of treatment; if it is undetectable, the triple regimen must be continued until week 12 followed by 12 additional weeks with PegIFN /RBV; if HCV-RNA is detectable -but below 25 IU/mL- we recommend to continue with PegIFN /RBV + SMV until week 12 followed by 36 weeks of PegIFN/RBV. Nonresponders should be treated with PegIFN /RBV + SMV by 12 weeks followed by 36 additional weeks of PegIFN/RBV.137
Treatment regimens and evaluation of response to BOC and SMV in this group of patients are shown in table 13. Table 14 summarizes the indications for triple therapy discontinuation.
RECOMMENDATIONS
- •
In non-cirrhotic patients with HCV genotype 1 chronic hepatitis and previous treatment failure the first option of treatment is: triple therapy with PegIFN/RBV + and protease inhibitor (BOC or SMV). [A1]
- •
A triple regimen including BOC and SMV should be used according to the previous response, the viral kinetic “on therapy” and following the rules of therapy discontinuation. [A1]
- •
Genotype 2 and 3 cirrhotic and non-cirrhotic patients with treatment failure to PegIFN/RBV. Retreating these patients with PegIFN /RBV has to be evaluated individually in each case. If attempted, a SVR rate > 50% is expected in relapsers if treatment is administered by longer duration (48 weeks); but in PR and non-responders, SVR rates are very low and “to wait” new treatment options is recommended.139 BOC is not approved for use in patients with infection due to genotypes 2 and 3.67 In other countries, there are other available therapeutic options such as sofosbuvir (SOF)/RBV with or without PegIFN, among others (See table 15).
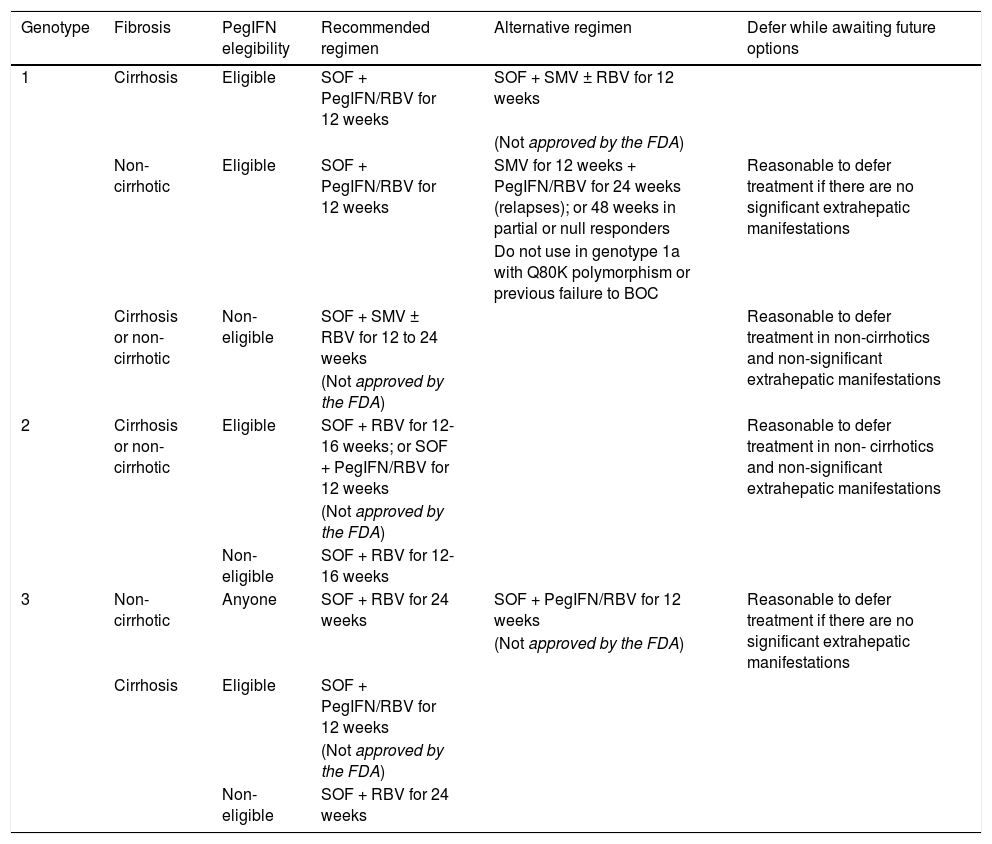
Table 15.Recommended regimens for patients with HCV chronic hepatitis and previous treatment failure in the United States – divided according to genotype.140
Genotype Fibrosis PegIFN elegibility Recommended regimen Alternative regimen Defer while awaiting future options 1 Cirrhosis Eligible SOF + PegIFN/RBV for 12 weeks SOF + SMV ± RBV for 12 weeks (Not approved by the FDA) Non-cirrhotic Eligible SOF + PegIFN/RBV for 12 weeks SMV for 12 weeks + PegIFN/RBV for 24 weeks (relapses); or 48 weeks in partial or null responders Reasonable to defer treatment if there are no significant extrahepatic manifestations Do not use in genotype 1a with Q80K polymorphism or previous failure to BOC Cirrhosis or non-cirrhotic Non- eligible SOF + SMV ± RBV for 12 to 24 weeks Reasonable to defer treatment in non-cirrhotics and non-significant extrahepatic manifestations (Not approved by the FDA) 2 Cirrhosis or non-cirrhotic Eligible SOF + RBV for 12-16 weeks; or SOF + PegIFN/RBV for 12 weeks Reasonable to defer treatment in non- cirrhotics and non-significant extrahepatic manifestations (Not approved by the FDA) Non- eligible SOF + RBV for 12-16 weeks 3 Non-cirrhotic Anyone SOF + RBV for 24 weeks SOF + PegIFN/RBV for 12 weeks Reasonable to defer treatment if there are no significant extrahepatic manifestations (Not approved by the FDA) Cirrhosis Eligible SOF + PegIFN/RBV for 12 weeks (Not approved by the FDA) Non- eligible SOF + RBV for 24 weeks Dose: PegIFN alpha 2a: 180 mcg. SC per week; or PegIFN alpha 2b: 1.5 mcg. SC per week. RBV: ribavirin 1,000 mg PO/day if < 75 kg, or 1,200 mg PO/ day if > 75 kg, divided in two doses, with meals. SMV: simeprevir 150 mg PO/day, with meals. SOF: sofosbuvir 400 mg PO/day. Note: Sofosbuvir or simeprevir should not be used as monotherapy or at low doses. Neither of these drugs should be reinitiated in case of discontinuation. IFN ineligible or intolerance criteria: platelet count <75,000/mm3, decompensated cirrhosis (Child-Pugh B or C), significant mental abnormalities that may be exacerbated with the use of interferon or poorly responsive to medical treatment, autoimmune disease potentially exacerbated by the use PegIFN, inability to comply with medical treatments or poor tolerance to IFN in a previously administered treatment regimen.
