Mirizzi’s syndrome (MS) is a rare complication of the inveterate biliary lithiasis. Diagnostic and therapeutic standardization is still missing, especially since laparoscopic cholecystectomy has become the gold standard approach for symptomatic cholelithiasis. Our study is a retrospective analysis based on a case-series. It considered 370 cholecystectomies performed from 2006 to 2011. We selected 11 patients affected by MS (2.97%). We divided them according to Csendes’ classification. Endoscopic Retrograde Cholangio-Pancreatography (ERCP) was used for biliary drainage when the patient suffered jaundice and/or cholangitis and, preoperatively, to confirm the suspicion of MS obtained through Magnetic Resonance Cholangio-Pancreatography (MRCP). We found it useful to exploit nasobiliary drainage (NBD) for intra-operative check of the biliary tree. In all 5 patients of the type 1 group MS was discovered intraoperatively and treated with Laparoscopic Sub-total Cholecystectomy (LSC). One patient suffered from biliary leakage, solved with NBD positioning. The type 2 group was made up of 2 women and 1 man. All of them were preoperatively submitted to ERCP and NBD positioning. Two underwent LSC and one was converted to laparotomy. The type 3 was represented by a 63-year-old woman suffering from recurrent cholangitis. She was submitted to MRCP, ERCP and then underwent LSC. The 2 patients affected by type 4 underwent open biliary reconstruction. In conclusion, every attempt should be made to identify MS prior to LCS since it will allow NBD insertion by ERCP. Once LCS is initiated, if MS is identified intra-operatively, we can provide the most practical surgical options.
In 1948 the Argentinian surgeon Pablo Luis Mirizzi described the case of a patient showing a partial obstruction of the common hepatic duct, due to the extrinsic compression of a gallstone lodged in the cystic duct or infundibulum, causing the associated inflammation.1 This condition was named after him, even though Kehr and Ruge had already described it in the early 1900’s.2,3
Mirizzi’s syndrome (MS) is a rare complication of the inveterate biliary lithiasis, with a prevalence among patients with colelithiasis between 0.5 and 1.4%.4,5 that can rise to 2.7% in some ethnic groups, such as Navajo Native Americans.6 The overall incidence of MS is low, reported in 0.7-2.53% of all patients undergoing cholecystectomy.7,8 In spite of intermittent symptoms and signs,9 the clinical presentation of MS can be outlined in 4 possibilities.10–12
- •
Obstructive jaundice (76% of cases).
- •
History of recurrent acute cholecystitis and/or cholangitis (35.3% of cases).
- •
Acute abdomen, due to a biliary peritonitis.
- •
Asymptomatic or paucisymptomatic.
With this variability of clinical pictures we can face a range of patients in whom it could be difficult to suspect MS before evaluation in the operative field,13 exposing the surgeon to the risk of facing unexpected conditions and a difficult operation, just like observed in MS type 1.
In 1982 McSherry provided a better description of the syndrome thanks to the use of Endoscopic Retrograde Cholangio-Pancreatography (ERCP) and distinguished between MS type 1, characterized by the extrinsic compression of the common bile duct (CBD) due to a trapped gallstone in the infundibulum or in the cystic duct and subsequent inflammation, and MS type 2, characterized by the presence of a cholecystocholedochal fistula.14 In 1989 Csendes further distinguished MS type 2 into 3 subtypes: type 2, with the cholecystocholedochal fistula involving 1/3 of the CBD diameter; type 3, with the fistula involving 2/3 of the CBD diameter; type 4, with the fistula involving the whole CBD diameter.15 In 2012 Beltràn introduced a new classification including a fifth case with complex cholecystobiliary fistulas and associated cholecystoenteric fistulas.16
Until the end of the eighties, iatrogenic lesions of the CBD were a well defined17 and rather rare entity, with an incidence between 0.2 and 0.5%.18 In the early nineties the rapid development of laparoscopic cholecystectomy suddenly determined an abrupt increase of CBD iatrogenic lesion incidence.19 According to Csendes, one of the most important factors involved in the increase of morbidity due to laparoscopic cholecystectomy is the dissection of Calot’s triangle (hepatocystic triangle), in patients with existing or previous acute cholecystitis.15 As guidelines haven’t yet been laid down, a diagnostic and therapeutic algorithm should be carefully planned for MS, with the aim of reducing the probability of encountering an unexpected complicated intraoperative anatomic situation. Our study is a retrospective analysis based on a case-series which aims at standardizing the MS diagnostic and therapeutic approach, while suggesting some technical notes to complete cholecystectomy with laparoscopy in known and unexpected cases.