- •
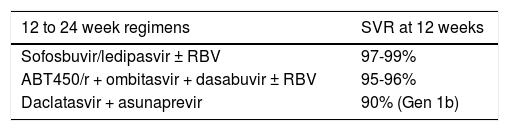
Patient with genotype 1 and triple therapy failure with PegIFN/RBV and protease inhibitor. There are new IFN-free regimens -submited for approval in other countries- that have been used in patients with cirrhosis and previous treatment failure, such as: ABT/450/r-ombitasvir and dasabuvir with RBV, leading to a significant increase in SVR of up to 95-100%.138 Another regimen undergoing evaluation combines ledipasvir/sofosbuvir in previously treated patients, yielding SVR between 94-99% if administered for 12-24 weeks, respectively.141
RECOMMENDATIONS
- •
In genotype 1 and treatment failure with triple therapy (including a PI), a change of PI is not re-commended.139[C1]
- •
This special group of patients should a wait for the availability of new effective regimens. (A1)
HCV is a well-recognized cause of hepatitis in pediatric patients worldwide. Unfortunately, the prevalence of hepatitis C in the Mexican pediatric population is unknown. A tertiary care hospital reported a frequency of infection due to this virus of 2%, in pediatric patients treated for hepatitis over a 5 year period.142
The most common form of HCV transmission in the pediatric population is vertical, via chronically infected mothers.26 There is currently no drug of proven efficacy that decreases the risk of vertical transmission.143 The evaluation of anti-HCV antibodies in children of seropositive mothers is recommended after 15-18 months of age.144
RECOMMENDATIONS
- •
Children of hepatitis C seropositive mothers should be evaluated for anti-HCV antibodies at 15-18 months of age in order to exclude the presence of remaining maternal antibodies. [A1]
Little is known on the characteristics of chronic infection in children. However, we know that the course of HCV infection tends to be asymptomatic and that 25 to 40% of cases of babies infected vertically resolve spontaneously; nevertheless, 2% of patients may rapidly progress to cirrhosis at a pediatric age. Chronic hepatitis is associated to various histological disease patterns and usually, its course is not as severe as in adults. The progression of liver injury due to HCV depends on several factors including the viral load, the level of aminotransferases, gender, ethnicity, obesity, environmental factors and other comorbidities such as anemia, immunosuppression or concomitant HBV or HIV infection. Genetic factors such as the presence of single-nucleotide polymorphisms at IL28B gene also play an important role.145–147
RECOMMENDATIONS
- •
Although HCV infection in children may resolve spontaneously, a small percentage may rapidly progress to cirrhosis at a pediatric age; hence, all HCV positive children should be periodically evaluated. [A1]
The diagnosis of HCV infection should be suspected in children with chronic liver disease, children of mothers infected with HCV and those with other risk factors such as hemodialysis, HIV infection or a previous transplant. Diagnosis, evaluation and follow-up is no different to that in the adult population.147–148
RECOMMENDATIONS
- •
After confirming chronic HCV infection in the pediatric population, the patient(s) should be evaluated in terms of viral load (HCV-RNA), HCV genotype, subtype and hepatic fibrosis. [A1]
- •
For treatment and follow-up purposes, evaluation of the disease is performed as in adults. [A1]
- •
The evaluation of hepatic fibrosis in the pediatric population may be performed with invasive methods - liver biopsy-or other validated non-invasive methods - ej. Elastography. [B2]
Antiviral treatment of HCV infection in pediatric patients remains controversial and should be individualized. Deciding to initiate antiviral therapy in children with chronic HCV infection is still a challenge. There is a possibility of spontaneous viral clearance before the age of 3 (10-20%), particularly in the case of genotype 3. Regardless, treatment should be individualized and based on the patient’s characteristics (such as age), viral characteristics (such as genotype), the stage of hepatic fibrosis and the future development of powerful antiviral agents with improved antiviral activity, thus decreasing the need for IFN.144,147,151,152
Most of the information on the subject in pediatric patients has become available through a few studies conducted in children; but predictors of an unfavorable outcome, treatment and evaluation guidelines as well as treatment response rates are similar to those in adults. In this group in particular, IFN has deleterious effects on the child’s growth especially in periods of maximum growth. However, children tolerate antiviral treatment well.
RECOMMENDATIONS
- •
Antiviral therapy is not recommended before the age of 3 due to the possibility of spontaneous viral clearance. [B1]
- •
In the case of genotype G3 infection, treatment should be further delayed until age 5 due to the possibility of spontaneous viral clearance. [B2]
- •
Standard therapy of pediatric patients with HCV between the ages of 3 and 18 is the combination of PegIFN/RBV. [A1]
The use of protease inhibitors is not currently recommended in the treatment of HCV infection in pediatric patients. [C2]
- •
Weight and height should be closely monitored if treatment is initiated. [A1]
- •
The dosage of RBV is 15 mg/kg/day divided in two doses. [A1]
- •
The dosage of interferon alpha 2b is 1.0 or 1.5 mcg/kg/dose and that of interferon alpha 2a is 10 mcg/m2/dose. [A1]
The most frequent indications for an orthotopic liver transplant (OLT) are chronic HCV infection, cirrhosis complications or the presence of a hepatocellular carcinoma (HCC). Unfortunately, in patients undergoing an OLT and with detectable HCV RNA at transplantation, recurrence is universal and immediate. The viral load is detectable a few hours after transplantation and significantly increases 10 to 20fold in comparison with the pre-transplant viral load. This infection commonly progresses to acute hepatitis, chronic hepatitis, cirrhosis and graft failure. Thirty percent (30%) of transplanted patients develop cirrhosis within 5 years,153 as well as associated clinical decompensation within the first year after diagnosis; thus, complete clearance of the virus is decisive, since a SVR could prevent recurrent graft infection.154 We have 2 antiviral therapeutic strategies: the first is to initiate treatment while on the waiting list and the second, to initiate treatment after OLT.
Treatment with PegIFN/RBV in patients on the waiting list is indicated in cases of compensated cirrhosis (Child-Pugh A and B) and it is effective in 20%.155 Treatment after liver transplant is effective in 30% of patients that have received antiviral therapy.156
Antiviral treatment in patients on the waiting listAntiviral therapy and a SVR while on the waiting list, prevent graft infection and its secondary complications. However, its applicability is limited since a great number of patients develop decompensated cirrhosis, a contraindication to therapy. Tolerance to treatment is also low and leads to the need to decrease or discontinue dosage and a subsequent lower viral response.
Treatment efficacy is also low since most patients have cirrhosis, they carry genotype 1 and some are elderly-all factors leading to a low probability of response.
In the setting of post-liver transplantion, before begining treatment before beginning treatment, a liver biopsy should be obtained in order to differentiate histological injury in case of graft rejection and to determine the severity of the viral relapse. The biopsy may be obtained via a trans-jugular approach with gradient measurements, if available.
Patients with a Child-Pugh A score and those with HCC should be treated regardless of the genotype. Patients with genotype 2 or 3 and a low viral load have a high probability of response and should also be treated. In patients with liver dysfunction (Child-Pugh B), treatment is recommended in those with a favorable virologic profile. These patients require close monitoring during therapy due to the development of secondary effects that may entail dosage modification. Surveillance of decompensation episodes and bacterial infections is pivotal during treatment. Studies have determined that shorter treatment courses lead to SVR after transplantation -they have shown a SVR in 50% of cases by week 12 if treatment is begun at least 16 weeks before transplantation.157 Living donor recipients are also treatment candidates since it is easier to calculate the duration of therapy– and since it is a programmed surgery, the date can always be modified.
Triple therapy combined with a PI –such as BOC– increases the efficacy of PegIFN/RBV double therapy, with SVR of 75% in previously untreated patients, 75-85% in patients in relapse, 50-60% in PR and only 15% in NR – those that are frequently on the OLT waiting lists.158,159 A study of triple therapy reported that HCV RNA was undetectable in 80.5% of cases after 8 weeks of treatment; by week 16, 74.8% of patients in relapse responded as did 66.2% of PR and 45.8% of NR.71 Triple therapy is indicated in patients with compensated cirrhosis and genotype 1. The induction period in patients on triple therapy identifies those that will achieve a SVR if it decreases ≥ 1 log in partial responders and relapsers with BOC 87% vs. ≤ 1 log with BOC 34%.160 Triple therapy in cirrhosis carries the risk of patients developing cytopenias, bacterial infections, liver function deterioration and death. Factors associated to severe complications such as death, severe infection and hepatic decompensation after 16 weeks of treatment were platelets ≤ 100,000 and albumin ≤ 3.5 g/dL.
We currently do not have new direct antivirals or IFN-free treatments in Mexico and it is precisely in these special groups that they are best indicated. The first study that reported the safety and efficacy of IFN-free therapy (SOF + RBV) was a phase II study in which 61 patients were treated while on the waiting list for a median duration of 17 weeks: 40 patients were transplanted and 37 (92%) had a HCV RNA < 25 UI/mL before transplantation. Twenty-six transplanted patients completed 12 weeks of followup and 18 (69%) showed a SVR by week 12. The efficacy, safety and tolerance of the treatment were excellent. Adverse events were mild and only one patient discontinued treatment due to anemia attributed to the RBV.161
RECOMMENDATIONS
- •
Antiviral therapy is recommended for patients on liver transplant waiting lists since it may prevent graft infection. [A1]
- •
Treatment is recommended in patients with preserved liver function (Child-Pugh A) or Child-Pugh B with a favorable virological profile - genotypes 2 and 3, or genotype 1 and a low viral load. [A1]
- •
Antiviral therapy is not recommended in Child-Pugh C patients or in those with a MELD score above18 points. [A1]
- •
Treatment is recommended in patients with preserved liver function and HCC or that will be living donor liver recipients. [A1]
- •
In Mexico, we currently have PegIFN/RBV double therapy for patients with genotype 1. At least 16 weeks of treatment are recommended. [A1]
- •
Another strategy is PegIFN/RBV + BOC triple therapy for genotype 1 with an increased SVR. [A1]
- •
Ribavirin will be adjusted according to the patient’s weight, renal function and anemia. [A1]
Our available therapy is the PegIFN/RBV combination, which eradicates HCV in 30% of cases. It decreases progression, decompensation and mortality while increasing survival.156 This treatment should be offered in the acute hepatitis phase in case of severe acute hepatitis (histologically and biochemically documented) and in cholestatic or fibrosing cholestatic hepatitis; it is also recommended in case of significant fibrosis (score F ≥ 2 on METAVIR scale) or portal hypertension (HVPG) ≥ 6 mmHg. These are the best predictors of rapid HCV progression in the post-transplant period and are usually detected one year after transplantation. Non-invasive methods such as transition elastography (fibroscan) are useful in follow-up and a significant correlation has been found between 8.7 kilopascals (kPa) and an F2 score in the METAVIR scale and a HVPG ≥ 6 mmHg;162 Therefore, treatment is recommended in these patients since they are at high risk of decompensation and graft loss. Survival is improved in those achieving a SVR than in untreated patients or non-responders. Secondary effects such as anemia and leukopenia are frequent, so erythropoietin and granulocyte-stimulating growth factor are recommended - increasing treatment tolerance and efficacy. Another problem is the induction of graft rejection with treatment, even chronic rejection. The rate of rejection varies between 0 and 35%.163
During post-OLT treatment, triple therapy has been recently shown to improve SVR when compared with double therapy (Verna EC; unpublished data). A multicenter study conducted by the CRUSH-C group164 in patients with genotype 1, revealed that in the post-transplant period, 43% of patients developed a transitional phase between fibrosis and cirrhosis and 10% developed cholestatic HCV. Immunosuppression regimens included cyclosporine in 66% of cases, tacrolimus in 23%, steroids in 27% and mycophenolate mofetil in 72%. Median treatment duration was 136 days. The SVR in this group was 41.2%, of which 70% had an eRVR. With triple therapy, 49% required transfusions and 32% had creatinine elevations ≥ 0.5 mg/dl. Growth factors were required in 86% of patients and the PegIFN and RVB doses were decreased in 27% and 78% of cases, respectively. Hospitalizations resulting from adverse events were recorded in 21% of cases, 2 patients rejected the graft and there were 2 deaths.
A European study evaluating the efficacy and safety of triple therapy165 -PegIFN/RBV and BOC or telaprevir- in patients with genotype 1 and HCV relapse after liver transplant defined as F ≥ 1 in the METAVIR scale, concluded that among patients treated with BOC, 83% achieved a complete early virologic response by week 12 compared to 61% of those treated with telaprevir. In the group treated with BOC, 82% responded by the end of treatment on week 48 vs. 38% of those treated with telaprevir. In both groups, some patients abandoned treatment, there were relapses and 2 deaths per group. In Mexico, telaprevir is unavailable.
When using these treatment strategies, colony-stimulating factors may be used depending on the developing adverse effects (anemia, neutropenia, thrombocytopenia); the use of mycophenolate mofetil and sirolimus should be reconsidered due to their myelosuppressive effects. Immunosuppressor dosages should be modified since drug interactions may lead to complications due to increased levels and toxicity.