Material and MethodsOur experience considered 370 cholecystectomies, 315 laparoscopic ones (85.14%) and 55 open ones (14.86%), performed from 2006 to 2011 at the Unit of Endocrine, Digestive and Emergency Surgery, Department of Biomedical Sciences and Human Oncology, Section of General and Oncologic Surgery, University Medical School of Bari “A. Moro”.
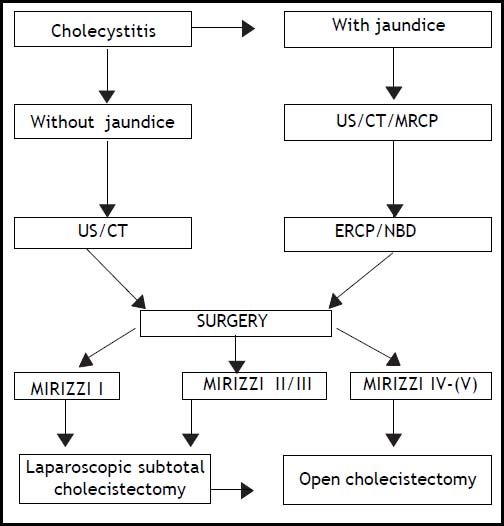
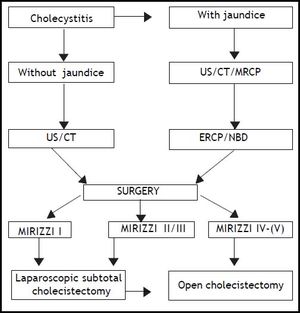
We retrospectively analyzed 11 patients affected by MS (2.97% of all cholecystectomies), 6 females and 5 males between 43 and 70 years old (average age: 63). We divided them into 4 groups according to Csendes’ classification. We examined the diagnostic approach that had been advocated and conducted since patient admittance. All patients admitted for cholecystitis were initially studied with UltraSound (US). If they presented an associate obstructive jaundice or cholangitis, a Computed Tomography (CT) scan was performed and then they underwent ERCP, sphincterotomy with stenting of the CBD and/or nasobiliary drainage (NBD) placement. Magnetic Resonance Cholangio-Pancreatography (MRCP) was performed in all patients with a history of recurrent acute cholecystitis without obstructive jaundice, with increased levels of hepatic enzymes (AST/ALT) and in cases of cholangitis or pancreatitis. On the contrary patients admitted with cholecystitis, first episode, without signs of CBD obstruction, were directly forwarded to laparoscopic cholecystectomy. When MS was discovered by MRCP, patients underwent ERCP, considered as a mere therapeutic procedure, in order to place a stent or an NBD. We found it useful to exploit this device intraoperatively for the identification of the CBD and to perform an intraoperative cholangiography. In our experience it allowed postoperative T-tube placement to be avoided. The only efficient diagnostic strategy for MS type 1 was laparoscopy.
All patients affected by MS type 1 were introduced to laparoscopic cholecistectomy whereas patients affected by MS type 2 and 3 were scheduled for laparoscopic subtotal cholecystectomy (LSC) broadly speaking 2 to 7 days after the endoscopic drainage. In the end MS type 4 was submitted to an open approach. The approach to cholecystectomy was the same even in the case of MS recognized intraoperatively (MS type 1) (Figure 1): indeed when Calot’s triangle (hepatocystic triangle) dissection was too difficult and it was impossible to recognize and dissect the cystic duct and artery contained in the inflammatory tissue by using the “critical view of safety” as described by Strasberg,20 we decided to perform the LSC as described below. Conversion to laparotomy was reserved to those cases with dense adhesions to colon and/or duodenum.