IFN-free therapies are ideal in this patient population due to their safety profile, tolerance and efficacy; they are still unavailable in Mexico. Preliminary results obtained in 45 patients treated with SOF and RBV for compassionate reasons after severe relapse -including cholestatic fibrosing hepatitis- reported a 50% SVR by week 12.166
RECOMMENDATIONS
- •
Before initiating treatment, a liver biopsy must be obtained to differentiate histological injury due to the virus from that due to rejection as well as to determine the severity of viral relapse. [A1]
- •
Initiating treatment must be considered in patients with evidence of severe HCV infection recurrence (acute hepatitis, cholestatic fibrosing hepatitis or evidence of severe necrotic-inflammatory activity). [A1]
- •
In phases of chronic hepatitis, the presence of significant fibrosis -grade F2 in METAVIR scale- suggests severe recurrence and mandates treatment initiation. [A1]
- •
Follow-up of fibrosis progression in transplanted patients due to HCV can be achieved with liver biopsy or non-invasive methods – such as transition elastography (fibroscan). Upon detection of 8.7 kPa and/ or F ≥ 2 on the METAVIR scale, treatment should be initiated. [B1]
- •
Post-transplant antiviral therapy is based on IFN/RBV. However, adding another direct action antiviral drug such as boceprevir may improve the probabilities of viral eradication. [A1]
- •
Due to the pharmacokinetic interactions complicating the treatment paradigms, boceprevir must be used cautiously in transplanted patients with hepatitis C; close clinical and biochemical surveillance of the immunosuppressive treatment and its interaction with the other drugs should be emphasized. [A1]
- •
Based on the results obtained to date with direct antiviral and IFN free regimens –less adverse effects, no drug interactions, shorter treatment duration and greater SVR– they are recommended in this subgroup of patients as soon as they are available. [A1]
Hepatitis C in HIV-infected patients
As in HCV mono-infected patients, the same detection, evaluation and follow-up recommendations should be applied to this group of patients. Screening for hepatitis A and B viruses is also recommended as is applying the respective vaccines to prevent infection, if negative.
Although the same evaluation schema is advised, obtaining a liver biopsy is controversial and its need questionable before initiating therapy, since over 8590% of HIV/HCV co-infected patients already harbor a certain degree of fibrosis. A cost-benefit analysis concluded that treatment efficacy is the most profitable strategy, so the suggestion is to treat all candidates without considering the liver biopsy results.
In case of identifying patients with no fibrosis (F0) or in its initial phases (F1), treatment initiation may be delayed. Non-invasive methods predicting fibrosis have also been included in the evaluation of this group, but there are doubts on the management of intermediate fibrosis.63,64
Treatment determination follows the same guidelines as in mono-infected patients: considering comorbidities, genotype, depression, pregnancy and drug interactions.
RECOMMENDATIONS
- •
Due to its impact, routine evaluation should include anti-HCV antibodies. [A1]
- •
If positive, determine blood HCV-RNA to confirm or exclude active infection. [A1]
- •
Disease evaluation is similar to that conducted in HCV mono-infected patients. [A1]
- •
Treatment is not recommended in patients: [A1]
- •
With F0-F1 fibrosis.
- •
With severe immune suppression and advanced disease.
- •
With low response probabilities, similar to mono-infected patients.
- •
With decompensated cirrhosis.
- •
In general, treatment of patients with a CD4+ lymphocyte count below 200 cells/uL should be avoided. In case the CD4+ count is below 350 cells/uL, antiviral treatment should be initiated; if HIV-RNA undetectable, then the hepatitis C virus may be treated.
In infections with genotypes 2 and 3, standard therapy is based on:
- •
PegIFN alpha 2a or 2b-180 µg per week or 1.5 µg/kg/week, respectively and RBV 800 mg/day for 24 weeks.
In infections due to genotype 1, calculate the RBV dose according to patient weight and consider the same indications and contraindications as in mono-infected patients; treatment duration will depend on viral kinetics:68,167
- •
If < 75 kg: 1,000 mg/day.
- •
If > 75 kg: 1,200 mg/day.
In cases of decompensated cirrhosis, IFN-based regimens are contraindicated due to the risk of decompensation. Therefore, patients with greater degrees of fibrosis or cirrhosis are treatment priorities.64,167 As in mono-infected patients, the same recommendation guidelines should be followed as in those with genotype 1 in this special group of patients.
RECOMMENDATIONS
- •
In general, treatment of patients with a CD4+ lymphocyte count below 200 cells/μL should be avoided. [A1]
- •
In case the patient has a CD4+ count below 350 cells/μL, anti-retroviral therapy should be initiated; if HIV-RNA is undetectable, the hepatitis C virus can be treated. [A1]
- •
The combination of PegIFN/RBV is the most available therapy and yields similar virological response rates as in mono-infected patients. [A1]
- •
In previously untreated patients with compensated cirrhosis (Child Pugh A), the same treatment as for patients without cirrhosis is recommended. [A1]
The worldwide prevalence of HIV/HCV co-infection ranges between 10% and 50%, and is particularly high among intravenous drug users (IDU). It is estimated that 60 - 90% of patients that contracted HIV by IDU also carry HCV, as do those patients treated with contaminated blood products. After the introduction of highly active anti-retroviral therapy (HAART) in 1996, the prognosis of HIV-infected patients improved considerably, with the associated decrease in morbidity and mortality. Since then, chronic hepatitis C became the main cause of death in this group of patients; this was proven in the DAD study (Data collection on Adverse events of Anti-HIV Drugs) that concluded that hepatic abnormalities are the main non-HIV related cause of death.168,169
HIV infection modifies the natural history of hepatitis C by different mechanisms:
- 1.
It increases HCV viremia and hence, transmission.
- 2.
It accelerates the progression of fibrosis to cirrhosis, advanced liver disease and hepatocellular carcinoma.170
The course of chronic hepatitis in patients coinfected with HIV is more severe. HCV clearance in acute hepatitis (acute HCV) is only 5% vs. 15-35% in mono-infected patients and even lower in patients with low CD4+ lymphocyte counts; this leads to greater HCV chronicity.171
Factors accelerating the progression of fibrosis in patients without HAART in chronic infection are: CD4+ lymphocytes < 200 cells/mm3, alcohol ingestion > 50 g/dL and age of HCV infection > 25 years. Patients on HAART and with a higher CD4 + lymphocyte count, a longer duration of undetectable HIV-RNA and less progression to fibrosis, have lower possibilities of decompensation and death.168
If viral replication is well controlled and patients are on antiretroviral therapy, HIV/HCV co-infected individuals have better immunity, an improved overall survival, less liver disease progression and a lower risk of complications and death due to liver disease. HIV has the ability to infect liver stellate cells and promote their activation by increasing collagen synthesis. This suggests that HIV replication contributes directly to increased hepatic fibrosis in co-infected patients, so early control of HIV replication is necessary as well as the maintenance of an adequate immune status.172
RECOMMENDATIONS
- •
In the absence of contraindications, all coinfected patients should be considered candidates for hepatitis C treatment, since co-infection affects various systems and disease progression may be faster. [A1]
- •
In patients with no previous anti-retroviral therapy and good immunity, it is convenient to initiate anti-HCV treatment if the CD4+ lymphocyte count is above 350 cells/μΣ. [A2]
- •
In patients co-infected with HCV, HAART treatment should be hastened if the CD4+ count is above 350 cells/μL, but the decision must be individualized in terms of virologic, histological and patient motivation variables. [C1]
To initiate treatment, we suggest considering the presence of hepatotoxicity -particularly in patients on HAART and with advanced liver disease- since toxicity increases further in the presence of advanced fibrosis (METAVIR grades F3-F4).
In HIV/HCV co-infected patients, treatment of hepatitis C notably decreases the risk of liver toxicity.64
RECOMMENDATIONS
- •
The stage of fibrosis should be evaluated if possible, in patients co-infected with HCV since it may influence the choice of antiretroviral therapy. [C1]
- •
No anti-retroviral is contraindicated in cases of HCV or HBV co-infection if liver function is preserved [B1]; but choosing the anti-retroviral(s) with the least liver toxicity should be a priority. [C1]
- •
A change in anti-retrovirals should be considered in cases of symptomatic or asymptomatic hepatitis if mitochondrial toxicity, hypersensitivity reactions or hypertransaminasemia are suspected. [C1]
Chronic liver disease may alter the metabolism and bioavailability of ARV, thus increasing their toxicity and altering viral activity; this is very frequent in patients with chronic hepatitis without hepatocellular failure. The accumulated experience does not preclude the use of ARV, but they are contraindicated in hepatocellular failure due to decreased P450-mediated metabolism and glucuronide conjugation.
RECOMMENDATIONS
- •
ARV can be used at the usual dose in cases of HCV without hepatocellular failure or mild hepatocellular failure (Child Pugh A), but with strict surveillance of hepatotoxicity. [B1]
- •
Adjust ARV in case of chronic liver disease and signs of hepatocellular failure, measuring plasma drug levels or according to recommendations. [C1]
Treatment of HCV provides the opportunity of eradicating the virus in a defined time period; therefore, every patient should be considered for treatment when benefits outweigh the risks and no contraindications are present. Anti-HCV therapy must be provided as early as possible in patients co-infected with HIV/HCV. Treatment of HIV/HCV co-infection is based on the combination of PegIFN and RBV; their simultaneous use with ARV affects the safety and efficacy of both drugs. In HIV/HCV co-infected patients with CD4 + cells > 500 cells/ mm3 and low HIV RNA levels (> 50,000 copies/mL), anti-HCV therapy must be initiated first.173
RECOMMENDATIONS
- •
If possible, treat chronic hepatitis before initiating ARV therapy. [C1]
- •
ARV therapy should not be initiated simultaneously with anti-HCV treatment. [C1]
- •
When simultaneously treating HIV and HCV, follow-up should be stringent in order to detect adverse reactions. [B1]
- •
Do not associate RBV with didanosine. [B1]
- •
Avoid the association of RBV and zidovudine (AZT). [B1]
- •
It is not necessary to modify HIV monitoring during simultaneous treatment of HCV infection. [C1]
Treatment of hepatitis C is the same as in mono-infected patients: combination therapy with PegIFN/ RBV therapy. In the published literature, the dose of RBV is not clearly defined for patients with genotype 1. However a RBV dose of 1 to 1.2 mg/day is not clearly superior to fixed 800 mg/day doses as in genotypes 2 and 3. High RBV doses are associated with hemoglobin decreases.68,174
Viral kinetics should be monitored during treatment and it should be adjusted according to the virologic response by weeks 4 and 12. In patients with genotypes 2 or 3, a negative HCV-RNA by week 24 suggests sufficient treatment; in patients with undetectable HCV-RNA by week 12, treatment should be continued until week 48. Generally, the SVR is lower in co-infected than in mono-infected patients.68,173,175
Treatment with direct-acting antivirals (DAA) and protease inhibitors (PI) -such as BOC- was approved in 2011 in the United States and Europe in patients with chronic HCV genotype 1, in combination with PegIFN and RBV. SVR varies from 69 to 75%, but secondary effects increase in mono-infected patients.67,168,174 Other molecules with powerful antiviral activity have improved safety profiles and dosing. Many DAA specifically target a HCV enzyme, such as the NS5A polymerase inhibitor, but few studies have been conducted in this group of patients.
- •
DAA evaluation in HIV. The use of DAA in patients co-infected with HIV/HCV is complicated. Some prognostic factors have been implemented in HIV/HCV co-infected individuals:
Patients co-infected with HIV/HCV genotype 1 –either previously untreated or treated for HCV– must be considered for treatment with PegIFN alpha + RBV + telaprevir or BOC. In terms of the PI, different studies have shown should be selective of certain ARV leading to a better SVR, such as efavirenz, raltegravir and some ritonavir-boosted PIs.68,175
RECOMMENDATIONS64
- •
Treatment indications in co-infected HIV/ HCV patients are the same as in mono-infected patients. [A1]
- •
The treatment regimen is the same in mono-infected patients as in HIV/HCV co-infected patients. [A1]
- •
Boceprevir in HIV/HCV co-infected patients.67,168–175 In the phase II study of PegIFN /RBV + BOC in previously untreated genotype 1 patients, the SVR was from 24 to 31%.
There are limitations to triple therapy such as:
- •
Inconvenient PI dosing since it is administered every 8 h.
- •
A large number of pills (2 every 8 h for telaprevir or 3 every 8 h if associated to efavirenz; 4 every 8 h if using BOC).
- •
Must be administered with meals.
- •
The number of ARV tablets.
This polypharmacy may be associated to poor compliance, leading to resistance to the selected drug and thus increasing PI treatment failure rates. Overlapping toxicity of the HCV and HIV drugs may negatively compromise the efficacy of the PI used for HCV in this population.