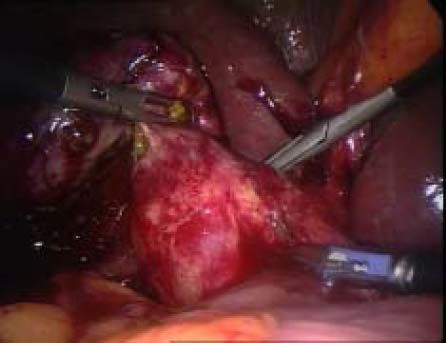
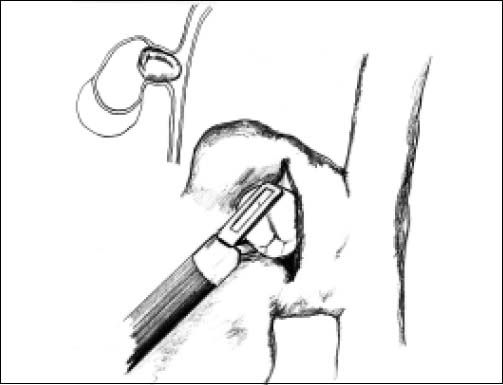
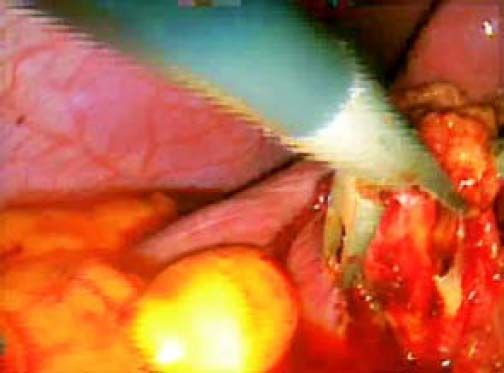
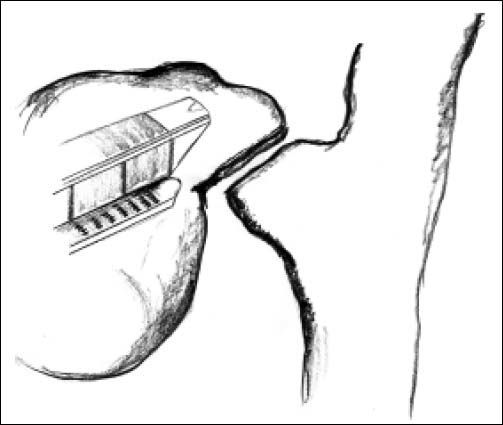
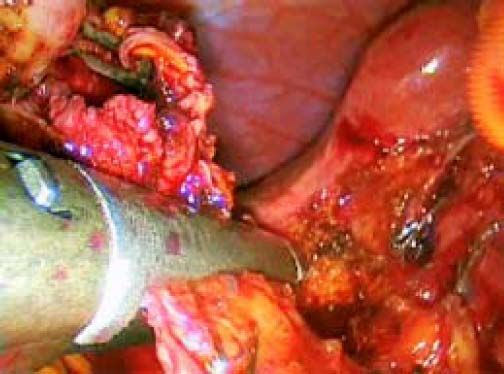
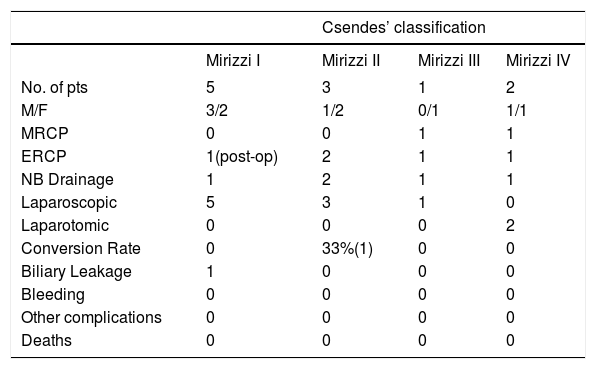
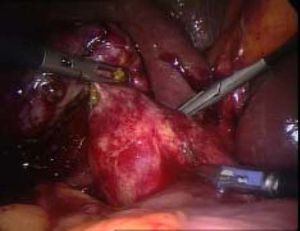
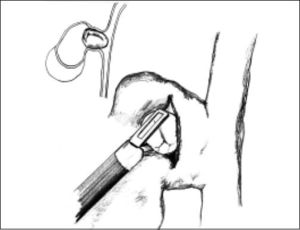
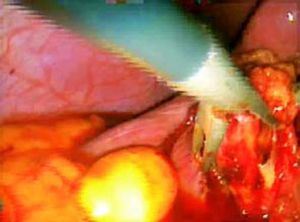
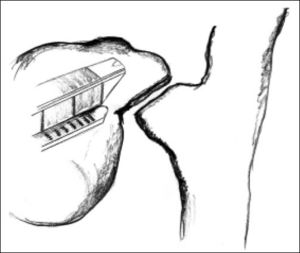
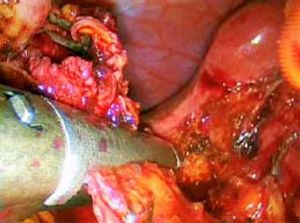
ResultsAccording to Csendes’ classification we identified: 5 cases of type 1, 3 cases of type 2, 1 case of type 3 and 2 cases of type 4. In all 5 patients of the type 1 group MS were discovered and confirmed intraoperatively. This group was treated with LSC: after identification and opening of the gallbladder fundus we remove gallstones in a bag. Then, by using an “inside approach” as described by Hubert19 (Figures 2 and 3), we explored the Hartmann’s pouch with the optic and detached the gallbladder starting from the fundus and until the rear of the infundibulum, where later the stump was closed by applying a linear endoscopic stapler at the Hartmann’s pouch (Figures 4 and 5). Drainage was left in the abdomen in all cases. In the cases of MS type 1 discovered intraoperatively we never needed a T-Tube, in the other cases the preoperative ERCP and NBD placement avoided the intra-operative T-Tube placement.
One patient suffered from biliary leakage (20%), solved by sphincterotomy and NBD positioning.
The type 2 group was made up of 2 women aged 54 and 68 presenting obstructive jaundice, and 1 man aged 70 with transient jaundice. All of them were preoperatively submitted to ERCP with sphincterotomy, NBD placement and diagnosis of MS type 2. Two of them underwent LSC without complications. In one case (33%) we converted the procedure to laparotomy because a dense inflammatory tissue around the gallbladder involved the duodenum. Then we performed a subtotal cholecystectomy, with closure of the biliary defect using the gallbladder infundibulum wall.
The type 3 group was represented by a 63 year old woman with cholelithiasis suffering from recurrent cholangitis. She was submitted to MRCP and MS type 3 was diagnosed. She underwent ERCP with sphincterotomy and NBD positioned in order to grant an intraoperative guide to identify the CBD. Two days later she was submitted to LSC with intraoperative cholangiography through the NBD to check the correct position of the stapler and the complete closure of the stump. The postoperative course was complication free.