There is no cross-reactivity or cross-resistance between HIV and HCV drugsBoth viruses share some biological similarities, hence increasing the possibility that anti-HIV drugs may induce HCV polymerase or protease changes or vice versa. In studies following theNS5B gene before and during the use of ARV, there was no evidence of selective resistance to the drug due to HCV polymerase mutation. Hence, the HCV polymerase is an RNA polymerase depending on RNA distant to the HIV reverse transcriptase, which is an inactive RNA-dependent DNA polymerase.67,175
Similarly, the HCV protease is a serine protease while the HIV protease is a structurally different aspartate protease. Thus, exposure to antivirals does not lead to changes fostering resistance.168
Drug interactions between DAA and ARV in HIV therapyMany patients with HIV and HCV are on ARV. But when patients with HIV age, they are administered other drugs for associated comorbidities such as hypertension, dyslipidemia, diabetes mellitus, mood disorders and others; the potential drug interaction between DAA and other medications must be analyzed before initiating HCV treatment.68
- 1.
Intolerance between PegIFN/RBV and ARV. These interactions are limited but major, particularly with the use of RBV and zidovudine, didanosine and stavudine; they are therefore contraindicated due to the development of toxic secondary effects such as anemia and mitochondrial toxicity. Hyperbilirrubinemia may be more pronounced in patients on RBV + atazanavir 68,168
The role of abacavir is controversial since recent publications do not preclude its use with RBV.63,68
- 2.
Interaction between HCV, protease inhibitor and ARV. There is scant data available, but there are some recommendations.
BOC is metabolized by the enzyme aldo-keto reductase, affecting CYP3A4 less. It does not contribute to substitution of BOC metabolism and/or elimination.67,68,168
There is no significant increase in BOC exposure if combined with low-dose ritonavir and it need not be adjusted with tenofovir.67 The BOC + efavirenz combination should be avoided. No interactions have been detected with raltegravir.167 Recent studies have reported no abnormalities with etravirine, rilpivirine and maraviroc, and whether there is any effect when associated to oral contraceptives is unknown (Table 16).68
Table 16.Drug-drug interaction between HIV and HCV therapies
NRTIS VIH Boceprevir PegIFN alpha Ribavirin Abacavir ○ ○ ○ Didanosine ○ ○ • Emtricitabine ○ ○ ○ Stavudine ○ ○ ○ Zidovudine ○ • • HIV protease inhibitors ○ ⊕ ○ Atazanavir ○ ⊕ ⊕ Darunavir ○ ⊕ ⊕ Fosamprenavir ○ ⊕ ⊕ Indinavir ○ ⊕ ⊕ Lopinavir ○ ⊕ ⊕ Nelfinavir ○ ⊕ ⊕ Ritonavir ○ ⊕ ⊕ Saquinavir ○ ⊕ ⊕ Tipranavir ○ ⊕ ⊕ NNRTIS VIH Boceprevir PegIFN alpha Ribavirin Delavirdine ○ ⊕ ⊕ Efavirenz ○ ⊕ ⊕ Etravirine ○ ⊕ ¤ Nevirapine ○ ⊕ ⊕ Rilpivirine ¤ ⊕ ¤ Entry integrase inhibitors Elvitegravir/ Cobicistat ○ ¤ ¤ Raltegravir ¤ ⊕ ¤ Maraviroc ○ ¤ ¤ - 3.
Interaction between polymerase inhibitors, HCV and ARV. There are not many studies on these pharmacodynamic interactions. May be more probable as a result of competitive inhibition of the drug that completes the phosphorylation pathway – seen with lamivudine and emtricitabine.68,167
- •
Drug resistance develops faster than in HIV leading to a low resistance barrier to new HCV drugs (nucleoside or nucleotide analogues inhibiting the HCV polymerase site). Consequently, there is no place for monotherapy and early viral kinetics can predict the usefulness of HCV treatment.
- •
As in HIV, there is broad cross-resistance between HCV drugs belonging to the same family except for non-nucleoside polymerase inhibitors. HCV variability increases resistance patterns in terms of specific measurements and viral sub-type, particularly after exposure to BOC and telaprevir. The most frequent mutations are in codons 36 and 155 in HCV genotype 1a, and in codons 54, 156 and 170 in HCV genotype 1b.
RECOMMENDATIONS
- •
The same PegIFN/RBV regimen can be used in HIV/HCV patients and in those without HIV, although longer treatment may be considered in patients with genotype 2 or 3 and a slow virologic kinetic response. [B2]
- •
Patients with HIV co-infected with HCV genotype 1 should be considered candidates for triple therapy, including BOC, but with special care to minimize or prevent drug-drug interactions. [B1]
INTERVENTIONS
- 1.
Needles, syringes and other instruments for drug use.
- 2.
Opiate replacement therapy and other treatments of drug dependence.
- 3.
HIV tests and counseling.
- 4.
Antiretroviral therapy.
- 5.
Prevention and treatment of sexually transmitted diseases.
- 6.
Condom use programs for the drug-using population and their sexual partners.
- 7.
Specific information, education and communication means for the drug-using population and their sexual partners.
- 8.
Vaccines, diagnosis and treatment of viral hepatitis.
- 9.
Prevention, diagnosis and treatment of tuberculosis.
Recently, the American Association for the Study of Liver Diseases in collaboration with the American Society of Infectious Diseases published their recommendations for the treatment of hepatitis C in different populations, such as HIV/HCV co-infected patients and according to their genotype.
They emphasize that treatment regimens by genotype may or may not include IFN and RBV, and treatment duration may be shorter. Drug eligibility and treatment duration depend, aside from the virologic kinetics genotype and treatment response to previous therapy, on the type of previously administered therapy and the lack thereof. In genotype 1a, they recommend perform the Q80K resistance testing and if using alternative therapies, consider whether the mutation is present.
Another point to emphasize is the permitted ARV therapies. In the case of direct antivirals –such as SOF– all are allowed except didanosine, zidovudine or tipranavir; in the case of SMV, the choice is limited to raltegravir, rilpivirine, maraviroc, enfuvirtide, tenofovir, emtricitabine, lamivudine and abacavir.
The use of BOC or telaprevir is no longer recommended in these treatment regimens. However, we must consider that currently, other recommended second generation direct activity antivirals are unavailable in Mexico. It is definitively expected that, in a short term, these antivirals will become available.177
Acute hepatitis CThere are two groups of patients with a high prevalence of acute HCV: intravenous drug users (IDU) –up to 48%– and men who have sex with men (MSM). The diagnosis may be difficult because most patients are asymptomatic. Anti-HCV antibodies must be determined to identify cases of acute hepatitis and its presence must be suspected within the first 6 months after exposure.63,64
Most patients with acute hepatitis C are asymptomatic (60-75%). Some factors involving spontaneous viral clearance have been suggested such as: female gender, young age, IL28B, etc.63,64 The earliest marker of acute infection is viral RNA, that can be detected as of week 1 or 2. Antibodies may take weeks and up to 9 months to become apparent. Symptoms may appear between weeks 2 and 8 and are indistinguishable from those of acute hepatitis due to virus A or B.63
Knowledge on HCV acute hepatitis has been limited for 2 reasons:64
- 1.