The type 4 group was made up of a 70 year old man, and a 60 year old woman. The man, with recurrent acute cholecystitis and a diagnosis of MS type 4 obtained through MRCP, was submitted to open cholecystectomy and reconstruction of the CBD with resection of the fistula, mobilization of the duodeno-pancreatic bloc and end-to-end anastomosis over a T-tube drainage. The postoperative course was uneventful and the T-tube was removed after 3 months. The woman, with a preoperative diagnosis of cholelitiasis and jaundice (bilirubin level: 4 mg/dL) underwent ERCP and obtained a diagnosis of MS type 4: a NBD was positioned. She was then submitted to open cholecystectomy and hepatic-jejunal anastomosis without biliary drainage. The postoperative course was uneventful.
Summarizing, as can be seen from the table 1, among the 9 LSCs performed, we reported only one case of biliary leakage (11.11%) and an overall conversion rate of 11.11%.
Results.
| Csendes’ classification | ||||
|---|---|---|---|---|
| Mirizzi I | Mirizzi II | Mirizzi III | Mirizzi IV | |
| No. of pts | 5 | 3 | 1 | 2 |
| M/F | 3/2 | 1/2 | 0/1 | 1/1 |
| MRCP | 0 | 0 | 1 | 1 |
| ERCP | 1(post-op) | 2 | 1 | 1 |
| NB Drainage | 1 | 2 | 1 | 1 |
| Laparoscopic | 5 | 3 | 1 | 0 |
| Laparotomic | 0 | 0 | 0 | 2 |
| Conversion Rate | 0 | 33%(1) | 0 | 0 |
| Biliary Leakage | 1 | 0 | 0 | 0 |
| Bleeding | 0 | 0 | 0 | 0 |
| Other complications | 0 | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 | 0 |
There is no consensus on the management of MS, in terms of both diagnostic and surgical choices. Neither in the classification nor in the definition of the various anatomo-pathologic pictures can we see a uniformity of view. Some authors still prefer to use McSherry’s classification, while a great deal remains to be done from a physiopathological viewpoint in order to define the first grade of MS in all classifications. In clinical practice the anatomic modification of the biliary tract due to Mirizzi’s syndrome predisposes to iatrogenic lesions of the CBD during laparoscopic cholecystectomy and, in addition, the chronic inflammation and subsequent perivisceral fibrosis represents an obstacle to the safe dissection of Calot’s triangle (hepatocystic triangle).