Most cases of acute HCV are asymptomatic.
- 2.
Its identification and follow-up in groups at highrisk of infection -such as IDU- have been difficult.
Traditionally, the acute phase of HCV infection is defined as the 6 months following viral contact. The exact principle defining a case is the detection of HCV-RNA in an anti-HCV negative individual that subsequently seroconverts to the antibody, as well as increased serum alanine aminotransferase (ALT) levels and clinical signs of hepatitis such as jaundice – the second diagnostic criterion, present in only 15-30% of cases.63
The frequency of HCV clearance in mono-infected patients is 25%. Some cohort studies mention HCV predictive factors such as: young females (up to 50%), non-White individuals and symptomatic disease. Those with the CC IL28B genotype are 3 times more likely to clear the virus than those with the CT or TT genotype; the IL28B status is less important when there is associated jaundice – as is the case in HIV infection.63,68
Patients with acute HCV should be considered for antiviral treatment to prevent progression to chronic hepatitis C. A SVR has been reported in over 90% of cases treated with PegIFN alpha monotherapy.68
RECOMMENDATIONS
- •
The diagnosis of acute HCV must be established by detectable HCV-RNA. [A1]
Although the ideal moment to begin therapy has yet to be clearly established, some investigators estimate that the rise in ALT –with or without clinical symptoms– may be the ideal point to begin treatment. They have also suggested that patients should be periodically monitored with HCV RNA quantifications and only those still HCV-RNA detectable after 12 weeks of treatment should be treated. There is currently no indication for the administration of prophylactic IFN alpha after exposure.178
Further, the consensus on acute HCV of the European AIDS Treatment Network or NEAT, recommends that HIV positive patients with HCV without at least a 2 log decrease by week 4 or that remain HCV-RNA detectable by week 12, should be treat- ed.172
In acute HCV mono-infected patients, monotherapy with PegIFN for 24 weeks yielded a 90% SVR if they were fully compliant. Combined PegIFN/RBV should be offered to patients if there is doubt on whether it is acute HCV vs. chronic HCV; moreover, in patients on monotherapy that are not undetectable by week 4, RBV should be added.172
In co-infected patients, combined therapy with PegIFN/RBV has only yielded SVR in 60-80% of cases. In 2001, the acute HCV consensus guidelines in HIV patients made the same recommendations: only adjust the RBV dosage according to the patient’s weight. Treatment duration depends on the viral kinetic response. In patients with a RVR, treatment duration is 24 weeks while in those with a delayed viral response it should be extended for 48 weeks.172
RECOMMENDATIONS
- •
Treatment with PegIFN at the usual dose should be initiated in cases that have not resolved spontaneously 12 weeks after diagnosis. [A1]
- •
PegIFN monotherapy may be used (2a: 180 μg./week; or 2b: 1.5 μg/kg/week) for 24weeks in patients with acute HCV; SVR will be achieved in 90% of cases. [A1]
- •
PegIFN (2a: 180 μg/week; or 2b: 1.5 μg/kg/ week) should be combined with RBV based on the patient’s daily weight (1,000 or 1,200 mg. in patients < 75 kg or >75 kg, respectively) for 24 weeks in patients with acute HCVco-infected with HIV. [B1]
Finally, although there is no available data on IFN-free regimens, it could theoretically be used in this group of patients and high SVR rates might be expected. Dosage and treatment duration would be similar to those used in chronic HCV until new data suggest that intensive therapy should be shorter and/or sufficient to achieve high cure rates.
HBV/HCV co-infectionPatients with HBV/HCV co-infection usually have low or undetectable viral DNA levels, although these may fluctuate; HCV is the main virus leading to chronic, active inflammation. The status of viral replication of both viruses must be known and the Delta virus should also be determined if HBV is positive.63
In case the HCV is replicating and there is evidence of liver disease, the patient should be treated with PegIFN/RBV, following the same rules as in mono-infected patients. The SVR in this group is comparable to that in mono-infected patients although they are at high risk of HBV reactivation. If there is HBV significantly detectable replication or it persists after HCV clearance, therapy with nucleoside/nucleotide analogs should be added.64
RECOMMENDATIONS
- •
HBV/HCV co-infected patients should be treated with PegIFN /RBV, following the same rules as in mono-infected patients. [B1]
- •
If there are significant HBV replication during or after HCV clearance, they can be simultaneously treated for HBV with nucleoside/nucleotide analogs. [B1]
Hepatitis C virus is the most common blood-transmitted infection among intravenous drug users (IDU), occurring in up to 67% of cases. Another form of HCV transmission is by intra-nasal drug use.
Sharing contaminated needles and syringes among injected drug users is the cause of most new HCV infection cases, so decreasing the risk of transmission is paramount in the control and care of this infection.63,64
In addition, HAV, HBV and HIV detection should also be performed. One must consider that the IDU population with chronic hepatitis is at greater risk of developing cognitive dysfunction, fatigue and depression in association with possible viral replication in the brain - this leads to greater neuropsychiatric abnormalities with the use and/or withdrawal of drugs.68,170
RECOMMENDATIONS FOR THE PREVENTION OF HCV INFECTION AMONG IDU (4; WORLD HEALTH ORGANIZATION)
- •
Offer a rapid HBV vaccination schedule.
- •
Offer incentives to increase compliance and completion of the HBV vaccination schedule.
- •
Implement sterile needle and syringe programs and provide a container to discard used syringes.
- •
Offer replacement therapy with opiates to treat dependence.
- •
Educate the IDU on risky behaviors fostering HCV infection.
Obstacles to treatment initiation in this group of patients include irregular monitoring, drug costs and the lack of appropriate follow-up facilities. One must also consider poor compliance, the possibility of reinfection, psychological and neuropsychiatric abnormalities due to PegIFN and other toxic reactions; the decision to treat should be individualized on a case-by-case basis.64,68,179
The IDU should also be counseled on the benefits of moderate alcohol ingestion or abstinence and on the moderate use of cannabis or abstinence, if there is evidence of advanced liver disease.