Because of its nonspecific characteristics at imaging, there is no optimal diagnostic technique for MS. Becker, et al., as well as for Erben, et al., showing that preoperative recognition for MS using imaging is problematic, inconsistent and limited: indeed MS is documented preoperatively in only 50% of cases.21,22 However, in common practice, the assessment of a diagnostic flowchart must consider US as the first step in the assessment of biliary lithiasis even though its sensitivity for MS is estimated to be between 8.3% and 27%.23–25
A CT scan does not show any additional information compared to US, but it is, in our opinion, essential in order to differentiate MS from a malignant bilio-pancreatic obstruction.26–28
ERCP is mandatory in all cases of MS arising with obstructive jaundice, whereas MRCP, with more or less the same diagnostic accuracy as ERCP, is recommended in all cases of clinical suspicion of MS without obstructive jaundice. However, MRCP is weak in localizing a cholecystocholedochal fistula.13 Moreover it is our opinion that all non-invasive methods, US and CT and MRCP, must be systematically used with clinical good sense to improve pre-operative diagnostic data. At the moment there is no consensus about the use of one versus another diagnostic tool.
According to a recent systematic review by Antoniou, et al., the mean preoperative diagnosis rate of MS is 66.1%, with most authors reporting ERCP as the favorite diagnostic procedure; this tool has a satisfactory mean sensitivity rate of 76.2%.29
Many different ways of managing MS have been described in literature. Both on the diagnostic and on the therapeutic side there is a lack of defined guidelines. The anatomo-pathologic aspect of the biliary tract in MS can question the appropriateness of a laparoscopic cholecystectomy, because of the increased risk of even not immediately recognizable iatrogenic lesions of the CBD. Therefore, it is often mandatory to recur to traditional open surgery techniques. Approaching the literature and looking for the best technique in “open” surgery for MS, a subtotal cholecystectomy with an anterograde dissection, opening of the fundus, removal of the gallstone, reconstruction of the CBD using the remaining portion of the gallbladder and placing Ttube drainage in the CBD, was one of the first options to be described.30,31 Sometimes the only appropriate “open” procedure is the hepatic-jejunal anastomosis with Roux en Y loop after the removal of the gallbladder and part of the hepato-choledochus. The surgical procedures that take into consideration Calot’s triangle (hepatocystic triangle) dissection, like total laparoscopic cholecystectomy, are penalized by a higher conversion rate than open surgery and a higher risk of CBD lesions or jejunal lacerations.9,32 On the contrary, subtotal laparoscopic cholecystectomy, using an Endo-GIA, is more frequently completed without conversion26,33–34 and its complications are represented mainly by biliary leakage.31
From 1997 to 2003 Waisberg, et al., analyzed 8 patients with MS and successfully applied a subtotal cholecystectomy with the placement of a T-tube drainage to a patient with MS type 2, while in two patients with MS type 1 and 3 they performed a cholecystectomy and side-to-side choledochoduodenostomy. The same procedure, despite leaving the gallbladder in situ, was performed in a patient with MS type 4. In the remaining 4 cases, all MS type 1, they applied a total cholecystectomy.35
From 1995 to 1999 Schafer, et al. analyzed 13,000 laparoscopic cholecystectomies and found 39 cases of MS, classified according to McSherry into 34 MS type 1 and 5 MS type 2 ones. MS was preoperatively supposed only in 18 cases out of 39. ERCP played a key role in the preoperative assessment, being performed in each patient showing obstructive jaundice, while MRCP was performed only in few cases. The conversion rate was 74% in all patients with MS type 2 and 24 patients with MS type 1. As regards the surgical procedure, the dissection of Calot’s triangle (hepatocystic triangle) was always performed. This procedure was followed by: a total cholecystectomy in 23 cases; a T-tube implantation in 13 cases; hepatic-jejunal anastomosis with Roux en Y loop in 3 cases. In 5 cases a subtotal cholecystectomy was performed, because of the impossibility of a complete removal. The intraoperative complication rate was 7.7%: one CBD lesion, one jejunal laceration, one incident of local bleeding. The overall complication rate was 10.3%.36
From 1994 to 2005 Gomez, et al. considered 33 patients with MS. A laparoscopic cholecystectomy with classical dissection of the cystic duct was chosen to be the appropriate treatment for MS type 1, although their conversion rate was 56%. Regarding MS type 2 they performed the hepatic-jejunal anastomosis with Roux en Y loop. The Authors stressed the importance of MRCP in the preoperative assessment, conferring to ERCP a merely preoperative interventional role, when necessary. They also indicated how an intraoperative cholangiography could improve the chances of completing laparoscopic cholecystectomy without conversion.27
From 2003 to 2005 Sinha, et al. performed 889 laparoscopic cholecystectomies, with the aim of establishing an appropriate alternative to conversion to open surgery in complicated cases, and in 28 cases they carried out a subtotal cholecystectomy, without the dissection of Calot’s triangle (hepatocystic triangle) and without the ligation of the cystic duct. The conversion rate was reduced to 0.3%, with the additional advantage of conservative management of bile leaks, thanks to postoperative ERCP with endoscopic drainage.37
From 2005 to 2006 Rohatgi, et al. examined 5 cases of MS, among 323 laparoscopic cholecystectomies, suggesting a minimally invasive approach: opening fundus, removing the gallstones, exploring the potential fistula, and subtotal cholecystectomy performed with Endo-GIA if necessary. T-tube drainage was not always used.34
From 1991 to 2001 Yeh, et al. had previously analyzed 11 patients with MS: they had already successfully utilized an Endo-GIA to close the cystic duct stump in 2 of them.28
In 1995, Strasberg described a method to identify the hidden cystic duct during a laparoscopic cholecystectomy in order to reduce the risk of iatrogenic lesions of the CBD: the “critical view of safety”,20 consisting of the dissection of the apex of Calot’s triangle (hepatocystic triangle) between the infundibulum and the hepatic border, showing two and only two structures entering the gallbladder (cystic duct and artery). This approach is, in our opinion, really useful to avoid CBD damage, especially in difficult situations, like MS, when the inflammatory process has altered the normal anatomy of the gallbladder pedicle. Another alternative to the classic Calot’s triangle dissection was described by Hubert et al. for severe cholecystitis: in their “inside approach” they suggested the incision of the ventral/peritoneal surface of the gallbladder, from the fundus to Hartmann’s pouch, the evacuation of its content and its dissection from the liver bed from inside and outside, enabling an easier determination of the precise limits of the gallbladder wall and a safer dissection.19
ConclusionOur policy is that ERCP is mandatory in cases of obstructive jaundice, pancreatitis and MS diagnosed through MRCP. With this procedure we are able to perform a sphincterotomy and to place an NBD, considered to be very useful intraoperatively to identify the CBD and to perform an intraoperative colangiography. MS type 4 should be treated directly with a laparotomy. According to our experience, the surgical approach for MS type 1, 2, and 3 on the other hand should be laparoscopic, performing a subtotal cholecystectomy as described before. The dissection of Calot’s triangle (hepatocystic triangle) in patients with MS should be prescribed. Laparoscopic cholecystectomy is a widespread and successful minimally invasive approach to cholelithiasis, but it should not, therefore, be considered a simple technique to treat MS. Moreover, it is desirable to achieve a widespread knowledge of the various clinical presentations of MS as well as managing a flowchart, like the one we propose (Figure 6), capable of permitting a totally endoscopic approach in almost all cases.
AcknowledgementsWe would like to thank Prof Malcolm CLARK (B.A.) for the English version.
FundingNot supported.
Ethical ApprovalNo needed.
Competing InterestNo benefits in any form have been recived or will be received from a commercial party related directly or indirectly to the subject of this article.