The treatment of HCV infection is cost-effective in IDU. Hence, the WHO recommend that all users should be evaluated for antiviral treatment since this may represent a good preventive measure by decreasing transmission. Treatment should be individualized.68,170
Recent studies have proven the viability and efficacy of treatment even in patients that have not discontinued drug use while on therapy.170,180
Potential drug-drug interactions between prescription and non-prescription drugs should be considered. Methadone levels may decrease in individuals treated with PegIFN/RBV. Although this interaction is usually sub-clinical, it requires withdrawal symptom monitoring.180
A recently conducted study of patients in a methadone detoxification program and treated with IFN -as monotherapy and in combination with RBVyielded a SVR of 36%; there was no difference in the results between those who remained abstinent while treated and those that relapsed. During treatment, they found that pre- existing neuropsychiatric ab- normalities were the most determining factor in hindering treatment response. Therefore, patients should be evaluated and treated until stabilized before initiating HCV therapy.181
In clinical development programs of antiviral drugs, individuals actively using non-prescription drugs were excluded; however, many studies have included patients on opiate replacement therapy although safety results have yet to be presented. Among drug-drug interaction studies of SOF, SMV, methadone and buprenorphine, no clinically significant interactions have been reported. Studies on daclatasvir and methadone/buprenorphine interaction are still in progress.64,68
SMV increases orally administered midazolam or triazolam concentrations, so care should be taken when prescribing them. Finally, there is scant available data on daclatasvir.181
RECOMMENDATIONS
- •
IDU should be routinely and voluntarily evaluated in order to detect anti-HCV; if negative, testing should be repeated every 6-12 months. [B1]
- •
Access to opiate replacement therapy is recommended as part of the integral program to decrease generalized injury. [B1]
- •
HCV treatment in IDU should be individualized within a multidisciplinary team. [A1]
- •
Anti-HCV regimens that can be used are the same as in non-IDU. [B1]
- •
Determining treatment in IDU is an individualized decision; those with early liver disease may be warned while awaiting further data and/or the development of better therapeutic options. [B2]
We gratefully acknowledge all the physicians in the Work Group for sharing their knowledge and experience in the development of this National Consensus on Hepatitis C.
Our special and sincere gratitude to Celia Mercedes Alpuche Aranda Ph.D., Past-President of the Asociación Mexicana de Infectologíay Microbiología Clínica, A.C. and to Miguel Ángel Valdovinos Díaz MD, Past-President of the Asociación Mexicana de Gastroenterología, for their support in this endeavor and for endorsing the final document.
We particularly thank Margarita Dehesa Violante M.D. and Enrique Wolpert Barraza M.D. for their generous contributions in time and effort and most importantly, for their experience in writing and reviewing the manuscript.
Dr. Juan Francisco Sánchez-Ávila
Work Group, National Consensus on Hepatitis C
Dr. Juan Francisco Sánchez-Ávila
Past-President, Mexican Association of Hepatology
Dr. Francisco Bosques-Padilla
Associate Professor, C. Facultad de Medicina y Hospital Universitario J.E.
González. UANL, Monterrey, Nuevo Leon, Mexico
Dr. Mauricio Castillo-Barradas
Department of Gastroenterology, CMN La Raza, IMSS.
Mexico City, Mexico
Dr. Graciela Castro-Narro
Department of Gastroenterology, INCMNSZ. Mexico City, Mexico
Dr. Laura Cisneros-Garza
Liver Disease Clinic, Hospital San José TEC de Monterrey.
Monterrey, Nuevo Leon, Mexico
Dr. Ruby Ann Chirino-Sprung
Gastroenterology, Hospital Ángeles. Mexico City, Mexico
Dr. Margarita Dehesa-Violante
Ex President, Mexican Association of Hepatology.
Mexico City, Mexico
Dr. Ignacio García-Juárez
Department of Gastroenterology, INCMNSZ.
Mexico City, Mexico
Dr. Ma. Saraí González-Huezo
Head, Department of Gastroenterology, ISSEMYM
Toluca, Estado de Mexico, Mexico
Dr. René Malé-Velázquez
Medical Director, Instituto de Salud Digestiva y Hepáticas
Head, Department of Gastroenterology, Hospital del Carmen
Guadalajara, Jalisco, Mexico
Prof. Nahum Méndez-Sánchez
Liver Research Unit. Medica Sur Clinic & Foundation.
Mexico City, Mexico
Dr. Rosalba Moreno-Alcántar
Head, Department of Gastroenterology, Hospital de Especialidades CMN
SXXI, IMSS. Mexico City, Mexico
Dr. Linda Muñoz-Espinosa
Head, Liver Unit, Hospital Universitario J.E. González. UANL
Monterrey, Nuevo Leon, Mexico
Dr. Mayra Ramos-Gómez
Head, Department of Gastroenterology, CMN 20 de Noviembre, ISSSTE,
Mexico City, Mexico
Dr. Ma. Teresa Rizo-Robles
Department of Gastroenterology, CMN La Raza, IMSS
Vicepresident, Mexican Association of Hepatology. Mexico City, Mexico
Dr. Juan Francisco Sánchez-Ávila
Department of Gastroenterology, INCMNSZ
Past-President, Mexican Association of Hepatology. Mexico City, Mexico
Dr. Ricardo Sandoval-Salas
Department of Gastroenterology, Hospital de Especialidades,
CMN Siglo XXI, IMSS. Mexico City, Mexico
Dr. Juan Sierra-Madero
Head, Department of Infectious Disease, INCMNSZ. Mexico City, Mexico
Dr. María del Rocío Torres-Ibarra
Department of Infectious Disease, Hospital de Infectología, CMN La Raza,
IMSS. Mexico City, Mexico
Dr. Rodrigo Vázquez-Frías
Department of Gastroenterology, Hospital Infantil de México “Federico Gómez”, SSA. Mexico City, Mexico
Dr. Enrique Wolpert-Barraza
Clínica Lomas Altas
Ex President, Mexican Association of Gastroenterology.
Mexico City, Mexico
7. Glossary- •
ALT: alanine transaminase, also called alanine aminotransferase.
- •
ANA: antinuclear antibodies.
- •
ARV: antiretroviral.
- •
AZT: zidovudine.
- •
BOC: boceprevir.
- •
CBC: complete blood count.
- •
DAA: direct-action antivirus.
- •
EIA: enzyme immunoassay.
- •
eRVR: extended rapid viral response.
- •
HAART: highly active antiretroviral therapy.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
HIV: human immunodeficiency virus.
- •
HVPG: hepatic venous pressure gradient.
- •
IDU: intravenous drug use/user.
- •
IFN: interferon.
- •
IL28B: interleukin-28B.
- •
INR: International Normalized Ratio.
- •
LFT: liver function test.
- •
MELD: model for end-stage liver disease.
- •
NR: null response.
- •
OLT: orthotopic liver transplant.
- •
PCR: polymerase chain reaction.
- •
PegIFN: Pegylated interferon.
- •
PI: protease inhibitor.
- •
PR: partial response.
- •
PT: prothrombin time.
- •
RBV: ribavirin.
- •
RNA: ribonucleic acid.
- •
RVR: rapid viral response.
- •
SMV: simeprevir.
- •
SOF: sofosbuvir.
- •
SVR: sustained viral response.
- •
TSH: thyroid-stimulating hormone.
- •
US: ultrasound.